
Proposal for a new local recurrence score in patients with recurrent malignant pleural mesothelioma
Introduction
Patients diagnosed with malignant pleural mesothelioma (MPM) have a high risk of local recurrence (LR) even after multimodality therapy approach (1-4). The optimal treatment approach, for patient with histologically proven MPM, according to the latest ERS/ESTS/EACTS/ESTRO guidelines (5), is defined as induction chemotherapy followed by macroscopic complete resection (MCR). Prior to define this multimodality therapy approach, patients have to undergo mediastinal staging either via mediastinoscopy or via endobronchial ultrasound to rule out lymph node (LN) metastasis. Up to date the multimodality therapy approach consists of induction chemotherapy followed by MCR. The preferred systemic therapy for induction is still cisplatin/pemetrexed since the landmark trial by Vogelzang et al. (6) in 2003. There are recent immunotherapy phase II/III trials, that have been investigated and shown to improve outcome for resectable MPM. MCR is defined as either extrapleural pneumonectomy (EPP) or (extended) pleurectomy/decortication [(E)PD].
As the individual course of the disease is hard to predict, those who present with local hemithoracic relapse represent a particular challenge, since local therapy such as surgery or radiation therapy may be limited due to side effects, the extent and the localization of the recurrence. There are no standardized therapy regimens for recurrent MPM, but systemic and localized therapies are part of the current state. Nevertheless, the optimal treatment strategy balancing efficacy and morbidity is not yet defined in the present guidelines (2-4,7). For a better therapeutic allocation, a classification and evaluation of LR pattern would be helpful.
Based on the extension of peritoneal carcinomatosis established by Sugarbaker’s peritoneal cancer index (PCI), a thoracic spread pattern was mirrored and tailored for recurrent pleural mesothelioma (8). Sugarbaker’s PCI measures the tumor spread within the abdominal cavity by dividing it into 13 regions, although it was not primarily used for recurrent diseases of the abdominal cavity.
To apply this index to MPM, the surgical approach of (E)PD and EPP was analyzed. To achieve MCR, different anatomical structures have to be approached within these surgery types, as they are: parietal pleura, chest wall (CW) (in case of local infiltration), visceral pleura, diaphragm, mediastinal pleura, lung parenchyma (LP) in case of (E)PD, and LN. Based on this configuration and inspired by Sugarbaker’s PCI, we defined an MPM specific LR pattern within the thoracic cavity, and further established a local recurrence score (LRS).
The aim of this present study was to evaluate the prognostic impact of this newly investigated LRS, and to evaluate the impact on prognosis depending on second line treatments. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1628/rc).
Methods
This is an observational study of retrospective nature. The institutional prospective database was searched for MPM patients who obtained MCR through curative-intent surgery after induction chemotherapy and presenting with LR in the follow up period during the observation period of 2001–2017 (Figure 1). Follow-up was monitored by serial imaging every 4 months either by computed tomography (CT)-scan or positron-emission-tomography/computed tomography (PET/CT-scan) in the outpatient clinic. Recurrence was confirmed either histologically or radiologically with biopsy or increasing lesions over time, respectively.
LRS
The thoracic cavity was divided into the following sections: CW, mediastinum, diaphragm, LP, neo-pleural thickening (N-PT) and LN (Figure 2). Discrimination between the six locations is also demonstrated in Figure 2.
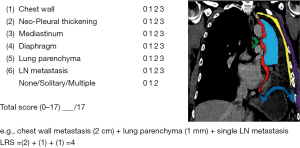
Each progressive progression in the sense of tumor growth seen in one of the above-mentioned regions on imaging was declared as a relapse/metastasis. In order to exclude an inflammatory process, radiological suspicion of recurrence was first raised after serial imaging sessions. In case of multiple lesions in one section, the biggest lesion was taken for calculation according to the lesion size score (LS). The LS was also adopted of Sugabaker’s lesion score for peritoneal carcinomatosis (8). LS =0 is defined as no visible tumor, LS 1 shows tumor up to 0.5 cm, LS 2 up to 5.0 cm and LS 3 >5.0 cm. In our cohort, each lesion was measured by the perpendicular diameter on axial CT imaging by two surgical staff members independently and the points 0–3 were given as depicted in Figure 2. Finally, the LRS is defined as the sum of the measured lesions at each recurrence site. In case of LN involvement, a different measurement method was used. LN metastasis was measured in short axis on CT-scans and were sub-divided into single and multiple LN metastases. The maximum number of points that could be reached was 17. Two independent thoracic surgeons, reviewed, measured and analyzed the images based on the radiologists’ report.
Statistical analysis
Regarding treatment parameters, descriptive statistics were calculated and results are reported as median and range for continuous variables and absolute and relative frequency for categorical covariates.
In a first approach, only LRS was used to fit the proportional hazard model. The proportional hazard assumption was tested and the assumption cannot be rejected. Lastly, LRS was adjusted according to second line treatment, the hazard ratio (HR) of each covariate was calculated including one interaction term for LRS and second line treatment.
We included LRS as a covariate adjusting in a regression setting with either surgery type [EPP, (E)PD] or one of the second line treatments, allowing for an interaction between the two variables.
Post recurrence survival (PRS) was calculated according to the Kaplan-Meier method and defined from first recurrence until death or lost to follow up. LRS was then used to evaluate the PRS time. Additionally, the median PRS time was adjusted according to LRS as well as type of surgery.
Comparison of differences in survival rates between patient groups were evaluated using a non-parametric log-rank test.
A P value of <0.05 was considered as statistically significant. For statistical analysis, R-software version 3.5.3 and SPSS version 25 was used.
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This retrospective analysis is approved by local ethics committee (Kantonale Ethikkomission) (Nos. StV 29-2009 and EK-ZH 2012-0094). This project was integrated into the new project BASEC—number: 2020-02566. Informed consent could not be obtained from all patients as the data collection goes back to 2001.
Results
Patient characteristics
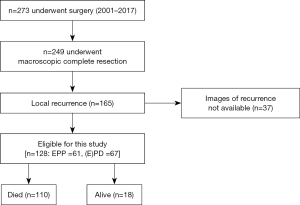
From 2001 until 2017, 273 MPM patients were diagnosed with MPM and underwent curative intent surgery and of those 249 patients achieved MCR after undergoing induction chemotherapy (Figure 1). Out of 249 patients, 165 patients were diagnosed with LR. LR was identified via serial imaging in an alternative manner by CT and PET and only histologically confirmed in two cases by CW biopsy and LN biopsy via endobronchial ultrasound. Of 165 LR patients, 37 patients have been excluded from analysis due to missing and incomplete data.
The final analysis was performed with 128 patients [EPP: n=61 and extended pleurectomy/decortication (E)PD: n=67]. Patient characteristics are shown in Table 1. Mean age at surgery was 63 (range, 33–77) years old. Male gender and epithelioid type were dominant (90.6% and 82.8%, respectively). Median PRS for all patients was 10.6 months (IQR, 4.8–18.5). Second line treatment was delivered in 104 patients (81.2%). The distribution of second line therapy was as followed: 65 patients had chemotherapy, 27 patients with radiotherapy, 11 patients with immunotherapy and 16 patients underwent surgery. At the time of analysis, 110 patients were dead and 18 patients are still alive. The median follow-up time was 58.6 months.
Table 1
| Variables | Overall (n=128) | (E)PD (n=67) | EPP (n=61) | P |
|---|---|---|---|---|
| Male gender (%) | 116 (90.6) | 62 (92.5) | 54 (88.5) | 0.635 |
| Age at surgery, mean [SD], years | 63 [59-66] | 64 [61-68] | 62 [58-66] | 0.102 |
| Right laterality of MPM (%) | 81 (63.3) | 47 (70.1) | 34 (55.7) | 0.132 |
| Surgery (%) | 128 (100.0) | 67 (100.0) | 61 (100.0) | <0.001 |
| Histological subtype pre-treatment (%) | 0.591 | |||
| Biphasic | 14 (10.9) | 5 (7.5) | 9 (14.8) | |
| Epithelioid | 106 (82.8) | 57 (85.1) | 49 (80.3) | |
| Sarcomatoid | 3 (2.3) | 2 (3.0) | 1 (1.6) | |
| Undefined | 4 (3.1) | 2 (3.0) | 2 (3.3) | |
| Unknown | 1 (0.8) | 1 (1.5) | 0 | |
| Induction chemotherapy (%) | <0.001 | |||
| No | 11 (8.6) | 10 (14.9) | 1 (1.6) | |
| Unknown | 10 (7.8) | 10 (14.9) | 0 | |
| Yes | 107 (83.6) | 47 (70.1) | 60 (98.4) | |
| Adjuvant radiotherapy (%) | <0.001 | |||
| No | 99 (77.3) | 57 (85.1) | 42 (68.9) | |
| Unknown | 11 (8.6) | 10 (14.9) | 1 (1.6) | |
| Yes | 18 (14.1) | 0 | 18 (29.5) | |
| Pathological staging surgery (%) | 127 (99.2) | 66 (98.5) | 61 (100.0) | 1 |
| IMIG stage surgery 7th-edition (%) | 0.584 | |||
| I | 9 (7.0) | 6 (9.0) | 3 (4.9) | |
| II | 19 (14.8) | 10 (14.9) | 9 (14.8) | |
| III | 79 (61.7) | 40 (59.7) | 39 (63.9) | |
| IV | 19 (14.8) | 9 (13.4) | 10 (16.4) | |
| Unknown | 2 (1.6) | 2 (3.0) | 0 | |
| IMIG stage surgery 8th-edition (%) | <0.001 | |||
| IA | 7 (5.5) | 4 (6.0) | 3 (4.9) | |
| IB | 60 (46.9) | 28 (41.8) | 32 (52.5) | |
| II | 1 (0.8) | 1 (1.5) | 0 | |
| IIIA | 26 (20.3) | 11 (16.4) | 15 (24.6) | |
| IIIB | 13 (10.2) | 3 (4.5) | 10 (16.4) | |
| Unknown | 21 (16.4) | 20 (29.9) | 1 (1.6) | |
| 2nd line treatment (%) | 0.12 | |||
| No | 22 (17.2) | 8 (11.9) | 14 (23.0) | |
| Unknown | 2 (1.6) | 2 (3.0) | 0 | |
| Yes | 104 (81.2) | 57 (85.1) | 47 (77.0) | |
*, initial multimodality therapy consisted of induction chemotherapy followed by surgery [EPP =41; (E)PD =49] and optional adjuvant radiotherapy (EPP =17) surgery followed by adjuvant chemotherapy [(E)PD and partial pleurectomy =4] and surgery followed by adjuvant radiotherapy (EPP =1, partial pleurectomy =1). (E)PD, (extended) pleurectomy/decortication; EPP, extrapleural pneumonectomy; MPM, malignant pleural mesothelioma; IMIG, international mesothelioma interest group.
The distribution of LR and LRS
Points summarized for the LRS depending of the type of surgery was 2–12 in case of an (E)PD and 1–8 in case of an EPP. The median LRS was significantly higher in the (E)PD group than in the EPP group (6 vs. 2, P<0.001).
The distribution of LR was N-PT 61.7%, LN 55.5%, CW 47.7%, mediastinum 38.3%, diaphragm 24.2% and LP 25.0%. In these six sections, there was a statistically significant worsening in the presence of recurrence for the respective section compared with no evidence of recurrence (CW: 9 vs. 16 months, P=0.05; LN: 9 vs. 17 months, P=0.02) (Table 2).
Table 2
| Recurrence site | Presence of recurrence | Total (n=128*) | PRS (months) | 95% CI | P value |
|---|---|---|---|---|---|
| CW | Positive | 61 | 9.00 | 4.90–13.10 | 0.05 |
| Negative | 67 | 16.00 | 10.19–21.81 | ||
| N-PT CW | Positive | 79 | 10.00 | 6.67–13.33 | 0.58 |
| Negative | 49 | 14.00 | 9.18–18.83 | ||
| N-PT mediastinum | Positive | 49 | 10.00 | 7.55–12.45 | 0.64 |
| Negative | 79 | 12.00 | 6.64–17.36 | ||
| Diaphragm site | Positive | 31 | 9.00 | 5.60–12.05 | 0.11 |
| Negative | 97 | 12.00 | 8.34–15.66 | ||
| LP [only (E)PD] | Positive | 32 | 16.00 | 9.19–22.81 | 0.65 |
| Negative | 35 | 14.00 | 5.63–22.37 | ||
| Mediastinal LNs | Positive | 71 | 9.00 | 6.50–11.50 | 0.02 |
| Negative | 56 | 17.00 | 14.37–19.63 |
Positive: presence of recurrence for the respective section; Negative: no evidence of recurrence for the respective section. *, except missing values. PRS, post recurrence survival; CW, chest wall; N-PT, neo-pleural thickening; LP, lung parenchyma; (E)PD, (extended) pleurectomy/decortication; LN, lymph node.
Prognostic impact of LRS and PRS
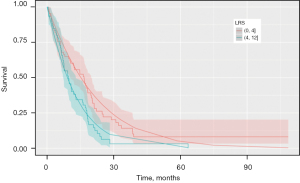
Primary analysis revealed a significant cut-off up to an LRS of 6 (P=0.04) having a prognostic impact on survival. However, a cut-off value at LRS 4 was determined by leaving out the highest values. Therefore, the cut-off at 4 seemed to be robust according to our analysis (P=0.04, not shown).
In survival analysis regardless of the type of surgery, PRS was significantly longer in patient with LRS ≤4 than for patients with LRS >4 (HR =1.68; 95% CI: 1.07–2.60; P=0.023) PRS according to the type of surgery revealed, a longer PRS of 12.4 months (IQR, 6.45–20.32; P=0.03) compared to 9.3 months (IQR, 2.93–17.40) for (E)PD and EPP, respectively (Figures 3,4).
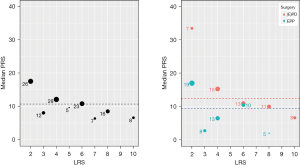
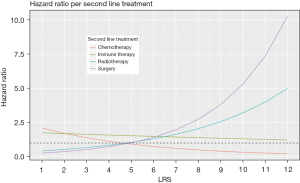
Prognostic impact between LRS and second line treatment
The HR according to second line treatment groups are shown in Figure 5. This analysis revealed that radiotherapy seemed to be beneficial for patients with an LRS ≤5, whereas it seemed not to be the right choice for patients with LRS >5 (P<0.03). Similar, but opposite results hold true for the group undergoing chemotherapy for second line treatment, which showed a favorable effect for patients treated with chemotherapy and LRS >4 (P=0.002). Patients with LRS ≤4 showed a longer survival after radiotherapy or local surgery.
Discussion
The present study established and revealed a score for patients with LR of MPM after multimodality therapy, namely LRS. We showed that LR can be allocated to a certain pattern inspired by Sugarbaker’s PCI and based on the anatomical structures approached by MCR.
The surgically approached anatomical structures are the ones that are incorporated in our LRS by dividing the hemithorax into 6 regions. These structures are always approached in a standardized way, at our institution. The first intraoperative step is to peel off the parietal pleura along the CW, followed by decortication of the lung and finished by partial resection of the pericardium and/or the resection of the diaphragm. Associated with each of these localizations is LN resection.
Therapy options for recurrent disease in patients with MPM for second- or third-line treatment are still not defined by default according to the ESMO and ASCO guidelines (2-4,7). In general, treatment for LR depends on the extent, as well as the distribution of the relapse. Nowadays, local therapy options such as resection or radiotherapy are mostly for limited localized recurrent diseases. However extensive recurrence pattern requires systemic treatment strategies to improve both local tumor control and survival (1,2,7,9-12).
The decision of the patient’s eligibility for second line therapy is challenging and requires expertise and interdisciplinary collaboration. Eligibility criteria and risk factors are known for patients with MPM undergoing surgery as first attempt, as there are higher age, worse performance status, male gender, non-epithelioid, that are associated with poor prognosis and represent predictors for overall survival (13-15). However, to our knowledge, independent prognostic factors for a better risk stratification for second line treatment allocation are missing.
In this analysis, patients with LRS ≤4 showed to benefit from radiotherapy and surgery as a second line therapy compared to patients with an LRS >4. Radiotherapy at this time, is used as part of a multimodality treatment approach (neo- or adjuvant setting), but in general not for curative second line treatment. However, it can be used in a palliative setting, for example for pain reduction (7,16). There are only a few reports about local radiotherapy as a salvage strategy for recurrent MPM. Our group recently investigated the feasibility of stereotactic body radiation (SBRT) for oligoprogressive recurrent MPM with a median progression free survival after first SBRT of 6 months (17).
In the presence of localized MPM CW recurrence, selected cases can be managed surgically as shown in a study by Burt et al. (18), where CW resection as a second line therapy after first progression for patients with localized MPM had an improved prognosis and overall survival after second surgery according to their histology was 20.4 months for epithelioid and 7.4 months for biphasic type (18). Another study by Politi and Borzellino, showed similar results for redo surgery for recurrent MPM after EPP with a median overall survival time of 14.5 months (6 to 29 months) (19). We already reported comparable results in patients undergoing redo surgery for second line therapy after relapse. They had a significantly longer median PRS compared with patients receiving other types of second-line therapy (16 vs. 9 months) (20). Additionally, based on this present analysis, radiotherapy and surgery should be the preferred approach in patients with limited extent. Another point that supports these therapeutic approaches is the distribution of the recurrence if situated at the CW or LN and if represented in an LRS of ≤4. Surgery, in particular, as a treatment modality for recurrent MPM should be applied very selectively. A reason that LR seems to be more common at lateral neo-pleura and LN than at the mediastinal pleura might be an easier access for histological biopsies leading to an “overdiagnosis” of these localizations. Secondly, we can speculate, surgically approaching the lateral pleura is easier and more radical by pleurectomy than the mediastinal pleura and therefore microscopic residues may remain easier there.
Generally, patients with progressive disease are treated with second line chemotherapy either using the same drugs as applied for induction, in case of response, or with alternative drugs for non-responder including patients with stable disease. A new approach in the treatment of advanced MPM is immunotherapy (21). These novel agents are still under investigation and its role is not yet defined. A recent published multicenter randomized phase III trial (PROMISE-meso) investigated pembrolizumab compared to standard chemotherapy for advanced pre-treated MPM (NCT02991482). Unfortunately, there was no overall improvement for pembrolizumab over chemotherapy (HR =1.04; 95% CI: 0.66–1.67; P=0.85). In this present analysis, immunotherapy as second line therapy was investigated and showed, that patients receiving immunotherapy for second line therapy seemed to be beneficial if they had an LRS >4.
We are aware of the limitations of this study and the interpretation must be done with caution due to the limited number of patients, its retrospective nature, as well as the initial two different surgical approaches. In some cases, especially the ones that have been referred from external institutions, coronal or sagittal slices were absent. Axial slices are universally available on all CT scans. To ensure a broad and worldwide application, all measurements were therefore performed on axial image slices. Additionally, acting as a referral center, it was not possible to obtain all available data and of equal quality for each patient from referring centers. Therefore, 37 of 165 patients were excluded due to insufficient data and this might also be considered a limitation of this study as a possible bias. Furthermore, inhomogeneity regarding surgical and multimodality approach is seen in the cohort. The different localization and the extent of the patient’s recurrence may influence the second line treatment allocation and must be interpreted with caution.
In this challenging disease, pathological findings are well known as a prognostic factor in any therapeutic approaches. The absence of pathological factors is one of the most considerable issues in this study. However, the definitive pathological diagnosis of LR is often challenging due to postoperative situations.
The local therapies for LR such as surgical excision is still controversial, however several reports revealed the efficacy of local therapies in such a situation (17-20). The decision which patients should be operated on in case of recurrence is crucial and stays an exception. The decision was always be made consensual at our weekly based interdisciplinary tumorboard and always with the same and most experienced thoracic surgeon in MPM surgery.
Furthermore, adjuvant radiotherapy as a therapy option, was recently published by our group, where 21 consecutive patients have been treated with hypofractionated radiotherapy for oligoprogressive MPM. In this retrospective single-institution study the feasibility of an SBRT approach for oligorecurrent MPM was proven (17).
However, to our knowledge, this is the first report, which established a LR-pattern and LR-score with a potential prognostic impact on second line treatment allocation and survival, which needs to be further evaluated in a prospective manner.
Acknowledgments
We thank Dr. Chloe Spichiger for her support in the edition of the article, Dr. Martina Friess for editing the tables, and Alessandra Matter for data collection.
Funding: This work was funded in part by Swiss National Science Foundation (No. PP00P3_159269).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1628/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1628/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1628/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1628/coif). WW reports that he is on the Advisory Board and a speaker of Astra Zeneca and a speaker of Covidien (Medtronic) which he received a teaching and speaker grant. IO reports receiving institutional grants from Roche and Medtronic and speakers’ fees from Roche and AstraZeneca. She is on Advisory Boards of AstraZeneca and MSD. She is Treasurer and President Elect for ESTS and in the Program Committee of ESTS, IASLC and ISHLT. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This retrospective analysis is approved by local ethics committee (Kantonale Ethikkomission) (Nos. StV 29-2009 and EK-ZH 2012-0094) and informed consent was not mandatory for this retrospective analysis as data collection went back to 2001.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Scherpereel A, Astoul P, Baas P, et al. Guidelines of the European Respiratory Society and the European Society of Thoracic Surgeons for the management of malignant pleural mesothelioma. Eur Respir J 2010;35:479-95. [Crossref] [PubMed]
- Kindler HL, Ismaila N, Armato SG 3rd, et al. Treatment of Malignant Pleural Mesothelioma: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018;36:1343-73. [Crossref] [PubMed]
- Batirel HF, Metintas M, Caglar HB, et al. Adoption of pleurectomy and decortication for malignant mesothelioma leads to similar survival as extrapleural pneumonectomy. J Thorac Cardiovasc Surg 2016;151:478-84. [Crossref] [PubMed]
- Flores RM, Pass HI, Seshan VE, et al. Extrapleural pneumonectomy versus pleurectomy/decortication in the surgical management of malignant pleural mesothelioma: results in 663 patients. J Thorac Cardiovasc Surg 2008;135:620-6, 626.e1-3.
- Opitz I, Scherpereel A, Berghmans T, et al. ERS/ESTS/EACTS/ESTRO guidelines for the management of malignant pleural mesothelioma. Eur J Cardiothorac Surg 2020;58:1-24. [Crossref] [PubMed]
- Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003;21:2636-44. [Crossref] [PubMed]
- Baas P, Fennell D, Kerr KM, et al. Malignant pleural mesothelioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26:v31-9. [Crossref] [PubMed]
- Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res 1996;82:359-74. [Crossref] [PubMed]
- Rimner A, Simone CB 2nd, Zauderer MG, et al. Hemithoracic radiotherapy for mesothelioma: lack of benefit or lack of statistical power? Lancet Oncol 2016;17:e43-4. [Crossref] [PubMed]
- Nowak AK, Millward MJ, Creaney J, et al. A phase II study of intermittent sunitinib malate as second-line therapy in progressive malignant pleural mesothelioma. J Thorac Oncol 2012;7:1449-56. [Crossref] [PubMed]
- Maio M, Scherpereel A, Calabrò L, et al. Tremelimumab as second-line or third-line treatment in relapsed malignant mesothelioma (DETERMINE): a multicentre, int andomized, randomised, double-blind, placebo-controlled phase 2b trial. Lancet Oncol 2017;18:1261-73. [Crossref] [PubMed]
- Grosso F, Roveta A, Gallizzi G, et al. Management of recurrent pleural mesothelioma: Successful rechallenge with nintedanib in combination with chemotherapy. Clin Case Rep 2018;6:2000-4. [Crossref] [PubMed]
- Curran D, Sahmoud T, Therasse P, et al. Prognostic factors in patients with pleural mesothelioma: the European Organization for Research and Treatment of Cancer experience. J Clin Oncol 1998;16:145-52. [Crossref] [PubMed]
- Ceresoli GL, Grosso F, Zucali PA, et al. Prognostic factors in elderly patients with malignant pleural mesothelioma: results of a multicenter survey. Br J Cancer 2014;111:220-6. [Crossref] [PubMed]
- Herndon JE, Green MR, Chahinian AP, et al. Factors predictive of survival among 337 patients with mesothelioma treated between 1984 and 1994 by the Cancer and Leukemia Group B. Chest 1998;113:723-31. [Crossref] [PubMed]
- Münter MW, Nill S, Thilmann C, et al. Stereotactic intensity-modulated radiation therapy (IMRT) and inverse treatment planning for advanced pleural mesothelioma. Feasibility and initial results. Strahlenther Onkol 2003;179:535-41. [Crossref] [PubMed]
- Schröder C, Opitz I, Guckenberger M, et al. Stereotactic Body Radiation Therapy (SBRT) as Salvage Therapy for Oligorecurrent Pleural Mesothelioma After Multi-Modality Therapy. Front Oncol 2019;9:961. [Crossref] [PubMed]
- Burt BM, Ali SO, DaSilva MC, et al. Clinical indications and results after chest wall resection for recurrent mesothelioma. J Thorac Cardiovasc Surg 2013;146:1373-9; discussion 1379-80. [Crossref] [PubMed]
- Politi L, Borzellino G. Second surgery for recurrence of malignant pleural mesothelioma after extrapleural pneumonectomy. Ann Thorac Surg 2010;89:207-10. [Crossref] [PubMed]
- Kostron A, Friess M, Crameri O, et al. Relapse pattern and second-line treatment following multimodality treatment for malignant pleural mesothelioma. Eur J Cardiothorac Surg 2016;49:1516-23. [Crossref] [PubMed]
- Dozier J, Zheng H, Adusumilli PS. Immunotherapy for malignant pleural mesothelioma: current status and future directions. Transl Lung Cancer Res 2017;6:315-24. [Crossref] [PubMed]


