Trends and costs of stereotactic body radiation therapy in metastatic non-small cell lung cancer
Introduction
An estimated 228,820 new cases of lung cancer were expected in the United States in 2020, and lung cancer is the leading cause of cancer deaths (1). Approximately 85% of all cases of lung cancer are non-small cell lung cancer (NSCLC) (2). Oligometastatic disease was first described in 1995 as cancers with a limited number of metastatic deposits (3). Although the precise prevalence of oligometastatic NSCLC is unknown, some series suggest that approximately 50% of NSCLC patients present with disease limited to the primary lung and nodal sites and ≤3 metastases confined to three or fewer organs (4). Up to 40% of patients treated for cure with localized lung cancer will eventually develop metastatic progression (5).
Metastatic NSCLC has traditionally been managed with systemic therapy; however, there is growing evidence that the addition of local therapy to oligometastases improves overall disease control in this population. Multiple retrospective studies have demonstrated longer median overall survival in carefully selected patients who undergo metastasectomy for oligometastatic NSCLC (6-8). Stereotactic body radiation therapy (SBRT) is a form of definitive local therapy that allows for the delivery of anatomically precise, ablative doses of radiation therapy, and is routinely used to treat early stage medically inoperable lung cancer (9). Three randomized phase II studies support the use of SBRT in oligometastatic NSCLC. A randomized phase II by Gomez et al. in patients with oligometastatic NSCLC that did not progress after front-line systemic therapy found that SBRT directed at oligometastases prolonged progression-free survival and overall survival (10). Similarly, Iyengar et al. showed that the use of consolidative SBRT prior to maintenance chemotherapy nearly tripled progression-free survival in patients with limited metastatic NSCLC compared with maintenance chemotherapy alone (11). Finally, a randomized phase II study by Palma et al. found that in patients with controlled primary tumors of various sites, the addition of SBRT improved 5-year overall survival by about 25% (12). These findings need to be validated in phase III trials, which are currently accruing. Outside of clinical trials, which selectively enroll good performance status patients with limited (oligometastatic) disease, the use and outcomes of SBRT among the general metastatic NSCLC population are not known.
While systemic therapy remains the backbone of therapy for metastatic NSCLC, the introduction of new and expensive systemic therapy agents has also raised significant concern regarding their consumption of health care resources (13). Indeed, the overall costs of cancer care in the United States were $183 billion in 2015 and projected to increase 34% to $246 billion by 2030 (14). First-line chemotherapy with paclitaxel, carboplatin and bevacizumab for metastatic NSCLC has been estimated to cost Medicare $90,044 dollars per patient over a 2-year time period (15). Adverse events and hospitalizations are common, as approximately 30% of patients receiving this regimen experience grade 4 toxicity (16). There is growing recognition that hospitalizations represent a substantial proportion of cancer expenditures, primarily related to complications from systemic therapy, such as neutropenia (14). Therefore, characterizing admitting diagnoses and costs from hospitalizations during cancer therapy is warranted.
Currently, the patterns of care utilizing SBRT in elderly metastatic NSCLC patients and the impact on survival are unclear. The impact potential of SBRT use on hospitalizations is also not known, and we hypothesize that improvements in disease control from SBRT may result in more time off chemotherapy, thereby decreasing hospitalizations. The purpose of this project is to describe early SBRT use and its association with survival in patients with metastatic NSCLC, as well as to compare hospitalizations and their costs to a matched subsample of patients receiving chemotherapy using population-based Medicare data in the United States. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1835/rc).
Methods
Data source and identification of cohort
We performed a historical cohort study using the National Cancer Institute’s (NCI) Surveillance, Epidemiology, and End Results (SEER)-Medicare linked database. The SEER program collects cancer incidence and survival information from population-based cancer registries encompassing 26% of the population of the United States (17). Linked Medicare claims for health care services, and linkage to Medicare claims is available for 93% of the SEER population who are ≥66 years old (18). The study received a “Not Human Subjects” designation from the University of Vermont IRB. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
A total of 116,998 patients ≥66 years diagnosed with stage IV NSCLC diagnosed from January 1, 2004 to December 31, 2013 and enrolled in the Medicare fee-for-service program, linked to claims for treatment and outcomes from January 1, 2003 to December 31, 2014 were identified as potentially eligible for the study. Allowing a full year of Medicare claims prior to cancer diagnosis enables identification of pre-existing co-morbidities and disability scores. Medicare claims codes after diagnosis were used determine initial cancer treatment. Codes for SBRT were introduced in 2004; codes used to identify both SBRT and systemic treatment are in Table S1 (19-21). We excluded patients who were diagnosed with NSCLC on autopsy, had brain metastases, received cancer-directed surgery. We also excluded who died within 30 days of diagnosis and did not receive cancer-directed treatment. Patients were also excluded if they were enrolled in a health maintenance organization (HMO) during the 12 months before and after diagnosis in order to ensure complete Medicare claims record. After all exclusions, there were 12,701 patients eligible for the study: 215 receiving SBRT and 12,486 receiving chemotherapy as initial treatment. Some patients (108 or less than 1% of the overall sample) subsequently received the other treatment and were retained in the study to ensure that the results were representative of treatment provided in real-world clinical settings. These were mostly SBRT patients. Among patients who received SBRT as initial treatment, 24.5% subsequently received chemotherapy. Among patients who received initial chemotherapy, only 0.5% subsequently received SBRT. The follow-up period for survival was from the date of diagnosis of metastatic disease until death, and ranged from 1 to 131 months, with a mean of 17.6 for the SBRT group and 12.7 for the chemotherapy group.
Construction of variables
Data elements in SEER included patient characteristics (age, gender, race, payer, vital status, urban/rural county of residence, zip code) and cancer-related information (stage, grade, histology). Median income was determined by linking the patients’ zip codes to their median income in the Zip Code Census File. We obtained hospital characteristics for patients who underwent treatment at a hospital-based outpatient center (profit vs. non-profit, whether the center was associated with a teaching hospital, and whether the hospital was an NCI-designated comprehensive cancer center or community cancer center). Specific systemic therapy regimens and the number of treatment cycles of first- and second-line chemotherapy regimens administered to each patient were determined using algorithms in previous claims-based studies of chemotherapy utilization (22-25).
Comorbidity is an independent prognostic factor in lung cancer and is associated with lower chemotherapy use (26-28). Comorbidity was measured by the use of claims billed between 24 and 3 months before cancer diagnosis. ICD9 codes that appear on inpatient claims or at least two outpatient/physician claims occurring at least 30 days apart were used. Using the comorbid conditions included in the Charlson Index, we computed the NCI Comorbidity Index, which is a cancer-specific version of the Charlson Index (29,30). In addition, disability—a patient characteristic related to comorbidity—has been shown to independently impact healthcare utilization. To control for this potential confounding, we also computed validated claims-based index (the Davidoff index) which is correlated with patient characteristics, including age, and socioeconomic status and is a significant predictor of cancer treatment (31).
Costs
Hospitalization costs for each patient were ascertained from Medicare inpatient claims data. The length of stay, total cost and admission diagnosis were determined for each hospitalization from the date of diagnosis to the date of death, or the end of the follow-up period. For each patient the total number of hospitalizations, total number of days of hospitalization and the total hospital cost were calculated. Adjustment for differences in survival was made by dividing by the number of months of follow-up.
Statistical analyses
Bivariate associations between treatment and the independent variables of interest (time, clinicopathologic characteristics, and comorbidity/age strata), and covariates were assessed using chi squared tests for categorical variables and student’s t-test for continuous variables. The trend in use of SBRT as first-line treatment over the study period was tested with a Cochran Armitage trend test. Logistic regression was performed to identify variables significantly associated with SBRT use. Overall survival following initial treatment with chemotherapy or SBRT was assessed the Kaplan-Meier estimator. Cox proportional hazards regression was used to compare overall survival in the chemotherapy and SBRT groups after adjustment for demographic and clinicopathologic variables that were significantly associated with survival. Missing data for covariates were included in the regression analysis as separate categories. To compare hospitalizations and their associated costs a subsample of chemotherapy patients who were similar to SBRT patients in terms of age, T, N, disability status and comorbidity status was selected using propensity score matching based on a logistic regression model, with 1:4 nearest neighbor matching without replacement. We then used linear models with matched group as a random effect to compare hospitalizations and their associated costs. Prior to matching, all patients that joined an HMO after diagnosis were excluded. To comply with the SEER-Medicare Data Use Agreement stipulation that cell counts <11 may not be directly reported or be derivable with more precision than “<11”, variables presented in a table or figure with a cell count <11 had cell counts for two categories either coarsened or suppressed. Statistical analyses were performed using SAS Version 9.4 statistical software (SAS Institute, Cary, NC, USA). All statistical tests were two-sided with α=0.05.
Results
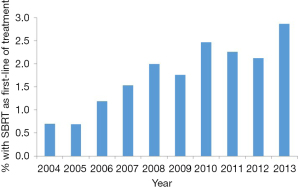
We observed a significant increase in the use of SBRT as first-line treatment from 0.7% to 3.0% over the study period (Figure 1, P<0.001). The median age for the entire sample was 73.0 years (interquartile range, 69–78 years) and comparisons of baseline characteristics in patients receiving SBRT and chemotherapy are shown in Table 1. As compared to patients treated with chemotherapy, those who received SBRT as first-line therapy had Charlson Comorbidity Scores ≥2 (P=0.005), were more likely to be female (P<0.001), had poor disability status (P<0.001), and lower T- and N-Stage (P<0.001). Logistic regression analysis indicated that older age [odds ratio (OR) 1.09 per year, P<0.001], female sex (OR 1.54, P=0.002), poor disability status (OR 2.09, P=0.001), and lower T- and N-stage (P<0.001) were independently associated with SBRT after adjustment for all other covariates (Table 2).
Table 1
| Characteristic | SBRT (n=215) | Chemo (n=12,486) | P value | |||
|---|---|---|---|---|---|---|
| No. | % | No. | % | |||
| Urban/rural | 0.71 | |||||
| Urban | >204 | >94.9 | 12,206 | 97.8 | ||
| Rural | <11 | <5.1 | 280 | 2.2 | ||
| Census tract poverty indicator | 0.90 | |||||
| <20% poverty | 174 | 80.9 | 9,963 | 79.8 | ||
| ≥20% poverty | >30 | >14.0 | 2,380 | 19.1 | ||
| Unknown | <11 | <5.1 | 143 | 1.1 | ||
| Charlson Comorbidity Score | 0.005 | |||||
| 0 | 68 | 31.6 | 4,982 | 39.9 | ||
| 1 | 54 | 25.1 | 3,378 | 27.0 | ||
| ≥2 | 93 | 43.3 | 4,126 | 33.0 | ||
| Race | 0.12 | |||||
| White | 192 | 89.3 | 10,951 | 87.7 | ||
| Black | >12 | >5.6 | 947 | 7.6 | ||
| Other | <11 | <5.1 | 588 | 4.7 | ||
| Sex | <0.001 | |||||
| Male | 95 | 44.2 | 7,025 | 56.3 | ||
| Female | 120 | 55.8 | 5,461 | 43.7 | ||
| Histology | 0.94 | |||||
| Squamous | 48 | 22.3 | 2,816 | 22.6 | ||
| Non-squamous | 167 | 77.7 | 9,670 | 77.4 | ||
| Disability status | <0.001 | |||||
| Poor | 26 | 12.1 | 567 | 4.5 | ||
| Good | 189 | 87.9 | 11,919 | 95.5 | ||
| Derived AJCC 6th ed., T | <0.001 | |||||
| T0/T1 | 51 | 23.7 | 1,199 | 9.6 | ||
| T2 | 49 | 22.8 | 2,879 | 23.1 | ||
| T3 | <11 | <5.1 | 740 | 5.9 | ||
| T4 | 85 | 39.5 | 5,850 | 46.8 | ||
| Unknown | >19 | >8.8 | 1,818 | 14.6 | ||
| Derived AJCC 6th ed., N | <0.001 | |||||
| N0 | 130 | 60.5 | 2,608 | 20.9 | ||
| N1 | 13 | 6 | 1,000 | 8.0 | ||
| N2 | 48 | 22.3 | 5,431 | 43.5 | ||
| N3 | 11 | 5.1 | 2,300 | 18.4 | ||
| Unknown | 13 | 6 | 1,147 | 9.2 | ||
| Median Income | 0.89 | |||||
| <$40,000 | 41 | 19.1 | 2,505 | 20.1 | ||
| $40,000–59,999 | 86 | 40 | 4,725 | 37.8 | ||
| $60,000–79,999 | 48 | 22.3 | 2,843 | 22.8 | ||
| ≥$80,000 | >29 | >13.5 | 2,202 | 17.6 | ||
| Unknown | <11 | <5.1 | 211 | 1.7 | ||
| Age at diagnosis, median [IQR] | 77 [73–82] | 73 [69–78] | <0.001 | |||
SBRT, stereotactic body radiation therapy; Chemo, chemotherapy; AJCC, American Joint Committee on Cancer; IQR, interquartile range.
Table 2
| Variables | Adjusted OR | 95% CI | P |
|---|---|---|---|
| Age at diagnosis | 1.09 | 1.07–1.12 | <0.001 |
| Derived AJCC 6th ed., N | <0.001 | ||
| N0 | Ref. | ||
| N1 | 0.29 | 0.16–0.52 | |
| N2 | 0.21 | 0.15–0.29 | |
| N3 | 0.12 | 0.06–0.22 | |
| NX | 0.29 | 0.16–0.52 | |
| Derived AJCC 6th ed., T | <0.001 | ||
| T0/T1 | Ref. | ||
| T2 | 0.45 | 0.30–0.68 | |
| T3 | 0.33 | 0.15–0.70 | |
| T4 | 0.43 | 0.30–0.62 | |
| TX | 0.35 | 0.21–0.60 | |
| Disability status | 0.001 | ||
| Good | Ref. | ||
| Poor | 2.09 | 1.35–3.25 | |
| Sex | 0.002 | ||
| Male | Ref. | Ref. | |
| Female | 1.54 | 1.16–2.03 | |
| Charlson Comorbidity Score | 0.33 | ||
| 0 | Ref. | ||
| 1 | 1.07 | 0.75–1.54 | |
| ≥2 | 1.27 | 0.92–1.76 | |
OR, odds ratio; CI, confidence interval; AJCC, American Joint Committee on Cancer.
The median survival for patients with SBRT as first-line treatment, and chemotherapy alone was 12 and 8 months, respectively (Figure 2, log-rank, P<0.001). SBRT was associated with longer overall survival [hazard ratio (HR) 0.72, 95% CI: 0.62–0.83, P<0.001] after adjustment for all covariates significantly associated with overall survival. Covariates independently associated with increased survival included female sex (HR 0.80, P<0.001) and higher income (≥$80,000, HR 0.85, P<0.001), while Charlson Comorbidity Score (≥2, HR 1.09, P<0.001), poor disability status (HR 1.22, P<0.001), higher T-stage (HR 1.31, P<0.001) and higher N-stage (N3, HR 1.21, P<0.001) were associated with decreased survival (Table 3). There was a significant interaction (P<0.001) between N-stage and treatment, indicating that the increased survival with SBRT was primarily attributable to patients with N0 disease (HR 0.59, 95% CI: 0.48–0.72).
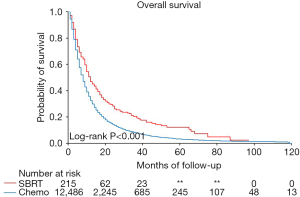
Table 3
| Variables | Unadjusted | Adjusted | |||||
|---|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P value | Hazard ratio | 95% CI | P value | ||
| First-line treatment | <0.001 | <0.001 | |||||
| Chemo | Ref. | Ref. | |||||
| SBRT | 0.67 | 0.57–0.77 | 0.72 | 0.62–0.83 | |||
| Urban/rural | 0.006 | – | |||||
| Rural | Ref. | – | – | ||||
| Urban | 0.84 | 0.75–0.95 | – | – | |||
| Census tract poverty indicator | 0.26 | – | |||||
| ≥20% poverty | Ref. | – | – | ||||
| <20% poverty | 0.96 | 0.92–1.01 | – | – | |||
| Unknown | 1.02 | 0.85–1.20 | – | – | |||
| Charlson Comorbidity Score | <0.001 | <0.001 | |||||
| 0 | Ref. | Ref. | |||||
| 1 | 1.03 | 0.99–1.08 | 1.03 | 0.99–1.08 | |||
| ≥2 | 1.11 | 1.07–1.16 | 1.09 | 1.05–1.14 | |||
| Race | <0.001 | <0.001 | |||||
| White | Ref. | Ref. | |||||
| Black | 0.98 | 0.92–1.05 | 0.93 | 0.87–1.00 | |||
| Other | 0.81 | 0.75–0.89 | 0.80 | 0.73–0.87 | |||
| Sex | <0.001 | <0.001 | |||||
| Male | Ref. | Ref. | |||||
| Female | 0.80 | 0.77–0.83 | 0.80 | 0.78–0.83 | |||
| Histology | 0.003 | – | |||||
| Non-squamous | Ref. | – | – | ||||
| Squamous | 1.07 | 1.02–1.11 | – | – | |||
| Disability status | <0.001 | <0.001 | |||||
| Good | Ref. | Ref. | |||||
| Poor | 1.16 | 1.07–1.26 | 1.22 | 1.12–1.33 | |||
| Age at diagnosis (per 1 year increase) | 1.009 | 1.006–1.013 | <0.001 | 1.012 | 1.008–1.015 | <0.001 | |
| Median Income | <0.001 | <0.001 | |||||
| <$40,000 | Ref. | Ref. | |||||
| $40,000–59,999 | 0.95 | 0.90–0.99 | 0.96 | 0.91–1.01 | |||
| $60,000–79,999 | 0.90 | 0.85–0.95 | 0.91 | 0.86–0.96 | |||
| ≥$80,000 | 0.84 | 0.79–0.89 | 0.85 | 0.80–0.90 | |||
| Unknown | 0.98 | 0.84–1.12 | 0.98 | 0.85–1.13 | |||
| AJCC 6th ed., N | <0.001 | <0.001 | |||||
| N0 | Ref. | Ref. | |||||
| N1 | 1.18 | 1.10–1.27 | 1.16 | 1.07–1.25 | |||
| N2 | 1.26 | 1.20–1.32 | 1.24 | 1.18–1.30 | |||
| N3 | 1.22 | 1.16–1.30 | 1.21 | 1.15–1.29 | |||
| NX | 1.18 | 1.10–1.27 | 1.16 | 1.08–1.25 | |||
| AJCC 6th ed., T | <0.001 | <0.001 | |||||
| T0/T1 | Ref. | Ref. | |||||
| T2 | 1.21 | 1.13–1.29 | 1.17 | 1.09–1.25 | |||
| T3 | 1.37 | 1.25–1.50 | 1.32 | 1.20–1.45 | |||
| T4 | 1.34 | 1.26–1.43 | 1.31 | 1.23–1.40 | |||
| TX | 1.27 | 1.18–1.36 | 1.25 | 1.15–1.35 | |||
CI, confidence interval; Chemo, chemotherapy; SBRT, stereotactic body radiation therapy; AJCC, American Joint Committee on Cancer.
Among a sample matched on age, sex, Charlson comorbidity Score, disability status, T-stage and N-stage, fewer patients treated with first-line SBRT underwent hospitalization compared to chemotherapy patients (73% vs. 81%, P=0.02) (Table 4). The mean number of hospitalizations was similar for SBRT and chemotherapy patients, but was significantly lower when normalized by months of survival (0.22 vs. 0.28, P=0.009). Although SBRT patients incurred higher total hospitalization costs (mean $24,266 vs. $19,213, P=0.008), costs per months of survival were slightly lower and did not differ significantly from chemotherapy patients. Among those who had one or more hospitalizations, the average number of hospitalizations and number of days of hospitalization were significantly higher for SBRT than chemotherapy patients, but not when expressed as per months of survival. Similarly, the total cost of hospitalization was higher for SBRT patients ($33,063 vs. $23,865, P<0.001), but the cost per month of survival was nearly the same as for chemotherapy patients ($3,883 vs. $3,924, P=0.94). However, the average cost per hospitalization was significantly higher for SBRT patients ($13,647 vs. $10,432, P<0.001). Admission diagnoses are listed in Table S2, with the most common diagnoses being shortness of breath (27%) and pneumonia (20%), with shortness of breath being more common among SBRT patients, and infections being more common among chemotherapy patients (Table S2).
Table 4
| Variables | SBRT (N=213) | Chemo (N=631) | P value |
|---|---|---|---|
| Patients with ≥1 hospitalization, n [%] | 156 [73] | 509 [81] | 0.02 |
| All patients in matched sample, mean ± SE | |||
| Number of hospitalizations | 2.00±0.14 | 1.90±0.09 | 0.52 |
| Hospitalizations per month of survival | 0.22±0.02 | 0.28±0.01 | 0.009 |
| Total hospitalization cost ($) | 24,266±1,697 | 19,213±1,058 | 0.008 |
| Cost per month of survival ($) | 2,843±360 | 3,165±209 | 0.44 |
| Hospitalized patients only, mean ± SE | |||
| Number of hospitalizations | 2.73±0.16 | 2.36±0.09 | 0.04 |
| Hospitalizations per month of survival | 0.29±0.03 | 0.35±0.01 | 0.07 |
| Number of days of hospitalization | 16.64±1.11 | 13.38±0.63 | 0.01 |
| Days of hospitalization per month of survival | 1.87±0.24 | 2.25±0.13 | 0.16 |
| Total cost of hospitalization ($) | 33,063±1,947 | 23,865±1,128 | <0.001 |
| Cost per hospitalization ($) | 13,647±735 | 10,432±407 | <0.001 |
| Cost per month of survival ($) | 3,883±452 | 3,924±250 | 0.94 |
SBRT, stereotactic body radiation therapy; dx, diagnosis; Chemo, chemotherapy; SE, standard error.
Discussion
To our knowledge, this is the first study to assess the patterns of care of SBRT use in metastatic NSCLC. First-line SBRT use in patients with metastatic NSCLC is increasing and its use was associated with longer overall survival (HR 0.72) compared to patients treated with chemotherapy only. This survival difference persisted despite SBRT patients having a number of poor prognostic characteristics in our sample, including higher Charlson Comorbidity Scores, poor disability status and higher T- and N-stage. It is important to acknowledge that selection bias is likely contributing this observed difference in survival to some extent. This is because detailed information regarding the extent of metastatic disease, or number of metastatic sites, cannot be determined with certainty from the SEER-Medicare data. Therefore, systemic imbalances could exist with regard to metastatic disease burden and its influence treatment patterns. While we minimized selection bias in our comparisons of outcomes, hospitalizations and costs between SBRT and non-SBRT patients by controlling for available confounding variables, additional unmeasured confounders likely exist. An observational study design cannot establish causality, but we have nonetheless observed that early adoption of SBRT was associated with improved survival when adjusting for available confounding covariates. Our study thus supports further investigation of SBRT in this population. Indeed, multiple phase III randomized studies assessing the impact of SBRT on survival in metastatic cancer are ongoing (32-34).
Although fewer SBRT patients were hospitalized during follow-up, among those who were hospitalized, the average number of hospitalizations and days of hospitalization were higher in SBRT compared to chemotherapy due to their longer survival. We also observed increased hospitalization costs for those who received SBRT, which is primarily attributable to longer survival. When normalized by months of survival, the number of hospitalizations was lower in the SBRT patients and costs did not differ significantly from the chemotherapy patients. It should be emphasized that this is an observational and not causal relationship, meaning that the observed differences in healthcare utilization may reflect fundamental differences in these population, such as the extent of disease. However, it is notable that the average cost per hospitalization was significantly higher in the SBRT patients. It is not entirely clear why this is the case, but is possible that the observed increased hospitalization costs reflect more complex management of treatment-related complications. As more chemotherapy patients were admitted with intercurrent infections and SBRT patients were admitted with shortness of breath (possibly pneumonitis), it is plausible that SBRT-related complications different from chemotherapy, leading to different care needs for hospitalizations. Supporting this hypothesis, in the SABR-COMET phase II randomized trial, SBRT was associated with a 20% absolute increased risk of grade ≥2 adverse events (12). Despite increased toxicity, multiple cost-effectiveness analyses of available data have thus far shown SBRT to be a cost-effective treatment for metastatic cancer (35-37). This is consistent with our finding of somewhat lower costs per months of survival for SBRT.
Our finding that SBRT use is higher among patients with increased age and poor disability warrants discussion. In the three aforementioned randomized phase II of SBRT in oligometastatic cancer, the median age of SBRT patients was 64–67 years, with Eastern Cooperative Oncology Group performance status of 0–2 (10-12). Our study is limited to Medicare-eligible patients and hence an older population. Older, more frail patients receiving SBRT in our population could suggest that outside of clinical trials, SBRT is being used in patients who cannot tolerate systemic therapy. Regardless, it is plausible that survival advantage SBRT may be underestimated in our study, given the differences in baseline characteristics we observed compared to the phase II studies.
This study has substantial strengths. It used data representing real-world settings and sophisticated quantitative methods to determine the trends, determinants, and outcomes of treatment in metastatic lung cancer patients. Our results provide useful survival information for this population. Estimates of hospitalizations and costs in this manuscript are novel contributions to the literature and may help guide policy and clinical decisions pertaining to the diffusion and use of SBRT in this patient population. Our findings support current practice and further investigation of SBRT in patients with metastatic lung cancer.
This analysis also has several important limitations and challenges. In addition to the potential selection bias as discussed above, the study population is not a random selection of all cancer patients across the country; SEER data cover approximately 28% of all cancer patients, and some regions are not be represented in the sample. In addition, SEER-Medicare data only includes patients over the age of 65 and the results may not be applicable to younger lung cancer patients. While this could affect results, we feel the study is relevant for most cases of metastatic lung cancer given the prevalence of lung cancer among the elderly. In addition, SEER-Medicare does not contain data on patient performance status, which is a known independent prognostic factor for survival in lung cancer (38). That said, performance status is well approximated by disability status which we used in this study (31). Finally, given the time period available for study using this SEER-Medicare database (outcomes through 2014), we do not have any information regarding immunotherapy use, which has been shown in multiple randomized trials to improve overall survival in advanced-stage NSCLC, but whose practice-changing results were published after this study period (39-45). In addition, prospective data supporting the use of SBRT has accrued since this time (10-12). Nonetheless, there is growing evidence for clinical synergy between SBRT and immunotherapy, and thus it is plausible that a survival advantage with SBRT may improve with immunotherapy (46-51). The combination of SBRT and immunotherapy in advanced stage NSCLC is currently being evaluated in prospective studies (52-57).
In conclusion, we observed increased use of SBRT from 2004 to 2013 and its use was associated with longer survival. Although fewer SBRT patients were hospitalized, there was an increased cost associated with hospitalizations that was primarily due to their longer survival. Our findings support the ongoing evaluations of SBRT in combination with systemic therapy in elderly patients with metastatic NSCLC.
Acknowledgments
This work was presented as a poster at the American Society for Radiation Oncology 2021 Annual Meeting.
Funding: This work was supported by a Lake Champlain Cancer Research Organization Pilot Grant.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1835/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1835/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1835/coif). All authors report that this work was supported by a Lake Champlain Cancer Research Organization Pilot Grant. Payments were made to the University of Vermont. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7-30. [Crossref] [PubMed]
- Molina JR, Yang P, Cassivi SD, et al. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc 2008;83:584-94. [Crossref] [PubMed]
- Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol 1995;13:8-10. [Crossref] [PubMed]
- Mehta N, Mauer AM, Hellman S, et al. Analysis of further disease progression in metastatic non-small cell lung cancer: implications for locoregional treatment. Int J Oncol 2004;25:1677-83. [Crossref] [PubMed]
- Rusthoven KE, Hammerman SF, Kavanagh BD, et al. Is there a role for consolidative stereotactic body radiation therapy following first-line systemic therapy for metastatic lung cancer? A patterns-of-failure analysis. Acta Oncol 2009;48:578-83. [Crossref] [PubMed]
- Pfannschmidt J, Dienemann H. Surgical treatment of oligometastatic non-small cell lung cancer. Lung Cancer 2010;69:251-8. [Crossref] [PubMed]
- Tanvetyanon T, Robinson LA, Schell MJ, et al. Outcomes of adrenalectomy for isolated synchronous versus metachronous adrenal metastases in non-small-cell lung cancer: a systematic review and pooled analysis. J Clin Oncol 2008;26:1142-7. [Crossref] [PubMed]
- Tönnies M, Pfannschmidt J, Bauer TT, et al. Metastasectomy for synchronous solitary non-small cell lung cancer metastases. Ann Thorac Surg 2014;98:249-56. [Crossref] [PubMed]
- Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303:1070-6. [Crossref] [PubMed]
- Gomez DR, Tang C, Zhang J, et al. Local Consolidative Therapy Vs. Maintenance Therapy or Observation for Patients With Oligometastatic Non-Small-Cell Lung Cancer: Long-Term Results of a Multi-Institutional, Phase II, Randomized Study. J Clin Oncol 2019;37:1558-65. [Crossref] [PubMed]
- Iyengar P, Wardak Z, Gerber DE, et al. Consolidative Radiotherapy for Limited Metastatic Non-Small-Cell Lung Cancer: A Phase 2 Randomized Clinical Trial. JAMA Oncol 2018;4:e173501. [Crossref] [PubMed]
- Palma DA, Olson R, Harrow S, et al. Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of Oligometastatic Cancers: Long-Term Results of the SABR-COMET Phase II Randomized Trial. J Clin Oncol 2020;38:2830-8. [Crossref] [PubMed]
- Bach PB. Limits on Medicare's ability to control rising spending on cancer drugs. N Engl J Med 2009;360:626-33. [Crossref] [PubMed]
- Mariotto AB, Enewold L, Zhao J, et al. Medical Care Costs Associated with Cancer Survivorship in the United States. Cancer Epidemiol Biomarkers Prev 2020;29:1304-12. [Crossref] [PubMed]
- Klein R, Muehlenbein C, Liepa AM, et al. Cost-effectiveness of pemetrexed plus cisplatin as first-line therapy for advanced nonsquamous non-small cell lung cancer. J Thorac Oncol 2009;4:1404-14. [Crossref] [PubMed]
- Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006;355:2542-50. [Crossref] [PubMed]
- Yu JB, Gross CP, Wilson LD, et al. NCI SEER public-use data: applications and limitations in oncology research. Oncology (Williston Park) 2009;23:288-95. [PubMed]
- Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care 2002;40:IV-3-18. [Crossref] [PubMed]
- Shirvani SM, Jiang J, Chang JY, et al. Comparative effectiveness of 5 treatment strategies for early-stage non-small cell lung cancer in the elderly. Int J Radiat Oncol Biol Phys 2012;84:1060-70. [Crossref] [PubMed]
- Vest MT, Herrin J, Soulos PR, et al. Use of new treatment modalities for non-small cell lung cancer care in the Medicare population. Chest 2013;143:429-35. [Crossref] [PubMed]
- Virnig BA, Warren JL, Cooper GS, et al. Studying radiation therapy using SEER-Medicare-linked data. Med Care 2002;40:IV-49-54. [Crossref] [PubMed]
- Davidoff AJ, Tang M, Seal B, et al. Chemotherapy and survival benefit in elderly patients with advanced non-small-cell lung cancer. J Clin Oncol 2010;28:2191-7. [Crossref] [PubMed]
- Hoverman JR, Robertson SM. Lung cancer: a cost and outcome study based on physician practice patterns. Dis Manag 2004;7:112-23. [Crossref] [PubMed]
- Ramsey SD, Martins RG, Blough DK, et al. Second-line and third-line chemotherapy for lung cancer: use and cost. Am J Manag Care 2008;14:297-306. [PubMed]
- Weycker D, Malin J, Kim J, et al. Risk of hospitalization for neutropenic complications of chemotherapy in patients with primary solid tumors receiving pegfilgrastim or filgrastim prophylaxis: a retrospective cohort study. Clin Ther 2009;31:1069-81. [Crossref] [PubMed]
- Asmis TR, Ding K, Seymour L, et al. Age and comorbidity as independent prognostic factors in the treatment of non small-cell lung cancer: a review of National Cancer Institute of Canada Clinical Trials Group trials. J Clin Oncol 2008;26:54-9. [Crossref] [PubMed]
- Brown N, Melville M, Gray D, et al. Relevance of clinical trial results in myocardial infarction to medical practice: comparison of four year outcome in participants of a thrombolytic trial, patients receiving routine thrombolysis, and those deemed ineligible for thrombolysis. Heart 1999;81:598-602. [Crossref] [PubMed]
- Earle CC, Venditti LN, Neumann PJ, et al. Who gets chemotherapy for metastatic lung cancer? Chest 2000;117:1239-46. [Crossref] [PubMed]
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83. [Crossref] [PubMed]
- Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. J Clin Epidemiol 2000;53:1258-67. [Crossref] [PubMed]
- Davidoff AJ, Gardner LD, Zuckerman IH, et al. Validation of disability status, a claims-based measure of functional status for cancer treatment and outcomes studies. Med Care 2014;52:500-10. [Crossref] [PubMed]
- Olson R, Mathews L, Liu M, et al. Stereotactic ablative radiotherapy for the comprehensive treatment of 1-3 Oligometastatic tumors (SABR-COMET-3): study protocol for a randomized phase III trial. BMC Cancer 2020;20:380. [Crossref] [PubMed]
- Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy for the comprehensive treatment of 4-10 oligometastatic tumors (SABR-COMET-10): study protocol for a randomized phase III trial. BMC Cancer 2019;19:816. [Crossref] [PubMed]
-
Maintenance Chemotherapy with or Without Local Consolidative Therapy in Treating Patients With Stage IV Non-small Cell Lung Cancer - Kumar A, Straka C, Courtney PT, et al. Cost-Effectiveness Analysis of Stereotactic Ablative Radiation Therapy in Patients With Oligometastatic Cancer. Int J Radiat Oncol Biol Phys 2021;109:1185-94. [Crossref] [PubMed]
- Lester-Coll NH, Rutter CE, Bledsoe TJ, et al. Cost-Effectiveness of Surgery, Stereotactic Body Radiation Therapy, and Systemic Therapy for Pulmonary Oligometastases. Int J Radiat Oncol Biol Phys 2016;95:663-72. [Crossref] [PubMed]
- Qu XM, Chen Y, Zaric GS, et al. Is SABR Cost-Effective in Oligometastatic Cancer? An Economic Analysis of the SABR-COMET Randomized Trial. Int J Radiat Oncol Biol Phys 2021;109:1176-84. [Crossref] [PubMed]
- Kawaguchi T, Takada M, Kubo A, et al. Performance status and smoking status are independent favorable prognostic factors for survival in non-small cell lung cancer: a comprehensive analysis of 26,957 patients with NSCLC. J Thorac Oncol 2010;5:620-30. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:2078-92. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med 2018;379:2040-51. [Crossref] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Hwang WL, Niemierko A, Hwang KL, et al. Clinical Outcomes in Patients With Metastatic Lung Cancer Treated With PD-1/PD-L1 Inhibitors and Thoracic Radiotherapy. JAMA Oncol 2018;4:253-5. [Crossref] [PubMed]
- Luke JJ, Lemons JM, Karrison TG, et al. Safety and Clinical Activity of Pembrolizumab and Multisite Stereotactic Body Radiotherapy in Patients With Advanced Solid Tumors. J Clin Oncol 2018;36:1611-8. [Crossref] [PubMed]
- Shaverdian N, Lisberg AE, Bornazyan K, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol 2017;18:895-903. [Crossref] [PubMed]
- Tang C, Welsh JW, de Groot P, et al. Ipilimumab with Stereotactic Ablative Radiation Therapy: Phase I Results and Immunologic Correlates from Peripheral T Cells. Clin Cancer Res 2017;23:1388-96. [Crossref] [PubMed]
- Verma V, Cushman TR, Selek U, et al. Safety of Combined Immunotherapy and Thoracic Radiation Therapy: Analysis of 3 Single-Institutional Phase I/II Trials. Int J Radiat Oncol Biol Phys 2018;101:1141-8. [Crossref] [PubMed]
- Wang YS, Yang G, Wang YY, et al. Early efficacy of stereotactic body radiation therapy combined with adoptive immunotherapy for advanced malignancies. Mol Clin Oncol 2013;1:925-9. [Crossref] [PubMed]
- Radiation and Immune Checkpoints Blockade in Metastatic NSCLC (BMS # CA209-632). Available online: https://clinicaltrials.gov/ct2/show/NCT03168464
-
Pembrolizumab and Stereotactic Body Radiation Therapy or Non-Stereotactic Wide-Field Radiation Therapy in Treating Patients with Non-small Cell Lung Cancer - Phase Ib Study of Stereotactic Body Radiotherapy (SBRT) in Oligometastatic Non-small Lung Cancer (NSCLC) With Dual Immune Checkpoint Inhibition. Available online: https://clinicaltrials.gov/ct2/show/NCT03275597
-
Concurrent or Sequential Immunotherapy and Radiation Therapy in Patients with Metastatic Lung Cancer (COSINR) -
Priming Immunotherapy in Advanced Disease with Radiation - UCDCC#270: Avelumab and Stereotactic Ablative Radiotherapy in Non-responding and Progressing NSCLC Patients. Available online: https://clinicaltrials.gov/ct2/show/NCT03158883