
Bleeding and thrombotic complications associated with anticoagulation prior to lung transplantation: a case series
Introduction
Pulmonary transplant recipients possess a drastically heightened vulnerability for perioperative thrombotic complications. Estimates have reported that 17–30% of lung transplant recipients develop venous thromboembolism (VTE) by 30 days after transplantation, and VTE incidence rises up to 40–64% by 4 years (1-5). Early VTE is especially frequent in these patients, with a median time from lung transplantation to VTE diagnosis of 17–20 days (1,2). These remarkable rates of thrombotic complications surpass those reported after other surgical procedures, including thoracic surgery (12%) and other types of solid organ transplantation (3–17%) (6-9). Previously identified risk factors for VTE after lung transplantation include history of prior VTE, increased age, decreased mobility, indwelling lines, and pro-thrombotic immunosuppressive medications (10-13). Moreover, the lung transplantation procedure itself may induce a hypercoagulable state due to increased coagulation factor VIII, increased thrombomodulin resistance and decreased circulating proteins C and S (14). Despite this predilection for thromboses, there remains no best practice for anticoagulation (AC) management in these high-risk patients, and a wide degree of variability in practices and preferences exists among transplant centers (15).
Appropriate reversal of AC-associated coagulopathy at the time of surgery is imperative for minimizing blood transfusion requirements, which are associated with significantly worsened survival in lung transplant recipients (16,17). Standard recommendations for adequate international normalized ratio (INR) reduction preoperatively advocate for discontinuation of AC 5 days prior to surgery (18). However, the unplanned nature of donor availability precludes this practice in transplant recipients. Although no guidelines exist for lung transplantation, the International Society of Heart and Lung Transplantation recommends an INR ≤1.5 prior to heart transplantation (19). Different agents such as vitamin K, fresh frozen plasma (FFP), and prothrombin complex concentrate (PCC) have been used to achieve this goal. However, these reversal strategies have several limitations, such as delayed INR normalization with vitamin K administration. Additionally, while the onset of action of FFP is more rapid than vitamin K, it remains limited by a prolonged thawing time, larger necessary volumes, required ABO compatibility, and the possibility of transfusion-related lung injury. PCC is a more costly alternative to FFP that acts rapidly and eliminates the need for crossmatching, but the risks and benefits of PCC use have not been fully elucidated in this population.
We sought to quantify the prevalence of preoperative therapeutic AC use within our institutional cohort of lung transplant recipients and describe our experiences with pharmacologic reversal strategies. Additionally, we aimed to identify potential associations with bleeding and thrombotic complications within this cohort. To our knowledge, this is the largest study to date evaluating therapeutic AC use in recipients undergoing pulmonary transplantation and associated perioperative outcomes. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-300/rc).
Methods
We performed a single center, retrospective cohort study of all adult (≥18 years old) lung transplant recipients taking preoperative therapeutic AC up to the day of pulmonary transplantation between January 1, 2014 and July 1, 2021. Data was collected from our institutional transplant recipient database and from the electronic medical records.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was deemed exempt by the institutional review board of Washington University School of Medicine, and individual consent for this retrospective analysis was waived.
We reviewed preoperative patient factors, including sex, age, indication for lung transplant, and indication for AC. We also reviewed pharmacologic reversal agents administered prior to transplantation and coagulation studies. The last INR value prior to entering the operating room was utilized to evaluate adequacy of preoperative INR reversal, which was defined as INR ≤1.5. We assessed the use of cardiopulmonary bypass (CPB) or extracorporeal membrane oxygenation (ECMO) during transplantation, major bleeding and thrombotic complications intra- and postoperatively, and inpatient mortality. Major bleeding complications were defined as delayed sternal closure due to bleeding, or any of the following within 24 hours from the start of surgery: reoperation for bleeding, chest tube output ≥1,500 cc, transfusion ≥4 units packed red blood cells, ≥4 units platelets, or ≥5 units FFP (16). Blood products were transfused at the discretion of the transplant surgeons, anesthesia team, or critical care team. Major thrombotic complications were defined as acute deep vein thrombosis, pulmonary embolism, cerebrovascular accident, or other major thrombosis identified on imaging (such as extensive intra-cardiac thrombus or acute mesenteric ischemia due to thromboembolism) within 30 days after transplantation. All imaging was obtained due to clinical suspicion and no surveillance testing was performed.
Statistical analysis
Categorical variables were reported as counts and percentages. Results include average and range values and n values are provided in the results and tables. Survival curve was generated using Excel Microsoft (version 16.54) by calculating the Kaplan-Meyer survival time function.
Results
Patient characteristics
Of 602 lung transplant recipients, 10 patients taking preoperative warfarin were included in the study (Table 1). One patient taking enoxaparin was excluded. The most common underlying lung disease was interstitial lung disease (n=5, 50%) and the most common indication for preoperative AC was VTE (n=7, 70%), including deep venous thrombosis in 5 patients and pulmonary embolism in 4 patients (Table 1). Other indications for AC included atrial fibrillation (n=2, 20%) and pulmonary arterial hypertension (n=1, 10%). The distribution of cases was fairly consistent over time, with at least one patient who underwent transplantation each year between 2014 and 2020 (Table 2). Bilateral lung transplantation was performed in all cases except one patient who underwent single lung transplantation due to hemodynamic instability (patient 10 in Table 2).
Table 1
| Recipient factors | N |
|---|---|
| Lung transplant recipients | |
| Total | 602 |
| Preoperative warfarin | 10 (2%) |
| Diagnosis | |
| ILD | 5 (50%) |
| A1A | 1 |
| CF | 1 |
| COPD | 1 |
| PAH | 1 |
| SARC | 1 |
| Indication for AC | |
| VTE | 7 (70%) |
| AF | 2 |
| PAH | 1 |
ILD, interstitial lung disease; A1A, alpha-1-antitrypsin deficiency; CF, cystic fibrosis; COPD, chronic obstructive pulmonary disease; PAH, pulmonary arterial hypertension; SARC, sarcoidosis; AC, anticoagulation; VTE, venous thromboembolism; AF, atrial fibrillation.
Table 2
| Patient | Diagnosis | AC indication | Year | Surgery timea | ECMO | CPB | Admit labs | Reversal | Post-reversal | OR start | OR end | Bleeding event | Thrombotic event | Death | Survival (years) | Details |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | CF | VTE | 2014 | DAY | No | No | INR 1.7 | None | N/A | INR 1.8 | INR 1.6 | X | 4 | No events | ||
| PTT 27 | PTT 33 | PTT 29 | ||||||||||||||
| Hct 14 | Hct 47 | Hct 30 | ||||||||||||||
| Plt 360 | Plt 278 | Plt 276 | ||||||||||||||
| 2 | ILD | AF | 2015 | DAY | No | No | INR 1.9 | 10 mg IV vitamin K | INR 1.8 | INR 1.2 | INR 1.4 | X* | X* | 6 | Open chest, return to OR <24 hours for bleeding, 7 U RBC, 4 U platelets, CT 2,257 cc, DVT <30 days | |
| PTT 33 | PTT 42 | PTT 37 | ||||||||||||||
| Hct 43 | Hct 35 | Hct 29 | ||||||||||||||
| Plt 194 | Plt 176 | Plt 175 | ||||||||||||||
| 3 | A1A | VTE | 2016 | DAY | No | No | INR 2.4 | 2.5 mg PO + 3 mg IV vitamin K | INR 1.4 | INR 1.4 | INR 1.2 | X | X | 5 | DVT <30 days | |
| PTT 40 | PTT 30 | PTT 42 | ||||||||||||||
| Hct 43 | Hct 36 | Hct 33 | ||||||||||||||
| Plt 294 | Plt 257 | Plt 150 | ||||||||||||||
| 4 | SARC | PAH | 2016 | DAY | No | Yes | INR 2.5 | 2U FFP, 10 mg PO + 13 mg IV vitamin K | INR 1.6 | INR 1.6 | INR 1.9 | X* | 5 | Open chest, 11 U RBC, 9 U FFP, CT 1,790 cc | ||
| PTT 48 | PTT 51 | PTT 54 | ||||||||||||||
| Hct 43 | Hct 33 | Hct 27 | ||||||||||||||
| Plt 124 | Plt 118 | Plt 36 | ||||||||||||||
| 5 | ILD | VTE | 2017 | NIGHT | No | Yes | INR 2.3 | 2.5 mg PO vitamin K | N/A | – | INR 2.4 | X* | X* | X | 2 | Open chest, return to OR <24 hours for bleeding, 12 U RBC, 4 U platelets, 15 U FFP, CT 5,265 cc, DVT <30 days |
| PTT 37 | Hct 51 | Hct 20 | ||||||||||||||
| Hct 54 | – | – | ||||||||||||||
| Plt 218 | ACT 999 | ACT 124 | ||||||||||||||
| 6 | COPD | VTE | 2018 | DAY | No | No | INR 1.2 | 10 mg PO vitamin K | N/A | INR 1.2 | INR 1.7 | X | X | 3 | 4 U RBC, DVT <30 days | |
| PTT 30 | PTT 26 | PTT 37 | ||||||||||||||
| Hct 30 | Hct 23 | Hct 26 | ||||||||||||||
| Plt 354 | Plt 262 | Plt 125 | ||||||||||||||
| 7 | ILD | VTE | 2019 | DAY | No | No | INR 2.0 | 10 mg PO + 2 mg IV vitamin K | INR 1.4 | – | – | X | X | <1 | DVT <30 days, fatal acute mesenteric ischemia, received 1,154 U PCC on post operative day 2 due to continued bleeding | |
| PTT 36 | – | – | ||||||||||||||
| Hct 43 | Hct 41 | Hct 29 | ||||||||||||||
| Plt 177 | – | – | ||||||||||||||
| 8 | ILD | AF | 2019 | DAY | No | No | INR 2.1 | 10 mg PO + 2.5 mg IV vitamin K | N/A | INR 2.2 | INR 1.8 | 2 | No events | |||
| PTT 32 | – | – | ||||||||||||||
| Hct 35 | Hct 29 | Hct 33 | ||||||||||||||
| Plt 314 | Plt 247 | Plt 259 | ||||||||||||||
| 9 | ILD | VTE | 2020 | NIGHT | Yes | No | INR 2.5 | 10 mg PO + 2.5 mg IV vitamin K | INR 2.1 | – | INR 2.0 | X* | X* | 2 | 7 U RBC, 4 U platelets, 7 U FFP, CVA <30 days | |
| PTT 34 | Hct 44 | Hct 26 | ||||||||||||||
| Hct 52 | – | Plt 112 | ||||||||||||||
| Plt 141 | ACT 116 | ACT 271 | ||||||||||||||
| 10 | PAH | VTE | 2020 | DAY | Yes | Yes | INR 2.3 | 1,622U PCC + 10 mg PO + 15 mg IV vitamin K | INR 2.3 | – | INR 2.0 | X* | X* | X | <1 | Open chest, 5 U RBC, 4 U platelets, 1,643 U PCC (29 U/kg), fatal intracardiac thrombus |
| PTT 43 | Hct 23 | Hct 30 | ||||||||||||||
| Hct 27 | Plt 100 | Plt 120 | ||||||||||||||
| Plt 114 | ACT 144 | ACT 109 |
a, defined as daytime (DAY, between 05:00–18:00) or nighttime (NIGHT, between 18:00–05:00) based on time of incision (20); X denotes occurrences of major bleeding, thrombotic event, and/or death; *, complication with inadequate INR reversal, defined by INR ≤1.5 prior to transplantation. AC, anticoagulation; ECMO, extracorporeal membrane oxygenation; CBP, cardiopulmonary bypass; OR, operating room; CF, cystic fibrosis; VTE, venous thromboembolism; INR, international normalized ratio; PTT, partial thromboplastin time; Hct, hematocrit; Plt, Platelets; N/A, not available/obtained; ILD, interstitial lung disease; AF, atrial fibrillation; IV, intravenous; U, units; RBC, red blood cells; CT, chest tube; DVT, deep venous thrombosis; A1A, alpha-1-antitrypsin; PO, per os; SARC, sarcoidosis; PAH, pulmonary arterial hypertension; FFP, fresh frozen plasma; ACT, activated coagulation time; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; PCC, prothrombin complex concentrate.
Details regarding reversal strategies utilized for each patient are outlined in Table 2. Many patients took a dose of oral vitamin K (2.5–10 mg) when called in, per our institutional practice. Most INR levels upon hospital arrival exceeded standard recommendations (n=9, 90%). However, additional preoperative reversal was not always administered in these cases (n=7, 78%). There was wide variation in the total preoperative dose of reversal administered to each patient. Two patients received preoperative blood products in addition to vitamin K, including one patient who received two units of FFP (initial INR 2.5), and one patient who received 1,622 units of PCC (28 units/kg, initial INR 2.3). Despite these efforts, most patients did not achieve adequate INR reversal prior to lung transplantation (n=7, 70%). Notably, all patients who required mechanical circulatory support had inadequate INR reversal.
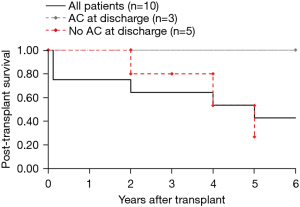
No patients developed severe primary graft dysfunction. Excluding the two cases of early mortality due to thrombotic complications (patients 7 and 10 in Table 2), posttransplant survival exceeded one year and appeared to be comparable to general outcomes following lung transplantation (Figure 1).
Bleeding complications
Major bleeding complications occurred in six patients (n=6, 60%). Importantly, all patients who suffered bleeding complications had inadequate INR reversal preoperatively except for one patient (Tables 3,4). There were four recipients with an open chest at the time of transplantation, which included one patient with an INR of 2.3 upon entering the operating room who required reoperation within 24 hours due to ongoing transfusion requirements with over five liters of chest tube output (Table 2). In summary, nearly half of all patients within the cohort required delayed closure or reoperation within 24 hours due to ongoing bleeding, which was always associated with inadequate INR reversal (n=4, 40%, INR 1.9–2.5). Within the first 24 hours, recipients who had inadequate INR reversal received an average of six units of red blood cells, two units of platelets, and five units of FFP (compared to an average of two units of red blood cells, zero units of platelets, and one unit of FFP in those with adequate INR reversal).
Table 3
| INR reversal | N [%] |
|---|---|
| Attempted INR reversal | 9 [90] |
| Adequate INR (≤1.5) before transplant | 3 [30] |
| Inadequate INR (>1.5) before transplant | 7 [70] |
AC, anticoagulation; INR, international normalized ratio.
Table 4
| Postoperative events | N [%] |
|---|---|
| Major bleeding event | |
| Total | 6 [60] |
| ECMO | 2 [33]* |
| CPB | 3 [50]* |
| Delayed sternal closure | 4 [40]* |
| Reoperation <24 hours for ongoing bleeding | 2 [20]* |
| Chest tube output >1,500 cc <24 hours | 3 [30]* |
| Transfusion ≥4 U RBC <24 hours | 6 [60] |
| Transfusion ≥4 U platelets <24 hours | 4 [40]* |
| Transfusion ≥5 U FFP <24 hours | 3 [30]* |
| Major thrombotic event | |
| Total | 7 [70] |
| ECMO | 2 [33]* |
| CPB | 2 [33]* |
| VTE <30 days | 5 [50] |
| Fatal thrombotic event | 2 [20]* |
*, all patients had inadequate INR reversal. ECMO, extracorporeal membrane oxygenation; CPB, cardiopulmonary bypass; RBC, red blood cells; U, units; FFP, fresh frozen plasma; VTE, venous thromboembolism; INR, international normalized ratio.
Thrombotic complications
Despite AC-induced coagulopathy on admission, major thrombotic complications (n=7, 70%) were more frequent than bleeding complications (Table 4). Acute deep venous thrombosis was the most common thrombotic event (n=5, 50%), followed by arterial thromboembolism in the setting of atrial fibrillation (n=2, 20%), and cerebrovascular accident (n=1, 10%) (Table 2). Importantly, all inpatient fatalities which occurred within the cohort (n=2, 20%) were associated with thrombotic, but not bleeding, complications. One inpatient death occurred within 48 hours after transplantation due to cardiac arrest during reoperation with extensive intra-cardiac thrombosis on transesophageal echocardiogram. Another inpatient death occurred approximately 2 weeks after transplantation after developing atrial fibrillation with hemodynamic instability and imaging findings consistent with acute mesenteric ischemia who died within 24 hours of these findings. Notably, none of the patients in this cohort were initially restarted on therapeutic AC postoperatively. Additionally, only 3 of the 8 patients who survived to hospital discharge were prescribed therapeutic AC (Figure 1).
Conclusions
This study highlights several important points to consider for patients taking preoperative therapeutic AC prior to lung transplantation. First, initial INR levels in these patients exceeded standard preoperative recommendations due to the unpredictable timing of transplantation. Second, adequate INR reversal was rarely achieved prior to transplantation, despite administration of pharmacologic reversal to nearly all patients. Third, inadequate INR reversal was frequently associated with major bleeding complications including higher transfusion requirements, delayed sternal closure, and urgent reoperation due to ongoing bleeding. Fourth, therapeutic AC was rarely restarted during hospitalization or at hospital discharge. Lastly, major thrombotic events were more frequent than bleeding complications and were associated with inpatient mortality.
There are no current guidelines regarding appropriate management and reversal of AC in lung transplant recipients. Only one prior study investigated preoperative AC and reversal in patients undergoing lung transplantation, which included four patients on warfarin who received PCC reversal (21). One other case report described a patient on rivaroxaban who underwent bilateral lung transplantation without reversal and no major bleeding events (22). No known study to date has compared AC agents or reversal strategies in patients undergoing lung transplantation. Administration of blood products such as FFP and PCC carry several risks relevant to transplant recipients. Interestingly, both patients within our cohort who developed fatal thrombotic complications received PCC perioperatively. Given the lack of available data addressing this challenging clinical dilemma, it is critical to elucidate best AC practices in order to reduce perioperative complications and mortality.
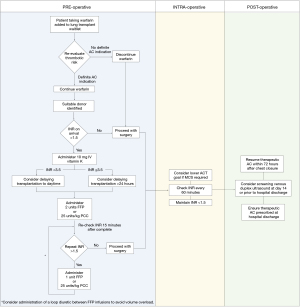
We have identified several perioperative management strategies to help mitigate the risks associated with preoperative AC before lung transplantation. For patients on the waiting list, AC indication should be carefully reviewed and AC should be discontinued in cases without a clear thrombotic indication (e.g., patient 4 in our series, Table 2). Currently, not enough evidence exists to support the use of certain AC agents over others in these patients, but future investigation is warranted in this area. For patients taking warfarin who are called in for lung transplantation, we strongly recommend the establishment of a defined protocol to ensure prompt reversal prior to surgery. We have outlined a perioperative protocol based on our experience (Figure 2) (19,23,24). Studies in other patient populations have demonstrated that PCC may be superior to FFP for successfully achieving INR reversal with more rapid onset and smaller fluid volumes (25,26). However, our own observations suggest that PCC may be associated with thrombotic complications in this patient population. Thus, we prefer the use of FFP for preoperative INR reversal over PCC in this clinical setting. Further studies evaluating the use of FFP and PCC in lung transplant recipients is crucial. Another important factor to consider in these patients is timing of transplantation. Our group has previously demonstrated that nighttime lung transplantation is associated with an increased risk of postoperative complications (20). While only two patients in our study underwent nighttime transplantation, both patients had inadequate INR reversal prior to surgery and subsequently experienced both bleeding and thrombotic complications (Table 2). Thus, deferring transplantation until daytime hours should be considered in these patients whenever possible due to the increased monitoring and treatment necessary to achieve adequate INR reversal. Our data suggests early resumption of AC therapy postoperatively may decrease adverse thrombotic events in these patients. Prior studies have documented alarmingly high rates of inpatient VTE in patients following lung transplantation without baseline increased risk, with estimates up to 64% despite mechanical and pharmacologic prophylaxis (3,4). Importantly, the development of VTE has been significantly associated with reduced survival following lung transplantation, even with upper extremity or below-the-knee deep venous thrombosis (1,3,4). Thus, routine surveillance with screening duplex ultrasound may be warranted in the acute postoperative period (1,2). While inferior vena cava filter insertion may be considered as an adjunct to therapy in these patients, AC provides additional benefit from other thrombotic complications and avoids the procedural risks related to filter placement. In summary, we believe that successful transplantation with favorable outcomes is feasible in patients taking preoperative AC, but adequate reversal of coagulopathy and proper resumption of AC is crucial.
Several limitations exist within our study, including the retrospective design which limits any definitive assumptions regarding outcomes, as well as the small sample size from a single institution, which limits the generalizability of our findings. Our study does not account for all variables which may have potentially impacted bleeding and thrombotic outcomes, such as surgeon experience. However, we do not believe this played a significant role in these patients, since all cases in this series were performed by thoracic surgery faculty with expertise in lung transplantation. Imaging to evaluate for postoperative VTE was only obtained in a subset of patients. Thus, the incidences of VTE in our cohort should represent minimum values, as surveillance screening was not performed and the sensitivity limitations of available diagnostic imaging. Additionally, no serial or longitudinal follow-up studies were obtained. Consequently, patients may have had undetected or subsequent VTE with potential adverse effects on survival which were not ascertained. The subjective, non-randomized clinical decision-making may have led to overestimation of major bleeding events. However, all except two patients who experienced major bleeding events met multiple criteria including objective measures such as chest tube output.
This is the largest study to date reporting the incidence of AC use in patients undergoing lung transplantation and the impact of AC-induced coagulopathy on outcomes after lung transplantation. Inadequate INR reversal appears to be associated with major bleeding complications. Indication for AC should be carefully reconsidered at time of listing and coagulopathy adequately corrected prior to transplantation to minimize this risk. While VTE incidence may not differ drastically from other patients after lung transplantation, patients with a preoperative indication for AC may suffer increased mortality with development of major thrombotic complications. Establishment of guidelines to ensure successful INR reversal preoperatively and prompt AC re-initiation postoperatively is paramount to improve clinical outcomes in patients taking therapeutic AC prior to lung transplantation.
Acknowledgments
The authors would like to thank Christy Hamilton for compiling the patient list used in this study.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-300/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-300/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-300/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-300/coif). RRH reports grants and personal fees from Bristol Myers Squibb, Mallinckrodt, UpToDate, CareDx, Natera, and Transmedics, outside of the submitted work. VP reports the following grants and personal fees: I01 HX002475, R01HL146856 and R01CA258681, PrecisCa and his spouse owns stock from Intuitive Surgical, outside of submitted work. DK serves as an unpaid editorial board member of Journal of Thoracic Disease from April 2022 to March 2024. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was deemed exempt by the institutional review board of Washington University School of Medicine, and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jorge A, Sanchez PG, Hayanga JWA, et al. Routine deep vein thrombosis screening after lung transplantation: Incidence and risk factors. J Thorac Cardiovasc Surg 2020;159:1142-50. [Crossref] [PubMed]
- Zheng M, Yousef I, Mamary AJ, et al. Venous thromboembolism in lung transplant recipients real world experience from a high volume center. J Heart Lung Transplant 2021;40:1145-52. [Crossref] [PubMed]
- Evans CF, Iacono AT, Sanchez PG, et al. Venous Thromboembolic Complications of Lung Transplantation: A Contemporary Single-Institution Review. Ann Thorac Surg 2015;100:2033-9; discussion 2039-40. [Crossref] [PubMed]
- Ribeiro Neto ML, Budev M, Culver DA, et al. Venous Thromboembolism After Adult Lung Transplantation: A Frequent Event Associated With Lower Survival. Transplantation 2018;102:681-7. [Crossref] [PubMed]
- Kanade R, Mohanka M, Bollineni S, et al. Characteristics and Outcomes Among Patients With Early Venous Thromboembolic Events After Lung Transplant. Transplant Proc 2021;53:303-10. [Crossref] [PubMed]
- Song C, Shargall Y, Li H, et al. Prevalence of venous thromboembolism after lung surgery in China: a single-centre, prospective cohort study involving patients undergoing lung resections without perioperative venous thromboembolism prophylaxis†. Eur J Cardiothorac Surg 2019;55:455-60. [Crossref] [PubMed]
- Kainuma A, Ning Y, Kurlansky PA, et al. Incidence of Deep Venous Thrombosis and its Impact on Outcomes after Heart Transplantation. The Journal of Heart and Lung Transplantation 2021;40:S212. [Crossref]
- Verhave JC, Tagalakis V, Suissa S, et al. The risk of thromboembolic events in kidney transplant patients. Kidney Int 2014;85:1454-60. [Crossref] [PubMed]
- Yip J, Bruno DA, Burmeister C, et al. Deep Vein Thrombosis and Pulmonary Embolism in Liver Transplant Patients: Risks and Prevention. Transplant Direct 2016;2:e68. [Crossref] [PubMed]
- Yegen HA, Lederer DJ, Barr RG, et al. Risk factors for venous thromboembolism after lung transplantation. Chest 2007;132:547-53. [Crossref] [PubMed]
- Ahya VN, McShane PJ, Baz MA, et al. Increased risk of venous thromboembolism with a sirolimus-based immunosuppression regimen in lung transplantation. J Heart Lung Transplant 2011;30:175-81. [Crossref] [PubMed]
- Izbicki G, Bairey O, Shitrit D, et al. Increased thromboembolic events after lung transplantation. Chest 2006;129:412-6. [Crossref] [PubMed]
- Patrassi GM, Sartori MT, Livi U, et al. Impairment of fibrinolytic potential in long-term steroid treatment after heart transplantation. Transplantation 1997;64:1610-4. [Crossref] [PubMed]
- Ruitenbeek K, Hugenholtz GC, Adelmeijer J, et al. Development of a hypercoagulable status in patients undergoing off-pump lung transplantation despite prolonged conventional coagulation tests. Am J Respir Crit Care Med 2015;191:230-3. [Crossref] [PubMed]
- Lichvar AB, Pierce DR, Salerno D, et al. Utilization of direct-acting oral anticoagulation in solid organ transplant patients: A national survey of institutional practices. Clin Transplant 2020;34:e13853. [Crossref] [PubMed]
- Weber D, Cottini SR, Locher P, et al. Association of intraoperative transfusion of blood products with mortality in lung transplant recipients. Perioper Med (Lond) 2013;2:20. [Crossref] [PubMed]
- Adelmann D, Koch S, Menger J, et al. Risk factors for early bleeding complications after lung transplantation – a retrospective cohort study. Transpl Int 2019;32:1313-21. [Crossref] [PubMed]
- Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141:e326S-50S.
- Costanzo MR, Dipchand A, Starling R, et al. The International Society of Heart and Lung Transplantation Guidelines for the care of heart transplant recipients. J Heart Lung Transplant 2010;29:914-56. [Crossref] [PubMed]
- Yang Z, Takahashi T, Gerull WD, et al. Impact of Nighttime Lung Transplantation on Outcomes and Costs. Ann Thorac Surg 2021;112:206-13. [Crossref] [PubMed]
- Barac YD, Klapper J, Pollack A, et al. Anticoagulation Strategies in the Perioperative Period for Lung Transplant. Ann Thorac Surg 2020;110:e23-5. [Crossref] [PubMed]
- Renner TA, Zalunardo MP, Weder W, et al. Bilateral lung transplantation in a patient receiving rivaroxaban anticoagulation. J Cardiothorac Vasc Anesth 2015;29:723-6. [Crossref] [PubMed]
- O’Shaughnessy DF, Atterbury C, Bolton Maggs P, et al. Guidelines for the use of fresh-frozen plasma, cryoprecipitate and cryosupernatant. Br J Haematol 2004;126:11-28. [Crossref] [PubMed]
- Fakheri RJ. Formula for fresh frozen plasma dosing for warfarin reversal. Mayo Clin Proc 2013;88:640. [Crossref] [PubMed]
- Goldstein JN, Refaai MA, Milling TJ Jr, et al. Four-factor prothrombin complex concentrate versus plasma for rapid vitamin K antagonist reversal in patients needing urgent surgical or invasive interventions: a phase 3b, open-label, non-inferiority, andomized trial. Lancet 2015;385:2077-87. [Crossref] [PubMed]
- Hickey M, Gatien M, Taljaard M, et al. Outcomes of urgent warfarin reversal with frozen plasma versus prothrombin complex concentrate in the emergency department. Circulation 2013;128:360-4. [Crossref] [PubMed]


