
Cardiothoracic surgery and peripheral endovascular intervention in cardiovascular damage from a cohort of orphan rheumatological diseases—epidemiological and survival analysis
Introduction
Aortic diseases are caused by traumatic, infectious, genetic, epigenetic, congenital or acquired disorders; however, aortic structure is affected in all of these (1,2).
Under normal conditions, the aorta has a tubular structure with cellular components originating from different embryonic structures, and this constitutes the basis for different cellular adaptation responses to any stimulus (3), and variable responses of smooth muscle cells during stimulation by inflammatory molecules and dysregulation of the redox state (4). Also, the genetic expression pattern could contribute to the aortic susceptibility to damage, determining the extent of injury and the presence or absence of aneurysms (5).
Aortic and cardiovascular damage occurs in a high percentage of so-called rare or orphan diseases, regardless of etiology as in Marfan syndrome (MS) or similar phenotypes such as Loeys-Dietz syndrome (6,7) and also in patients with vasculitis, such as Takayasu arteritis (TA) (8); patients with Takayasu’s arteritis may have complicated or catastrophic cardiac outcomes (9-11).
Dilation or dissection of the aorta occurs in up to 70% of cases with MS. Histological changes in the aorta are characterized by fragmentation of the elastic lamina, cystic necrosis of the media and fibrosis and loss of smooth muscle cells (12). The heart valves can also be affected and may coexist with other cardiovascular disorders (13). The aortic dilation and/or pectus excavatum chest deformity present in these patients may be associated with alterations in right ventricular morphology and function, which in turn lead to ventricular dysfunction and valve failure (14-16).
On the other hand, patients with TA also can be affected by occlusion or dilation of the aorta, albeit at a low frequency. In general, occlusive lesions predominate in TA. Although the pathophysiological pathways that initiate the process of aortic damage in TA and MS seem to be different, it has been found that both diseases have an inflammatory component, with accompanying alteration in the structural proteins of the endothelium and endothelial dysfunction (17).
Large arteries, mainly the aorta and its main branches, are affected by inflammation that progresses to stenosis, fibrosis and occlusion (18). In TA, aortic root or heart valves are affected, causing hemodynamic disturbance (19). Left ventricular (LV) systolic dysfunction has been reported in patients with TA (20-22) but little is known about the development of heart failure in these patients.
In some patients with TA, dilatation of the aortic root that further evolves to valvular regurgitation and a dilated ventricle has been found. However, surgical treatment improves the outcomes of patients with moderate to severe aortic regurgitation due to TA because a dilated left ventricle is associated with a worse prognosis (23).
Regarding surgical management, the correction of the dilatation or rupture of the aorta is resolved through various surgical techniques that are applied according to the requirements of the patient and the experience of the surgeon. The indications for surgical management of TA are diverse (24). Percutaneous stent placement for affected segments has been advocated (25); however, selection of the various complex medical, surgical/interventional or combined procedures in all of these patients is a challenge for the treating physician, as it requires a consensus to decide the best approach.
The National Institute of Cardiology is a reference center for patients of any Mexican state that have rheumatological diseases that evolve into cardiovascular damage. Although such diseases have different etiologic backgrounds, they require the specialized surgical care that this institute provides when occlusion, arterial dilation, aneurysms or valvular damage occur.
The objective of this study was to evaluate the epidemiological conditions, type of surgical management, evolution, outcomes and surgical results in patients with different rheumatological diseases. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1523/rc).
Methods
This is retrospective and descriptive cohort study spanning a period of 47 years. Only patients that had surgery or peripheral endovascular intervention were included. TA was diagnosed in adults when more than 3 criteria of the American College of Rheumatology were fulfilled; in children, the EULAR/PRINTO classification was used (26). The rest of the connective tissue diseases were classified according to Ghent’s criteria. Each case was selected and evaluated by expert specialists, Rheumatologist, Vascular interventionists, and vascular and cardiothoracic surgeons. Data were collected from the clinical record, from which demographic variables, follow-up time, type of surgical procedure and complications were obtained.
Statistical analysis
Categorical variables were summarized using absolute frequencies and percentages. Continuous variables were reported with mean ± standard deviations (SD) or with median and interquartile ranges (IQR) according to the distribution of data. Normality was evaluated with the Kolmogorov test. Differences between categorical variables were evaluated by means of the chi-squared test or Fisher’s exact test, as appropriate; Student t-test or Wilcoxon rank sum test was used for continuous variables, depending on the distribution of data. Log rank test was used to compare survival rates among different patient groups, considering P≤0.05 as statistically significant. Survival analysis was performed using Kaplan-Meier curves. Statistical analyses were performed using STATA version 16 software (College Station, TX, USA).
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics committee of the Ignacio Chávez National Institute of Cardiology board (No. 19-1140). Individual consent for this retrospective analysis was waived.
Results
A total of 212 patients were included, 77/364 with TA and 135/300 with other connective tissue diseases. The flowchart is shown in Figure 1.
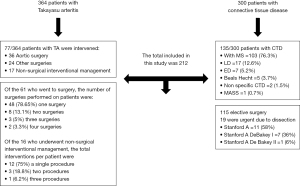
Patients with TA
The mean patient age was 31±14 years, and 60 patients (77.9%) were women.
Description of classification criteria, type of arterial injury and frequency of aneurysms according to gender are shown in Table 1. The main symptoms in adults were dyspnea (n=54, 70.1%), headache (n=46, 59.7%), dizziness (n=40, 51.9%), angina pectoris (n=32, 41.6%), blurred vision (n=29, 37.7%), paresthesia (n=26, 33.8%) and syncope (n=22, 28.6%).
Table 1
| Variables | Totala | Femalea | Malea | P |
|---|---|---|---|---|
| Total, n (%) | 77 (100.0) | 60 (77.9) | 17 (22.1) | |
| Age (mean ± SD) | 31±14 | 33±15 | 28±9 | NS |
| BMI (mean ± SD) | 22±4 | 23±4 | 23±3 | NS |
| Classification criteria variables (ACR, EULAR/PRINTO) | ||||
| Age <40 years | 57 (74.0) | 41 (68.3) | 16 (94.1) | 0.04 |
| Claudication | 43 (55.8) | 35 (58.3) | 8 (47.1) | NS |
| Heart murmur | 63 (81.8) | 49 (81.7) | 14 (82.4) | NS |
| Differences in systemic blood pressure | 68 (88.3) | 54 (90.0) | 14 (82.4) | NS |
| Absence of pulses | 70 (90.9) | 55 (91.7) | 15 (88.2) | NS |
| Type of arterial injury | ||||
| Type I | 1 (1.3) | 1 (1.7) | 0 | NS |
| Type I + P | 1 (1.3) | 1 (1.7) | 0 | NS |
| Type IIa | 3 (3.9) | 3 (5.0) | 0 | NS |
| Type IIb | 8 (10.4) | 6 (10.0) | 2 (11.8) | NS |
| Type IIb + p | 1 (1.3) | 1 (1.7) | 0 | NS |
| Type III | 8 (10.4) | 7 (11.7) | 1 (5.9) | NS |
| Type IV | 10 (13.0) | 7 (11.7) | 3 (17.6) | NS |
| Type IV+ C | 1 (1.3) | 1 (1.7) | 0 | NS |
| Type IV + P | 1 (1.3) | 1 (1.7) | 0 | NS |
| Type V | 29 (37.7) | 22 (36.7) | 7 (41.2) | NS |
| Type V + C | 6 (7.8) | 4 (6.7) | 2 (11.8) | NS |
| Type V + P | 5 (6.5) | 3 (5.0) | 2 (11.8) | NS |
| Type V + C + P | 3 (3.9) | 3 (5.0) | 0 | NS |
| Aneurysms | 11 (14.3) | 8 (13.3) | 3 (17.6) | NS |
| Laboratory parameters (median, range) | ||||
| Erythrocyte sedimentation rate (mm/h) | 27 [1.5–70] | 32 [1.5–70] | 10 [4–64] | NS |
| C-reactive protein (mg/L) | 4.8 [0.11–287] | 8.7 [0.11–287] | 2 [0.29–119] | NS |
| Comorbidities | ||||
| Diabetes | 4 (5.2) | 3 (5.0) | 1 (5.9) | NS |
| Hypertension | 55 (71.4) | 43 (71.7) | 12 (70.6) | NS |
| Smoking | 15 (19.5) | 9 (15.0) | 6 (35.3) | 0.08 |
| Alcoholism | 10 (13.0) | 3 (5.0) | 7 (41.2) | <0.001 |
| Patients with acute activity | 55 (71.4) | 48 (80.0) | 7 (41.2) | 0.01 |
| Age group | ||||
| Adults | 64 (83.1) | 50 (83.3) | 14 (82.4) | NS |
| Children | 13 (16.9) | 10 (16.7) | 3 (17.6) | NS |
a, n (%), unless otherwise specified. ACR, American College of Rheumatology; EULAR, European League Against Rheumatism; PRINTO, Paediatric Rheumatology International Trials Organisation; SD, standard deviations; NS, not significant; BMI, body mass index.
A total of 77 (21.2%) out of 364 patients had surgical or peripheral endovascular intervention procedures done, resulting in a mean of 1.6 procedures per year.
The median time from the moment of hospital admission to the diagnosis of TA was 2 months with a minimum of 1 day up to a period of 17 years.
In patients who required surgical treatment, a median time of 3 months (minimum of 1 day and maximum of 1 year 10 months) elapsed between the presentation of the case in surgical-medical session and the performance of the surgery.
The patients who were considered candidates for interventional treatment had a median time of 41 days (minimum of 1 day and maximum of 450 days) between the session and the procedure.
The type of cardiovascular surgery used according to the type of arterial injury is shown in Table 2. Aortic, mitral and tricuspid valve damage occurred in 8 (10.4%) patients, 4 (5.2%) patients and 1 (1.3%) patient, respectively.
Table 2
| G | Age | Type of arterial injury | Type of cardiovascular surgery | DD | Death |
|---|---|---|---|---|---|
| F | 11 | V + C | Coronary revascularization + femoro-femoral bypass | 16 | |
| F | 34 | V + C | Coronary revascularization + ascending aorta plasty + mitral valve replacement + aortic valve replacement | 2 | |
| F | 5 | V + C + P | Coronary revascularization | 108 | |
| F | 32 | V + C | Coronary revascularization + mitral valve replacement + aortic valve replacement | 36 | |
| M | 36 | V + C | Coronary revascularization | 1 | |
| F | 28 | IIa | Aortic valve replacement | 1 | |
| F | 26 | V | Aortic valve replacement + resection of discrete subaortic membrane | 0.1 | NSC |
| F | 25 | V | Aortic valve replacement + aortoplasty | 3 | |
| F | 15 | IV + C | Aortic valve replacement | 10 | |
| F | 43 | V | Aortic valve replacement | 36 | |
| M | 33 | V + P | Aortic valve replacement | 96 | |
| F | 49 | V | Aortic valve replacement + pacemaker | 12 | |
| F | 42 | V | Mitral valve replacement | 0 | SC |
| M | 35 | V | Mitral valve replacement + tricuspid valve plasty | 6 | |
| F | 64 | IIa | Pericardial window | 36 | |
| F | 34 | V + C + P | Pericardial window | 12 | |
| F | 76 | V | Pericardial window | 24 | |
| F | 36 | V | Bentall and DeBono + carotid and subclavian reimplantation | 5 | NSC |
| M | 31 | IIb | Bentall and DeBono + aortic valve replacement | 9 | NSC |
| M | 31 | V | Bentall and DeBono + mitral valve replacement + aortic valve replacement | 7 | |
| F | 30 | V | Bentall and DeBono + aortic valve replacement | 3 | |
| F | 30 | V + C | Bentall and DeBono + coronary revascularization | 144 | SC |
| F | 33 | V | Bentall and DeBono + mitral valve replacement + aortic valve replacement | 4 | NSC |
| F | 45 | III | Revascularization from ascending to infrarenal aorta | 25 | NSC |
| F | 22 | III | Revascularization from ascending to infrarenal aorta | 7 | |
| F | 12 | IV | Revascularization from descending to infrarenal aorta | 21 | |
| F | 23 | V | Revascularization from ascending to abdominal aorta | 2 | |
| M | 16 | IV | Revascularization from ascending to abdominal aorta | 1 | NSC |
| F | 59 | V + C + P | Revascularization of the abdominal aorta | 2 | SC |
| F | 60 | V + C + P | Revascularization of the abdominal and pelvic aorta and extra anatomical graft | 3 | NSC |
| F | 32 | V | Bifurcated thoracoabdominal replacement, reimplantation of lumbar vessels | 0 | NSC |
| M | 46 | V | Bypass from ascending aorta to infradiaphragmatic aorta | 3 | |
| F | 11 | IIa | Non-coronary sinus of Valsalva plasty | 12 | |
| F | 44 | V | Right carotid aortic bypass | 384 | |
| F | 30 | V | Resection of calcified VI aneurysm | 3 | |
| M | 33 | IIb | Resection of the left atrium and right ventricle | 60 |
G, gender; F, female; M, male; P, lung disease; C, coronary artery disease; DD, duration of disease (months); NSC, non-surgical cause; SC, surgical cause.
Table 3 shows other types of surgical interventions, depending on the type of arterial involvement present in various organs. Table 4 shows the types of peripheral endovascular intervention procedures that were done, according to the type of arterial injury.
Table 3
| G | Age | Type of arterial injury | Type of surgery | DD | Death |
|---|---|---|---|---|---|
| F | 22 | IIb | Left carotid revascularization | 23 | |
| M | 18 | V | Carotid revascularization | 0 | SC |
| M | 23 | III | Left renal revascularization | 36 | |
| F | 25 | V | Right and left renal revascularization | 306 | |
| F | 34 | III | Right and left renal revascularization | 396 | |
| F | 28 | IV | Right nephrectomy | 3 | NSC |
| F | 28 | IV | Right nephrectomy and aorto-renal graft | 4 | |
| F | 28 | V | Right nephrectomy | 3 | NSC |
| F | 43 | III | Right nephrectomy and right autotransplantation | 96 | |
| F | 45 | V | Left nephrectomy | 3 | |
| F | 25 | V | Left nephrectomy | 24 | |
| F | 17 | IV | Right renal autotransplantation to primitive iliac | 1 | |
| F | 18 | V | Left kidney autotransplantation | 1 | NSC |
| F | 16 | V | Right renal autotransplantation | 28 | |
| F | 40 | IV | Right renal autotransplantation and bypass of aortic coarctation | 17 | NSC |
| F | 43 | IV | Kidney transplant | 12 | |
| M | 34 | IV | Right renal autotransplantation | 1 | |
| M | 28 | V | Right renal autotransplantation | 0 | SC |
| F | 29 | V | Revascularization of the superior mesenteric artery | 348 | |
| F | 35 | V | Cesarean section | 24 | |
| F | 28 | V+C | Cesarean section | 36 | |
| M | 14 | V | Retroperitoneal tumor biopsy | 48 | |
| M | 19 | V | Exploratory surgery | 47 | |
| M | 16 | V+P | Right pelvic limb amputation | 210 |
G, gender; F, female; M, male; P, lung disease; C, coronary artery disease; DD, duration of disease (months); SC, surgical cause; NSC, non-surgical cause.
Table 4
| G | Age | Type of arterial injury | Type of vascular intervention | DD | Death |
|---|---|---|---|---|---|
| F | 56 | V | Balloon pulmonary angioplasty | 1 | NRI |
| F | 33 | V + P | Balloon pulmonary angioplasty | 84 | |
| F | 66 | IIb + P | Balloon pulmonary angioplasty | 1 | NRI |
| F | 16 | IIb | Stent graft in descending aorta | 108 | |
| F | 41 | IIb + P | Pulmonary artery stents | 0 | RI |
| F | 28 | V | Medicated stent grafting in the left kidney | 12 | |
| F | 27 | IV + P | Stent grafting in both renal vessels | 132 | |
| F | 11 | I | Stent grafting in left subclavian and angioplasty of right subclavian | 36 | |
| F | 42 | V | Abdominal, iliac and left subclavian stent grafting | 72 | |
| F | 2 | IIb | Palmaz stents in descending aorta | 48 | |
| F | 33 | IV | Two stents in abdominal aorta | 204 | |
| F | 20 | IV | Stents in descending and abdominal aorta | 204 | |
| F | 31 | IIb | Three stents in descending and abdominal aorta | 228 | |
| F | 47 | V + P | Left subclavian stent | 84 | |
| F | 33 | IIb | Left subclavian stent | 96 | NRI |
| M | 31 | V + C | Stents in bilateral renal arteries | 96 | |
| F | 45 | V | DDD pacemaker | 72 |
G, gender; F, female; M, male; P, lung disease; C, coronary artery disease; DD, duration of disease (months); NRI, not related to the intervention; RI, related to the intervention.
Of the 77 patients with TA that were included, 60 were treated with surgery and 17 with a peripheral endovascular intervention procedure; 52 (67.5%) survived, 21 (27.3%) died and 4 (5.2%) had an unknown outcome because they were lost to follow-up. Death was related to the surgical or peripheral endovascular intervention procedure in 6 cases and to other causes in 15 cases. Of the 21 patients that died, 17 were subjected to surgery and 4 to a peripheral endovascular intervention. Of the 17 patients that had surgery, 5 suffered death related to surgery and 12 died due to another cause. Of the 4 patients that were treated with a peripheral endovascular intervention, 1 suffered death related to the procedure and 3 died due to another cause.
The duration of extracorporeal circulation in patients treated with surgery was 148±53 min and the duration of aortic clamping was 98±52 min.
Of the 60 patients treated with surgery, 48 (78.6%) had one surgical procedure, 8 (13.1%) had two procedures, 3 (5.0%) had three procedures and 2 (3.3%) had four procedures. The corresponding number of patients that survived was 34, 4, 2 and 1. The corresponding number of deaths related to surgery was 3, 1, 0 and 0, and that related to other causes was 6, 3, 1 and 1. Five patients were lost to follow-up (all had one surgical procedure done).
Of the 17 patients treated with a peripheral endovascular intervention, 12 (75.0%) had one procedure, 3 (18.8%) had two procedures and 1 (6.2%) had four.
The corresponding number of deaths related to the procedures was 1, 0 and 0, and that related to other causes was 3, 0 and 0. One patient was lost to follow-up (one procedure was done in this patient).
Survival in patients with TA
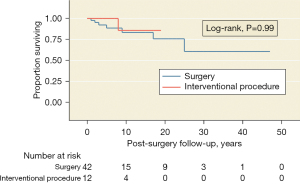
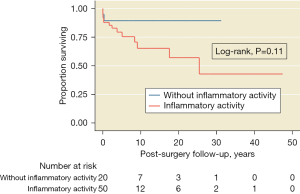
A median follow-up of 6 [2–12] years (maximum =47) was observed. The Kaplan-Meier estimates of the 5-year survival rate after treatment were 78% (95% CI: 62–87%) for the surgically treated group and 90% (95% CI: 50–98%) for the group treated with an endovascular interventionist procedure. There were no differences in the survival rates between surgical and peripheral endovascular intervention procedure groups (P=0.99, Figure 2) or between the group with TA activity vs. that without activity (P=0.11, Figure 3).
Patients with MS and similar conditions
A total of 135 patients were included: 77 (57.0%) were men and the male-to-female ratio was 1.3:1. MS was present in 103 (76.3%) patients, Loeys-Dietz syndrome in 17 (12.6%), Ehlers Danlos syndrome in 7 (5.2%), Beals-Hecht syndrome in 5 (3.7%), non-specific connective tissue disease in 2 (1.5%), and MASS (mitral, aorta, skin, skeletal) syndrome in 1 (0.7%). Age in years (median, range) at the time of surgery was 29 [8–58], 17 [6–43], 29 [4–49], 27 [17–61], 21 [19–23] and 22 years, respectively. Sixty-six patients (48.9%) had aortic dilation, 51 (37.8%) had dissection and 18 (13.3%) did not have aortic injury. Aortic, mitral and tricuspid valve damage occurred in 98 (72.6%), 50 (37.0%) and 20 (14.8%) patients, respectively.
Table 5 shows the frequency of Ghent criteria fulfilled in each of the syndromes. All patients with a diagnosis of MS met Ghent’s criteria; the genetic mutations sought were FBN1, FBN2, TGFBR1 and TGFBR2, COL3 and COL5. Of 135 surgical or interventional procedures, 115 were elective and 19 were urgent. One patient died while awaiting surgery. The types of surgical and interventional procedures done by gender are shown in Table 6.
Table 5
| Variable | Marfana (n=103) |
Loeys-Dietza (n=17) | Ehlers-Danlosa (n=7) | Beals-Hechta (n=5) | NSCTDa (n=2) |
MASSa,b (n=1) |
|---|---|---|---|---|---|---|
| No. Ghent criteria | 4 [2–5] | 2 [1–5] | 1 [1–3] | 2 [1–3] | 2 [2–3] | 2 |
| Family history | 70 (68.0) | 4 (23.5) | 1 (14.3) | 1 [20] | 1 [50] | 0 |
| Ophthalmologic | 73 (70.9) | 4 (23.5) | 0 | 0 | 0 | 0 |
| Systemic scorec | 91 (88.3) | 7 (41.2) | 1 (14.3) | 1 [20] | 2 [100] | 1 |
| Genetic test | 46 [45] | 14 [82] | 5 [71] | 3 [60] | 1 [50] | 1 [100] |
| Cardiovascular | 101 [98] | 17 [100] | 6 (85.7) | 5 [100] | 0 | 1 |
| Aortic dilation | 54 (52.4) | 8 (47.1) | 2 (28.6) | 2 [40] | 0 | 0 |
| Aortic ring diameter, mm (mean ± SD) | 33±12 | 33±11 | 41±23 | 30±5 | 22±4 | 18 |
| Valsalva sinus diameter, mm (mean ± SD) | 51±20 | 50±20 | 56±25 | 53±14 | 27±4 | 35 |
| Sinotubular union diameter, mm (mean ± SD) | 40±17 | 38±17 | 40±18 | 50±23 | 24±4 | 20 |
| Ascending aorta diameter, mm (mean ± SD) | 35±20 | 32±13 | 32±10 | 38±12 | 23±2 | 21 |
| Aortic dissection | 40 (38.8) | 7 (41.2) | 2 (28.6) | 2 [40] | 0 | 0 |
| Other cardiovascular | ||||||
| Aortic valve replacement | 61 (59.2) | 8 (47.1) | 4 (57.1) | 4 [80] | 0 | 1 |
| Mitral valve replacement | 17 (16.5) | 1 (5.9) | 3 (42.9) | 0 | 0 | 1 |
| Tricuspid valve replacement | 1 (1.0) | 0 | 1 (14.3) | 0 | 0 | 0 |
a, n (%), unless otherwise specified; b, since there was only one observation under the MASS category, summary of data does not apply; c, a score >7/20 was considered a positive systemic score. NSCTD, nonspecific connective tissue disease; MASS, Mitral, Aortic, Skeletal, Skin phenotype; SD, standard deviations.
Table 6
| Totala (n=135) | Malea (n=77) | Femalea (n=58) | |
|---|---|---|---|
| Elective surgery | 116 (85.9) | 68 (88.3) | 48 (82.8) |
| Urgent surgery | 19 (14.1) | 9 (11.7) | 10 (17.2) |
| Aortic dissection | 51 (37.8) | 29 (37.7) | 22 (37.9) |
| Reoperation | 29 (21.5) | 16 (20.8) | 13 (22.4) |
| Reoperation related to surgery | 12 (8.9) | 7 (9.1) | 5 (8.6) |
| Reoperation related to another cause | 17 (12.6) | 9 (11.7) | 8 (13.8) |
| Bentall and DeBono | 72 (53.3) | 46 (59.7) | 26 (44.8) |
| Bentall reoperation | 2 (1.5) | 1 (1.3) | 1 (1.7) |
| David | 15 (11.1) | 11 (14.3) | 4 (6.9) |
| David + mitral valve replacement | 2 (1.5) | 2 (2.6) | 0 |
| David + mitral plasty | 1 (0.7) | 0 | 1 (1.7) |
| David + aortic plasty | 1 (0.7) | 0 | 1 (1.7) |
| David + aortic plasty + ascending aorta replacement | 1 (0.7) | 1 (1.3) | 0 |
| David + aortic hemiarchal lining | 1 (0.7) | 0 | 1 (1.7) |
| David + aortic arch + subclavian revascularization | 1 (0.7) | 0 | 1 (1.7) |
| Florida sleeve | 1 (0.7) | 0 | 1 (1.7) |
| Yacoub | 1 (0.7) | 0 | 1 (1.7) |
| Mitral valve replacement | 5 (3.7) | 2 (2.6) | 3 (5.2) |
| Mitral valve replacement + TVC | 4 (3.0) | 2 (2.6) | 2 (3.4) |
| Mitral valve replacement + aortic valve replacement +TVC | 1 (0.7) | 0 | 1 (1.7) |
| Aortic valve replacement + thoracic abdominal replacement | 1 (0.7) | 0 | 1 (1.7) |
| Mitral plasty + Nuss correction procedure | 2 (1.5) | 1 (1.3) | 1 (1.7) |
| Supracoronarian replacement | 2 (1.5) | 0 | 2 (3.4) |
| Thoraco-abdominal aorta replacement and renal and mesenteric revascularization | 1 (0.7) | 0 | 1 (1.7) |
| Abdominal endoaneurysmorrhaphy | 2 (1.5) | 1 (1.3) | 1 (1.7) |
| Pectus excavatum Nuss correction procedure | 2 (1.5) | 2 (2.6) | 0 |
| Aortic endoprosthesis | 1 (0.7) | 1 (1.3) | 0 |
| Hybrid (surgery and interventionist procedure) | 1 (0.7) | 1 (1.3) | 0 |
| Pacemaker | 1 (0.7) | 0 | 1 (1.7) |
| Closure of atrial septal defect | 3 (2.2) | 0 | 3 (5.2) |
| Nuss surgery | 5 (3.7) | 5 (6.5) | 0 |
| Nuss surgery + mitral valve replacement | 1 (0.7) | 1 (1.3) | 0 |
| Pericardial window | 2 (1.5) | 1 (1.3) | 1 (1.7) |
| Cesarean section | 2 (1.5) | 0 | 2 (3.4) |
| Died while awaiting surgery | 1 (0.7) | 1 (1.3) | 0 |
| Duration of extracorporeal circulation, min (mean ± SD) | 205±68 | 210±70 | 199±65 |
| Duration of clamping, min (mean ± SD) | 144±44 | 150± 39 | 141±49 |
| Hospital stays in days, median [IQR] | 17 [0–223] | 15 [0–98] | 18 [3–23] |
| Duration of disease in months, median [IQR] | 132 [1–696] | 120 [1–696] | 138 [1–540] |
| Postsurgical follow-up in months, median [IQR] | 49 [1–324] | 60 [1–324] | 48 [1–252] |
a, n (%), unless otherwise specified; P value not significant for all comparisons. TVC, tricuspid valve change; SD, standard deviations; IQR, interquartile range.
Of the patients that had an aortic dissection, 19 presented with acute aortic dissection (Stanford A =11, Stanford A DeBakey I =7, Stanford A DeBakey II =1) that warranted emergency surgery; of these, 3 cases were related to pregnancy.
Survival in patients with MS and similar conditions
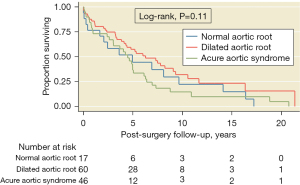
The median follow-up was 3.29 (0.42–6.62) years (maximum =21.37). The Kaplan-Meier estimates of the 5-year survival rate after surgical treatment were 82% (95% CI: 73–88%), and the restricted mean survival time after surgery was 15.83 years (95% CI: 13.7–17.8 years). There were no differences in the overall survival by clinical presentation, acute aortic syndrome, or presence of aortic aneurysm or dilation (Figure 4). There was no difference in all-cause mortality between the MS and similar conditions groups (P=0.44).
Discussion
Diseases of the ascending aorta and heart valves are the main manifestations within a wide variety of cardiovascular injuries that occur in patients with TA, MS and similar conditions, and are the main cause of morbidity and mortality in these patients (27).
The frequency of surgical care in patients with orphan rheumatological conditions was low in this study, very similar to previous reports (28), and is associated with the low frequency of these conditions. However, within the context of disease, the prevalence of complex surgical requirements can be very high when compared to other conditions of the aorta.
The dilation and dissection were found in similar percentages between patients with MS and similar conditions, but it is relevant to note that the average age in patients with Loeys-Dietz syndrome who had dissection was 26±11 years, which is significantly lower than the average found in patients with other similar conditions. This finding confirms that there is an increased risk for rapidly progressive dilatation in patients with Loeys Dietz syndrome (28).
Aneurysms in patients with MS and similar conditions have occurred in vessels other than the aorta, which adds to the extension and complexity of vascular damage commonly seen (29-32).
In MS and similar conditions, thoracic aortic aneurysms cause acute dissection and rupture, and are the leading cause of morbidity and mortality. Even within families, such aneurysms present wide phenotypic variability, sometimes leading to a late diagnosis that is only established when acute aortic rupture occurs. Unfortunately, catastrophic outcomes are not always averted because there are few preventive therapeutic options. For this reason, timely preventive therapy guided by early diagnosis and periodic monitoring to improve prognosis has been considered for these patients (33).
Acute aortic dissection is sometimes the initial presenting condition in patients with MS or similar conditions that leads to the recognition of such diagnoses. In this study, 19 (18.4%) patients with MS had acute aortic dissection when admitted to the hospital, but the reported prevalence in this condition varies by study (33).
Aortic dilation and dissection are reported in approximately 50% of pediatric MS patients and in 60–80% of adult MS patients, in whom aortic valve regurgitation may also be found. In some series these frequencies have not been different between age groups (34). Not all patients with MS have these cardiac complications, so the involvement of other genetic, environmental and physiological risk factors has been considered; besides, presence of aortic stiffness or increased carotid pulse pressure (surrogate marker of central pulse pressure) has been proposed as an additional risk factor (35).
In this series, 27 of 135 patients (20%) with MS and similar conditions were of pediatric age and 108 (80%) were adults. Aortic dilation was present in 18 (66.7%) pediatric patients and 48 (44.4%) adult patients, and dissection was found in 3 (11.1%) pediatric patients and 48 (44.4%) adult patients.
The risk of pregnancy-associated vascular complications in MS is uncertain due to verification bias, lack of knowledge of the prepartum diagnosis and insufficient peripartum imaging data. Nonetheless, it is generally accepted that there is an increased risk of acute aortic dilation and dissection of the ascending aorta in pregnant women with MS. Although management recommendations for pregnant patients vary between guidelines (36), preventive treatment is very important to lessen the probability of a poor outcome in both mother and child (37).
Vasculitides, which group together rheumatological diseases of varying etiologies, comprise another group of rare diseases. In this study, there were two patients with MS/similar conditions who had a surgical requirement due to acute aortic dissection during pregnancy. One patient had an acute aortic dissection induced by labor, and for this reason the pregnancy was interrupted, unfortunately without success for the product. The other patient was transferred to this center due to acute aortic dissection after pregnancy; she underwent Bentall surgery but mitral valve dysfunction was documented one year later, so she will require a reintervention. Both patients are currently alive.
Arterial damage in patients with TA is characterized by a chronic inflammatory process that leads to fibrosis and an associated occlusive state; however, aortic aneurysms can still occur, although at a lower incidence (38) and depending on ethnicity (39). The frequency of aneurysms in TA patients of this series was 14%. Isolated cases of aneurysms in other vascular beds, besides the aorta, that result in organ damage have been reported (40). In this study, 17 (22.1%) patients with renal artery involvement were found (one death related to the surgical event was observed), and coronary artery disease was found in five patients (all currently alive).
In TA, much of the variability of the aortic damage has been related to genetic susceptibility.
Our working group has previously found an association between HLA alleles and Takayasu’s arteritis in Mexican mestizo patients. HLA-B52 is a relevant susceptibility allele for Takayasu’s arteritis, and we found that HLA-B15 could be important as a disease marker in Mexican patients (41).
Alleles such as HLA-B52 and HLA-DR4 stand out in ethnically different populations, and we recently found the participation of an epitope located in the peptide binding site of the HLA-B molecule (positions 63 and 67) that appears to be shared by several disease-associated alleles (42,43).
Type 5 arterial injury predominates in Mexicans; in addition, one of the relevant clinical characteristics is arterial hypertension (44).
The long-term effects of surgical intervention depend on timely care and the selected approach, which varies by the complexity of the aortic injury or its large branches, and by any organic damage (45).
Survival after surgery in TA patients decreases with ongoing inflammatory activity (46); however, this issue was not investigated in this study. Although post hoc analyses did not suggest statistical differences between patients with ongoing disease activity versus those without, a tendency in a reduction of survival was observed in the former group (70% at 6 years of follow-up).
Surgery, peripheral endovascular intervention and hybrid management are key to the survival of patients with TA, MS and similar conditions. We found favorable long-term results and did not find outcome differences between surgery and peripheral endovascular intervention groups; these are in line with results of a previous meta-analysis (47).
Current advances in surgery and peripheral endovascular intervention are aimed to improve life expectancy. These strategies, in combination with medical therapy and adequate follow-up supported by the latest imaging technologies, help to improve the prognosis. Nonetheless, in order to positively influence preventive management, the study of injured tissue and its loss of functionality should continue to be investigated for a better understanding of damage mechanisms. The high costs of patient care are common, but few studies have studied this topic.
In developing countries, there are factors that influence the condition of orphanhood in these conditions, since there are factors that imply abandonment and include from ignorance of the diagnosis, which may be late. Other factors are the low influx of surgeons and interventional doctors with skills in managing damage in these conditions, which is required in the care of complex cases in patients with rare disease, and even more orphanhood can come from a low interest of those who regulate health policies, since they are generally unaware that although the prevalence is low, each of these conditions requires costly procedures for the patient, family and public care systems.
Surgery and intervention currently have state of the art techniques and methods that deserve to be known more widely, since the complications that arise in some patients with inflammatory rheumatic disease or of genetic origin, evolve to cardiovascular damage where aortic damage involves valve dysfunction, among others, and surgical, interventional or hybrid repair is successful regardless of etiology.
The major limitation of this study is its retrospective nature; bias may have mainly occurred during data entry in medical charts. Furthermore, few interventional and hybrid procedures were included. However, the findings support the importance of timely intervention in the advanced stages of the diseases studied.
Conclusions
In this center, good results were obtained in long-term survival with surgical and interventional treatment of patients with orphan diseases. The catastrophic results that can occur in the evolution of these patients merit a public health evaluation to institute policies that support efforts aimed at improving knowledge and recognition of the role of timely surgery and intervention within the complexity of these conditions.
Acknowledgments
This work was presented as an entry work project to the Mexican Academy of Surgery by Dr. MES, who was granted a distinction as a full member of the Rheumatology seat. All the authors thank the Mexican Academy of Surgery for this distinction.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1523/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1523/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1523/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics committee of the Ignacio Chávez National Institute of Cardiology board (No. 19-1140). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Restrepo MS, Turek JW, Reinking B, et al. Mycotic aneurysm in a child with history of coarctation of the aorta repair. Ann Pediatr Cardiol 2014;7:138-41. [Crossref] [PubMed]
- Faridhosseini R, Jabbari F, Shirkani A, et al. Multiple Right and Left Pulmonary Arteries and Subdivisions of Inferior Mesenteric Artery Aneurysms in Behcet's Disease Case: A Rare Clinical Presentation. Oman Med J 2014;29:e072. [Crossref] [PubMed]
- Okuyama K, Yaginuma G, Takahashi T, et al. The development of vasa vasorum of the human aorta in various conditions. A morphometric study. Arch Pathol Lab Med 1988;112:721-5. [PubMed]
- Gadson PF Jr, Dalton ML, Patterson E, et al. Differential response of mesoderm- and neural crest-derived smooth muscle to TGF-beta1: regulation of c-myb and alpha1 (I) procollagen genes. Exp Cell Res 1997;230:169-80. [Crossref] [PubMed]
- Absi TS, Sundt TM 3rd, Tung WS, et al. Altered patterns of gene expression distinguishing ascending aortic aneurysms from abdominal aortic aneurysms: complementary DNA expression profiling in the molecular characterization of aortic disease. J Thorac Cardiovasc Surg 2003;126:344-57; discission 357.
- Loeys BL, Schwarze U, Holm T, et al. Aneurysm syndromes caused by mutations in the TGF-beta receptor. N Engl J Med 2006;355:788-98. [Crossref] [PubMed]
- Siddiqi HK, Luminais SN, Montgomery D, et al. Chronobiology of Acute Aortic Dissection in the Marfan Syndrome (from the National Registry of Genetically Triggered Thoracic Aortic Aneurysms and Cardiovascular Conditions and the International Registry of Acute Aortic Dissection). Am J Cardiol 2017;119:785-9. [Crossref] [PubMed]
- Lie JT. Pathology of isolated nonclassical and catastrophic manifestations of Takayasu arteritis. Int J Cardiol 1998;66:S11-21. [Crossref] [PubMed]
- Mitrofanova LB, Rybakova MG. Causes and mechanisms of sudden cardiac death in children. Sud Med Ekspert 2021;64:43-9. [Crossref] [PubMed]
- Martín CE, Evangelista A, Teixidó G, et al. Aortic events in pregnant patients with Marfan syndrome. Lessons from a multicenter study. Rev Española Cardiol 2022. Available from: http://www.revespcardiol.org/en-aortic-events-in-pregnant-patients-avance-S1885585721002425
- Jedidi M, Chkirbene Y, Abdessayed N, et al. Sudden Death Due to Unusual Complication of Takayasu Arteritis: An Autopsy Case. Am J Forensic Med Pathol 2017;38:91-3. [Crossref] [PubMed]
- Collins MJ, Dev V, Strauss BH, et al. Variation in the histopathological features of patients with ascending aortic aneurysms: a study of 111 surgically excised cases. J Clin Pathol 2008;61:519-23. [Crossref] [PubMed]
- Rybczynski M, Mir TS, Sheikhzadeh S, et al. Frequency and age-related course of mitral valve dysfunction in the Marfan syndrome. Am J Cardiol 2010;106:1048-53. [Crossref] [PubMed]
- Oezcan S, Attenhofer Jost CH, Pfyffer M, et al. Pectus excavatum: echocardiography and cardiac MRI reveal frequent pericardial effusion and right-sided heart anomalies. Eur Heart J Cardiovasc Imaging 2012;13:673-9. [Crossref] [PubMed]
- Tandon A, Wallihan D, Lubert AM, et al. The effect of right ventricular compression on cardiac function in pediatric pectus excavatum. J Cardiovasc Magn Reson 2014;16:1-2. [Crossref]
- Kowalewski J, Brocki M, Dryjanski T, et al. Pectus excavatum: increase of right ventricular systolic, diastolic, and stroke volumes after surgical repair. J Thorac Cardiovasc Surg 1999;118:87-92; discussion 92-3. [Crossref] [PubMed]
- Soto ME, Soria-Castro E, Lans VG, et al. Analysis of oxidative stress enzymes and structural and functional proteins on human aortic tissue from different aortopathies. Oxid Med Cell Longev 2014;2014:760694. [Crossref] [PubMed]
- Numano F, Okawara M, Inomata H, et al. Takayasu's arteritis. Lancet 2000;356:1023-5. [Crossref] [PubMed]
- Zhang Y, Yang K, Meng X, et al. Cardiac Valve Involvement in Takayasu Arteritis Is Common: A Retrospective Study of 1,069 Patients Over 25 Years. Am J Med Sci 2018;356:357-64. [Crossref] [PubMed]
- Panja M, Kar AK, Dutta AL, et al. Cardiac involvement in non-specific aorto-arteritis. Int J Cardiol 1992;34:289-95. [Crossref] [PubMed]
- Talwar KK, Kumar K, Chopra P, et al. Cardiac involvement in nonspecific aortoarteritis (Takayasu's arteritis). Am Heart J 1991;122:1666-70. [Crossref] [PubMed]
- Lee GY, Jang SY, Ko SM, et al. Cardiovascular manifestations of Takayasu arteritis and their relationship to the disease activity: analysis of 204 Korean patients at a single center. Int J Cardiol 2012;159:14-20. [Crossref] [PubMed]
- Cheng X, Li Z, Dang A, et al. Different treatment options for Takayasu arteritis patients with moderate-to-severe aortic regurgitation: long-term outcomes. Rheumatology (Oxford) 2021;60:3134-43. [Crossref] [PubMed]
- Li C, Liu Y, Qi R, et al. Repair of Aortic Regurgitation due to Takayasu Arteritis. Heart Surg Forum. 2013;16:E24-6. [Crossref] [PubMed]
- Mason JC. Takayasu arteritis: surgical interventions. Curr Opin Rheumatol 2015;27:45-52. [Crossref] [PubMed]
- Ozen S, Pistorio A, Iusan SM, et al. EULAR/PRINTO/PRES criteria for Henoch-Schönlein purpura, childhood polyarteritis nodosa, childhood Wegener granulomatosis and childhood Takayasu arteritis: Ankara 2008. Part II: Final classification criteria. Ann Rheum Dis 2010;69:798-806. [Crossref] [PubMed]
- Groth KA, Stochholm K, Hove H, et al. Causes of Mortality in the Marfan Syndrome(from a Nationwide Register Study). Am J Cardiol 2018;122:1231-5. [Crossref] [PubMed]
- Price J, Magruder JT, Young A, et al. Long-term outcomes of aortic root operations for Marfan syndrome: A comparison of Bentall versus aortic valve-sparing procedures. J Thorac Cardiovasc Surg 2016;151:330-6. [Crossref] [PubMed]
- Latter DA, Ricci MA, Forbes RD, et al. Internal carotid artery aneurysm and Marfan's syndrome. Can J Surg 1989;32:463-6. [PubMed]
- Matsuda M, Matsuda I, Handa H, et al. Intracavernous giant aneurysm associated with Marfan's syndrome. Surg Neurol 1979;12:119-21. [PubMed]
- Carr SB, Imbarrato G, Breeze RE, et al. Clip ligation for ruptured intracranial aneurysm in a child with Loeys-Dietz syndrome: case report. J Neurosurg Pediatr 2018;21:375-9. [Crossref] [PubMed]
- Kim ST, Brinjikji W, Kallmes DF. Prevalence of Intracranial Aneurysms in Patients with Connective Tissue Diseases: A Retrospective Study. Am J Neuroradiol 2016;37:1422-6. [Crossref] [PubMed]
- Rurali E, Perrucci GL, Pilato CA, et al. Precise Therapy for Thoracic Aortic Aneurysm in Marfan Syndrome: A Puzzle Nearing Its Solution. Prog Cardiovasc Dis 2018;61:328-35. [Crossref] [PubMed]
- Wozniak-Mielczarek L, Sabiniewicz R, Drezek-Nojowicz M, et al. Differences in Cardiovascular Manifestation of Marfan Syndrome Between Children and Adults. Pediatr Cardiol 2018. Available online:
10.1007/s00246-018-2025-2 10.1007/s00246-018-2025-2 - Jondeau G, Boutouyrie P, Lacolley P, et al. Central pulse pressure is a major determinant of ascending aorta dilation in Marfan syndrome. Circulation 1999;99:2677-81. [Crossref] [PubMed]
- Narula N, Devereux RB, Malonga GP, et al. Pregnancy-Related Aortic Complications in Women With Marfan Syndrome. J Am Coll Cardiol 2021;78:870-9. [Crossref] [PubMed]
- Cox DA, Ginde S, Kuhlmann RS, et al. Management of the pregnant woman with Marfan syndrome complicated by ascending aorta dilation. Arch Gynecol Obstet 2014;290:797-802. [Crossref] [PubMed]
- Tanaka A, Afifi RO, Safi HJ, et al. Thoracoabdominal Aortic Aneurysm in a Patient With Takayasu Arteritis. Ann Thorac Surg 2020;109:e91-3. [Crossref] [PubMed]
- Yang KQ, Meng X, Zhang Y, et al. Aortic Aneurysm in Takayasu Arteritis. Am J Med Sci 2017;354:539-47. [Crossref] [PubMed]
- Ouali S, Kacem S, Ben Fradj F, et al. Takayasu arteritis with coronary aneurysms causing acute myocardial infarction in a young man. Tex Heart Inst J 2011;38:183-6. [PubMed]
- Vargas-Alarcón G, Flores-Domínguez C, Hernández-Pacheco G, et al. Immunogenetics and clinical aspects of Takayasu's arteritis patients in a Mexican Mestizo population. Clin Exp Rheumatol 2001;19:439-43. [PubMed]
- Flores-Domínguez C, Hernández-Pacheco G, Zúñiga J, et al. Alleles of the major histocompatibility system associated with susceptibility to the development of Takayasu's arteritis. Gac Med Mex 2002;138:177-83. [PubMed]
- Vargas-Alarcón G, Hernández-Pacheco G, Soto ME, et al. Comparative study of the residues 63 and 67 on the HLA-B molecule in patients with Takayasu's Arteritis. Immunol Lett 2005;96:225-9. [Crossref] [PubMed]
- Soto ME, Espinola N, Flores-Suarez LF, et al. Takayasu arteritis: clinical features in 110 Mexican Mestizo patients and cardiovascular impact on survival and prognosis. Clin Exp Rheumatol 2008;26:S9-15. [PubMed]
- Bartczak-Rutkowska A, Trojnarska O, Ciepłucha A, et al. Enlarging aneurysm of the ascending aorta in a pregnant woman with Takayasu arteritis. Kardiol Pol 2020;78:82-3. [Crossref] [PubMed]
- Rosa Neto NS, Shinjo SK, Levy-Neto M, et al. Vascular surgery: the main risk factor for mortality in 146 Takayasu arteritis patients. Rheumatol Int 2017;37:1065-73. [Crossref] [PubMed]
- Jung JH, Lee YH, Song GG, et al. Endovascular Versus Open Surgical Intervention in Patients with Takayasu's Arteritis: A Meta-analysis. Eur J Vasc Endovasc Surg 2018;55:888-99. [Crossref] [PubMed]


