
Development of a universal thoracic enhanced recover after surgery protocol for implementation across a diverse multi-hospital health system
IntroductionOther Section
Enhanced recovery after surgery (ERAS) pathways define a standard operating procedure for the peri-operative care of surgical patients. They were first developed for the colorectal surgical patient population and aimed at expediting bowel recovery (1,2). ERAS protocols outlined evidence-based elements of optimal management within all peri-operative care phases. Common elements sought to reduce operative stress and preserve anabolic homeostasis. Colorectal ERAS protocols have been successful in improving post-operative outcomes and decreasing patient length of stay, so these principals were extrapolated into ERAS protocols for other surgical subspecialties.
Several thoracic ERAS protocols have been described (3-5). While evidence-based tenants exist, we found little information in the literature on how to operationalize these recommendations, including what barriers and facilitators surround implementation. In 2019, the European Society of Thoracic Surgery (ESTS) released a comprehensive ERAS guideline based on extensive literature review and expert consensus (6). To the authors’ knowledge, no group has described the implementation process of this comprehensive ERAS protocol across a diverse health system comprised of several hospital types. Many factors prevent successful implementation of a new protocol, including provider resistance, lack of belief or knowledge of the effectiveness of a unified patient care protocol, lack of skills to implement the protocol, and inadequate organizational management support and resources (7,8). Thus, utilizing specific theories and strategies of Dissemination and Implementation (D&I) science to improve the adoption of new protocols into care is imperative (7,9).
The University of Colorado Health (UCHealth) system is comprised of six hospitals within three geographic regions along the front range of Colorado. One hospital is the site for the University of Colorado School of Medicine, with medical students, general surgery and cardiothoracic surgery residencies, and other aligned training programs. The other five hospitals range from university affiliated with residents but no students to completely community based with no trainees. About 500 anatomic pulmonary resections (lung segmentectomies, lobectomies, or pneumonectomies) are performed annually at UCHealth hospitals. While the majority of these operations are performed by five general thoracic surgeons, no standardized perioperative protocol previously existed. Therefore, heterogenous practice patterns between surgeons occurred. This led to inconsistent management of similar patients within and between hospitals and potentially suboptimal surgical outcomes. After reviewing system-wide outcomes data related to the target population, the authors saw the opportunity to standardize care and potentially improve patient postoperative outcomes. The purpose of this study was to describe the structured implementation of an evidence-based thoracic ERAS protocol for all patients undergoing anatomic pulmonary resection for quality improvement purposes. In this article we will describe the development of a system-wide, multidisciplinary group to lead implementation and identify facilitators and barriers encountered during implementation. Specific examples of implementation strategies are used to demonstrate how D&I science was used to support the implementation of the thoracic ERAS protocol (10). We present the following article in accordance with the SQUIRE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-518/rc).
MethodsOther Section
Ethical oversight
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study given quality improvement exemption by the Colorado Multiple Institutional Review Board, protocol number 20-3051, and individual consent for this study was waived as a quality improvement project within the healthcare system.
Step 1: establishing intent
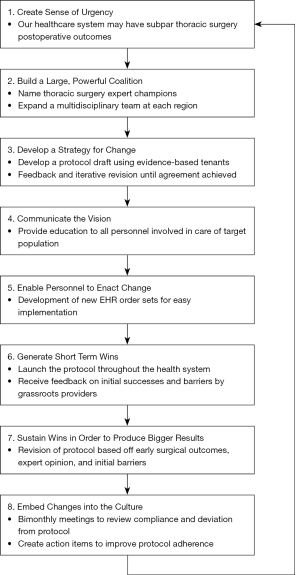
Figure 1 shows our implementation steps adapted from Kotter’s eight step model of change (11). We utilized Kotter’s eight step program for implementing change as this is validated and readily applies to the steps in ERAS implementation. Our intent to establish a thoracic ERAS protocol was prompted by feedback from national data benchmarking on postoperative outcomes with the target population. We identified opportunities to improve several postoperative outcomes. We sought to develop a thoracic ERAS protocol using evidence-based tenants provided in the ESTS guidelines. Once we established the target population, we engaged our health system’s Executive Leadership Committee to sponsor and expedite protocol development. Concurrently, we began the implementation process with a focus group of key stakeholders (subsequently expanded) where we reviewed the goals of implementation of a thoracic ERAS protocol, reviewed the current care processes at the different hospitals, introduced the proposed thoracic ERAS protocol (based on the ERAS society/ESTS thoracic ERAS guidelines) and discussed facilitators and barriers. This helped us select corresponding implementation strategies.
Step 2: identifying champions
A Director of Thoracic ERAS was established as the system-wide leader for this project. A Thoracic ERAS Implementation Specialist was established to engage in coordination of the Thoracic ERAS Steering Committee. Both would serve as the lead contacts of the program. An analysis of system-wide billing data using current procedural terminology (CPT) codes was performed for the three preceding years to identify which providers regularly performed anatomic lung resections across the healthcare system. It was necessary to identify local surgeon and anesthesia champions to spearhead the process at each of the six hospitals (Identify and prepare champions). Each local champion served as the point of contact for their respective specialties at each of the health system’s regions. Requirements of these champions were commitment to adaptation and implementation of the new protocol. Each had an intimate knowledge of their region’s external and internal culture, prior practice patterns, financial limitations, contact points for key personnel, and usual workflow practices.
Step 3: team expansion
The champions recruited stakeholders who were subject matter experts involved in quality improvement and/or involved in the clinical care of the target patient population at each individual location (Build a coalition). This included surgeons, anesthesiologists, advanced practice providers, quality improvement specialists, executive leadership liaisons, nurses, nutritionists, and respiratory, physical, and occupational therapists at each region. The key stakeholders established a bimonthly Thoracic ERAS Steering Committee meeting for development and implementation of the thoracic ERAS protocol. Agenda items for the first meeting were introductions, establishing roles and responsibilities, and establishing the implementation timeline (Create a learning collaborative).
Step 4: protocol development
We began protocol development with a rigorous literature review. The ESTS guidelines addressed all perioperative phases of care and outlined a standard operating procedure for anatomic lung resections6. Each item was rated both on the level of evidence compiled by the authors and the strength of recommendation given by the expert thoracic surgeon panel. However, some management tenants were vague (i.e., regional pain control should be used, but no specific approach is recommended). Effective elements from other studies were adopted, including specific management regarding early chest tube removal and specific agents for multimodal pain control (3-5). Experts within the healthcare system were also consulted for recommendations (Capture and share local knowledge). In addition to identifying evidence-based best practices, uniform management was prioritized to reduce irrational variation. The team’s approach to uncertainty in evidence was to seek general agreement and standardize with a commitment to modify when new evidence was presented. Once the group compiled recommendations for each principle, a draft was compiled for review by all key stakeholders.
Step 5: iterative revision
The protocol first draft was initially sent to the local surgeon and anesthesia champions for feedback (Audit and provide feedback). They provided context for individual hospital limitations, provider practice culture, patient population demographics, and structural factors that made several protocol tenants uniquely challenging at some locations while being amendable at others. For example, the presence of medical students, residents, and fellows inherently creates learner-based considerations at the academic hospitals that do not exist at the community-based hospitals, which are staffed predominantly by full-time board-certified physicians and their dedicated advanced practice provider teams. Large, dedicated specialist groups like multidisciplinary comprehensive cancer centers exist and meet with more regularity within some medical specialties at the quaternary academic center than the community-based hospitals. Each protocol element was scrutinized considering these hospital specific limitations. Multiple phases of feedback and iterative revision were performed until no additional changes were recommended. The same process was repeated for all five of the thoracic surgeons across the hospital system and interested stakeholders within the Thoracic ERAS Steering Committee until general consensus was achieved.
Step 6: infrastructure creation
After the system-specific thoracic ERAS protocol was established, we addressed the infrastructure changes required for implementation. The major need was creation of electronic health record (EHR) order sets specific to the thoracic ERAS protocol (Centralize technical assistance). Order sets were created to ensure uniformity, compliance, and ease of use. The Thoracic ERAS Implementation Specialist engaged with our healthcare system’s health information technology personnel to begin the build. Prior colorectal and gynecologic ERAS order sets provided the framework for these order sets with modifications made specific to the thoracic ERAS protocol. This required several meetings with subsequent additional iterative revisions. The order set rough draft was presented to the Thoracic ERAS Steering Committee for institution-specific feedback related to each hospital’s setting and culture. Workflow, culture, and infrastructural differences required specific order set adaptations for each healthcare system region. Adjustments were made to add, remove, or change items in order to reflect daily workflow while maintaining the goal of providing uniform, standardized care.
Step 7: resource acquisition
Initiation of some protocol aspects required acquisition of new resources including digital chest drainage systems and immunonutrition dietary supplementation. While there is mixed evidence regarding digital chest drainage systems’ efficacy, our group universally desired their acquisition to better monitor need for chest tubes. This request was reviewed by our healthcare system’s value and analysis (“Value Analysis”) team, which has accountability for assessing the acquisition of new equipment and evaluating the return on investment. The digital chest drainage system was not initially approved by Value Analysis but gained approval after re-engagement by the Thoracic ERAS Leadership. The evidence surrounding immunonutrition and its effect on reducing postoperative surgical site infections was sufficient to merit immediate acquisition with support from executive sponsors, and the administrative groups within the individual healthcare system regions provided these supplements to the patients free of charge.
Step 8: initial rollout
After executive sponsor approval, the protocol was rolled out on May 1, 2021. Prior to rollout, the protocol was shared with all staff engaged in care of patients undergoing anatomic lung resections within the health system by the local champions. Nurse educators provided teaching sessions to units involved in these patient’s care at each region (Conduct educational meetings). During the initial weeks after rollout, the care teams required assistance from the Thoracic ERAS Steering Committee and local champions to troubleshoot application of the protocol (Conduct ongoing training). Additional order set optimizations were made to address application of the order sets (e.g., differences in workflow patterns between hospitals that made seamless processes at once hospital burdensome at others). Timely and consistent review of protocol compliance and deviation was established through monthly meetings (Audit and provide feedback). A Thoracic ERAS data dashboard was developed to facilitate outcomes analysis. This provided each local champion an overview of their region’s thoracic ERAS protocol implementation metrics and patient outcomes.
ResultsOther Section
Implementation of the UCHealth thoracic ERAS protocol took 13 months from conception to rollout. The protocol has required several modifications since rollout. Reasons for modifications included workflow differences between hospitals requiring more specific regional adaptations, surgeon feedback of changes that facilitated postoperative complications, and engagement of stakeholders that were initially less engaged. As continued compliance data and outcomes measured are analyzed, additional granular optimizations are expected to occur.
Thoracic ERAS Protocol D&I Stakeholders
An analysis of system-wide billing data identified seven thoracic surgeons who performed anatomic lung resections (01/01/2018–06/30/2020). Of these, five were in practice during the thoracic ERAS protocol development and rollout. Table 1 lists the thoracic ERAS protocol stakeholders. The thoracic ERAS protocol development process identified successively larger groups of stakeholders. The UCHealth system is divided into regions- North, Central and South- each accounting for two hospitals where thoracic surgeons engage in anatomic lung resections. The stakeholders were initially limited to thoracic surgeons, cardiothoracic anesthesiologists, and associated team members at each region. The stakeholder group grew to include other subject matter experts: quality improvement specialists, perioperative administrators, perioperative nurses, postoperative nurses, inpatient advance practice providers, schedulers, medical assistants, dieticians, respiratory, physical and occupational therapists, regional anesthesia specialists, and health information technology specialists.
Table 1
| Thoracic ERAS Leadership | Thoracic ERAS Steering Committee | Key Stakeholders |
|---|---|---|
| Director of Thoracic ERAS | Region 1 Thoracic Surgeon Champion | Thoracic Surgeons |
| Thoracic ERAS Implementation Specialist | Region 1 Implementation Specialist | Thoracic Anesthesiologists |
| Region 1 Thoracic Anesthesiology Champion | Regional Anesthesiologists | |
| Region 2 Thoracic Surgeon Champion | Clinic Nurses | |
| Region 2 Implementation Specialist | Schedulers and Medical Assistants | |
| Region 2 Thoracic Anesthesiology Champion | Clinic Advanced Practice Providers | |
| Region 3 Thoracic Surgeon Champion | Inpatient Advanced Practice Providers | |
| Region 3 Implementation Specialist | Quality improvement specialists | |
| Region 3 Thoracic Anesthesiology Champion | Perioperative administrators | |
| System Perioperative Administrator | Perioperative nursing | |
| System ERAS Director | Postoperative nursing | |
| Inpatient advance practice providers | ||
| Nutritionists | ||
| Respiratory therapists | ||
| Physical therapists | ||
| Occupational therapists | ||
| Health information technology specialist |
ERAS, enhanced recovery after surgery.
Protocol development and approval
The process of the UCHealth-wide thoracic ERAS protocol development took longer than anticipated. The adoption of a pre-existing thoracic ERAS protocol and use of the existing framework for ERAS protocol implementation facilitated this process but did not make it seamless. Stakeholder engagement was slow at times, with little to no feedback garnered via email communication from some stakeholders. This necessitated additional communication and endorsement of the implementation plan by administrators. Items that generated prolonged discussion were intraoperative chest tube management, routine placement of chest tubes to water seal immediately after surgery, preoperative anesthesia and analgesia medication bundles, and postoperative analgesia protocols. The UCHealth Thoracic ERAS protocol is available in Appendix 1.
Facilitators
Our group was supported by an executive leadership committee who had charged one non-surgeon physician with heading all ERAS protocol implementations at UCHealth. Resource allocation by supportive leadership, including financial support for new technology acquisition, allowed for relatively smooth and seamless integration of new practice patterns. This support was driven by potential postoperative outcomes improvement and decreased hospital costs. Institutional commitment to ERAS and quality improvement helped to break down barriers and continue forward progress. Presence of other ERAS programs within our healthcare system and the associated infrastructure was vital to the success of this program.
A dedicated Thoracic ERAS Steering Committee was essential for operationalization of the thoracic ERAS protocol. While support from the leadership and champions was strong, multidisciplinary stakeholder support from all healthcare personnel involved prevented unforeseen persistent barriers in implementation. The knowledge of local workflow patterns, interactions between providers, and cultural differences between the hospitals in the UCHealth system were vital to uniform protocol implementation. A strong, multidisciplinary group’s participation in bi-monthly meetings and protocol iterative revisions facilitated rollout and implementation.
Incorporation of established guidelines hinged on the leadership of the regional physician champions. Engagement and active support amongst the regional champions made it possible to garner support from the majority of other surgeons, anesthesiologists, clinical staff, and supporting providers throughout the health system. Engagement by our information technology group to create order sets in our local EHR, Epic (Epic Systems Corporation, Verona, WI), assignment of a quality improvement specialist and data analysis for UCHealth, and participation from the Department of Surgery Director of Surgical Quality Improvement facilitated smooth protocol implementation. Through their support, data was obtained on compliance of elements in the protocol.
Barriers
Tracking of target population postoperative outcomes was a barrier throughout implementation. Identification and capture of patients varied based on of use of institutional billing data, CPT codes, diagnosis-related group (DRG) codes, and manual identification. While data from a hospital based national performance registry, Vizient, was readily available, it was difficult to identify the targeted population and abstract specific outcomes attributable to anatomic lung resection impacted by this ERAS pathway. Our healthcare system did not uniformly participate in specialty registries like the Society of Thoracic Surgeons General Thoracic Database. Review of compliance data and outcome measures started as a manual process with support from our internal data analytics team to capture data from the EHR.
Another barrier included acquisition of new technology and equipment. Initial review by the hospital’s “Value Analysis” team did not support these additional resources. Through partnership with our executive sponsor, we reviewed historical cases to highlight potential impact on patient outcomes and financial ramifications. This continued collaboration allowed for the request to be re-reviewed for consideration, and successfully approved for acquisition. Leading with the data and implications to patient care was essential in consideration of novel equipment or supplies.
A unique barrier unique to quality improvement efforts was the impact of the ongoing COVID-19 pandemic. This altered health system priorities and introduced resource scarcity. For example, all requests for information technology builds were deferred in favor of COVID-19 specific requests. Additionally, financial investment towards non-pandemic related expenditures became limited, and some of the stakeholders’ roles were revised to account for other system needs due to the pandemic.
Challenges
There was lack of consensus from key stakeholders (thoracic surgeons, thoracic anesthesiologists, regional pain anesthesiologists) about some interventions. Most were based on practice location and culture differences, hospital type (community versus academic), and patient disease process (lung cancer vs. chronic bronchiectasis). We were unable to achieve universal agreement on some items, including method of regional pain control, multimodal analgesia medication types, and certain intra-operative decisions. Thus, there remained a few regional protocol variations. This may present challenges in determining which interventions improved outcomes and prevented complications.
While lung malignancies account for the majority of patients undergoing anatomic lung resections across our healthcare system, the quaternary care referral academic hospital cares for patients with chronic mycobacteria lung infections and chronic bronchiectasis requiring surgical intervention. Patients with this pathology commonly undergo anatomic pulmonary resections. Their postoperative care requires special consideration to ensure persistent infections or pulmonary complications do not occur. The regional champions that care for these patients wanted to include these patients within the thoracic ERAS protocol to maximize benefit provided by the protocol. However, several allowances for rational deviance from the protocol for this group were imperative, particularly in the intra-operative and immediate postoperative setting, such as continuing chest tubes to suction after surgery to evacuate contaminated pleural fluid and minimize risk of postoperative empyema.
DiscussionOther Section
We developed and implemented a thoracic ERAS protocol across a diverse, multi-hospital health system. The protocol was specific to anatomic lung resections based on existing published guidelines. The key steps in this process were: identify and prepare champions; build a coalition; create a learning collaborative; capture and share local knowledge; centralize technical assistance; conduct ongoing training; and audit and provide feedback (10). The authors chose to focus on this patient population because of their expertise on the subject matter and the opportunities for quality improvement that were driving change.
Creation and implementation of a new ERAS protocol required engagement from key stakeholders and executive leadership within the hospital system. Stakeholders are more likely to participate in improvement efforts when it affects their clinical practices (12). Had one hospital been driving the efforts and implemented without input from regional colleagues, the support surrounding the protocol may have been less successful. While protocol elements are evidence-based and derived from international thoracic surgery experts recommendations, it was essential to identify and engage local subject matter experts to form a collective recommendation (12). However, had the protocol only been implemented at a single hospital site, we may have faced fewer barriers and achieved more widespread buy-in from stakeholders with less dissention and disagreement. While universal agreement on all ERAS protocol elements was not achievable, widespread buy-in to the rationale driving the protocol was achieved. This may have been amplified as the number of unique stakeholders increased. Based on initial adherence observations, providers who were most engaged in development have had the highest levels of protocol compliance. We did not engage patient representatives in the initial phases of the thoracic ERAS protocol implementation. However, based on their valuable input into our past efforts, we plan to engage patient partners in this program moving forward. We foresee their engagement may help with some of the challenges we have been facing in patient buy-in to taking perioperative immunonutrition supplements, for instance. Concurrently, we have been studying patient reported outcomes since the implementation of thoracic ERAS at our healthcare system, the topic of future analysis. We anticipate publishing these and the implementation results once we have amassed sufficient data.
Acquisition of resources and information technology builds were integral to protocol implementation. Several resources like preoperative immunonutrition supplements and templated order sets could translate and be implemented in other surgical services and patient populations. The impact of the COVID-19 pandemic hampered progress and necessitated team member persistence for continued progress in the setting of external constraints. While basic data tracking was available through one local database, more specific outcomes of interest were not trackable. This problem could be alleviated by accessing current procedural terminology (CPT) code data related to this group. This would facilitate compliance and outcomes analysis, which would provide the framework for iterative review and continued evolution of the protocol over time. However, access to EHR-based CPT code data has remained elusive. Our executive leadership was made aware of this problem and worked to rectify this issue. However, this may lead to deficits in understanding potential postoperative outcome improvements made on more granular outcomes of interest. Further development in automated patient identification within our EHR remains an area of opportunity for eliminating manual chart review and tracking postoperative outcomes and compliance data.
Inclusion of unique mycobacteria/bronchiectasis patients was important to the providers in the health system region who cared for them. Because they are included, they will receive several of the protocol benefits, including preoperative immunonutrition and preoperative multimodal pain bundle. This will hopefully lead to improved outcomes in this patient population but may skew some of our outcome measures during future analysis.
We found little existing literature describing the facilitators and barriers to the implementation of a thoracic ERAS protocol across a diverse health system. This study adds to the literature by detailing the specific implementation processes and strategies utilized for the creation and primary implementation of a thoracic ERAS protocol, and these processes could be used as a road map for future protocol implementation at large healthcare systems. However, this study has limitations that require consideration. Our health system includes a large quaternary academic medical center and several community hospitals. The size and maturity of our health system may have been a benefit in some implementation respects (i.e., availability of financial resources) and a barrier in others (i.e., protocol approval through a large group with different backgrounds and practice patterns). Additionally, our healthcare system primarily functions within urban and suburban settings, which may not reflect challenges rural healthcare systems encounter.
In conclusion, we developed and implemented a thoracic ERAS protocol for anatomic lung resections across a diverse healthcare system. An engaged multidisciplinary team aided by executive leadership support facilitated protocol creation, dissemination, and implementation. Broad stakeholder engagement supported tailoring the protocol to fit the needs and culture of each hospital. Our development, adoption, and implementation program could be used as blueprint for future quality improvement interventions. Future studies will evaluate protocol adherence, discuss methods to improve compliance, and assess the protocol’s impact on postoperative outcomes across this diverse healthcare system.
AcknowledgmentsOther Section
This study was presented at the STS 18th Annual Perioperative and Critical Care Conference in September 2021.
Funding: This work was supported by an internal grant from the Department of Surgery, University of Colorado School of Medicine. The funding organization had no role in the design and conduct of implementation; collection, management, analysis, and interpretation of the results; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
FootnoteOther Section
Reporting Checklist: The authors have completed the SQUIRE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-518/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-518/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-518/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-518/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study given quality improvement exemption by the Colorado Multiple Institutional Review Board, protocol number 20-3051, and individual consent for this study was waived as a quality improvement project within the healthcare system.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
ReferencesOther Section
- Fearon KC, Ljungqvist O, Von Meyenfeldt M, et al. Enhanced recovery after surgery: a consensus review of clinical care for patients undergoing colonic resection. Clin Nutr 2005;24:466-77. [Crossref] [PubMed]
- Wind J, Polle SW, Fung Kon Jin PH, et al. Systematic review of enhanced recovery programmes in colonic surgery. Br J Surg 2006;93:800-9. [Crossref] [PubMed]
- Brunelli A, Thomas C, Dinesh P, et al. Enhanced recovery pathway versus standard care in patients undergoing video-assisted thoracoscopic lobectomy. J Thorac Cardiovasc Surg 2017;154:2084-90. [Crossref] [PubMed]
- Dinic VD, Stojanovic MD, Markovic D, et al. Enhanced Recovery in Thoracic Surgery: A Review. Front Med (Lausanne) 2018;5:14. [Crossref] [PubMed]
- Semenkovich TR, Hudson JL, Subramanian M, et al. Enhanced Recovery After Surgery (ERAS) in Thoracic Surgery. Semin Thorac Cardiovasc Surg 2018;30:342-9. [Crossref] [PubMed]
- Batchelor TJP, Rasburn NJ, Abdelnour-Berchtold E, et al. Guidelines for enhanced recovery after lung surgery: recommendations of the Enhanced Recovery After Surgery (ERAS®) Society and the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothorac Surg 2019;55:91-115. [Crossref] [PubMed]
- Lambert-Kerzner AC, Aasen DM, Overbey DM, et al. Use of the consolidated framework for implementation research to guide dissemination and implementation of new technologies in surgery. J Thorac Dis 2019;11:S487-99. [Crossref] [PubMed]
- Bauer MS, Damschroder L, Hagedorn H, et al. An introduction to implementation science for the non-specialist. BMC Psychol 2015;3:32. [Crossref] [PubMed]
- Albright K, Gechter K, Kempe A. Importance of mixed methods in pragmatic trials and dissemination and implementation research. Acad Pediatr 2013;13:400-7. [Crossref] [PubMed]
- Powell BJ, Waltz TJ, Chinman MJ, et al. A refined compilation of implementation strategies: results from the Expert Recommendations for Implementing Change (ERIC) project. Implement Sci 2015;10:21. [Crossref] [PubMed]
- Kotter J. Leading Change. Harvard Business Review Press. 1996.
- Horev T, Babad YM. Healthcare reform implementation: stakeholders and their roles-the Israeli experience. Health Policy 2005;71:1-21. [Crossref] [PubMed]


