
A novel system for analyzing indocyanine green (ICG) fluorescence spectra enables deeper lung tumor localization during thoracoscopic surgery
Introduction
In recent years, widespread implementation of computed tomography (CT) screening has enabled the detection of small lung cancer lesions at an early stage, and rates of surgical treatment among patients with early-stage lung cancer have increased (1,2). Partial lung resection is often the treatment of choice for small lesions with ground-glass opacities. Previous studies have also reported prolonged survival after resection of metastatic lung tumors (3,4), and partial resection is often the treatment of choice in these cases.
In addition, recent advancements in video-assisted thoracoscopic surgery (VATS), robot-assisted thoracic surgery (RATS) (5,6), and uniport VATS (7,8) have popularized these methods as alternatives to conventional multiport VATS. Compared to thoracotomy, VATS results in a smaller wound and is less invasive, making it safer than open thoracotomy for older adults and high-risk patients (9). However, unlike a thoracotomy, VATS may not allow for direct palpation of the lesion depending on its diameter, morphology, and depth relative to the pleural wall. Therefore, such tumors are even more challenging to palpate during robotic surgery and uniport VATS. Thus, the development and implementation of methods for the reliable identification of tumors will play a key role in improving the quality of thoracoscopic surgery.
Various methods for tumor localization (identification) during thoracoscopic surgery have been proposed. Preoperative methods include (I) CT-guided percutaneous assisted localization, (II) transbronchial-guided assisted localization, and (III) 3D-CT-guided assisted localization (10). Recently, the virtual assisted lung mapping (VAL-MAP) method has been applied in clinical settings (11). Intraoperative marking methods include the intraoperative stamping method, and intraoperative CT-guided assisted localization (12,13). However, these methods are all associated with an array of complications, such as dye diffusion and implant displacement, adverse events (pleuralgia, pneumothorax, air embolism, etc.), the uncertainty of marking, postponement of surgery due to post-procedural fever, hypoxemia, and radiation exposure during the procedure, and the need for trans-bronchial lung biopsies. Several studies have also highlighted concerns regarding the necessity of these techniques, the limited interval between marking and surgery, and the cost of these complex procedures (10,11,14).
Given the difficulty in tumor localization during thoracoscopic surgery and the various complications associated with available methods, developing a simple and minimally invasive procedure that can be performed at any facility is urgently required. Therefore, the present study aimed to investigate the utility of a novel, non-invasive method that utilizes the fluorescent dye indocyanine green (ICG) and a probe to measure the spectrum of near-infrared (NIR) light emitted by ICG in tumor tissues. Pseudo-tumors combined with a sponge and porcine lungs were utilized in this preclinical study based on the methods used in previous studies attempting percutaneous or transbronchial tumor localization using ICG (15-17).
Importantly, although intravenous methods are often used during thoracic surgery to evaluate demarcation lines during area resection (18), the intravenous injection method of ICG for identifying tumors has only been investigated in clinical trials and has not been examined in clinical practice (19,20). In addition, Percutaneous or transbronchial ICG marking, which is currently performed in a limited number of centers, is a highly specialized procedure associated with the issues of uncertainty, invasiveness, and complications. Therefore, while the proposed spectral measurement system is intended for intravenous use, it may also aid in addressing issues related to current ICG-based marking methods.
Conventional NIR cameras can only capture ICG fluorescence with the naked eye; however, this new innovative system captures ICG fluorescence as a wavelength, enabling the identification of tumors at a greater depth.
Therefore, we hypothesized that this spectral measurement device would be able to capture fluorescence wavelengths deeper than conventional NIR cameras and conducted experiments to clarify this hypothesis. We present the following article in accordance with the STARD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-244/rc).
Methods
A novel system for the measurement of fluorescence spectra
We used the NIR fluorescence spectrum system (Advantest Corporation, Tokyo) described by Ebihara et al. (21). A laser diode (wavelength: 785 nm, maximum output power: 5 mW) excitation source was focused onto the center of a Y-shaped quartz coaxial fiber. A notch filter excluded reflected laser signals except for the fluorescence signal. A photonic multichannel analyzer (PMA) detected the isolated fluorescence signal. The NIR fluorescence from the ICG samples prepared in this study was collected through the outside of the coaxial fiber and measured using spectroscopy. The central wavelength of the fluorescence is approximately 840 nm, which is close to the excitation wavelength of 785 nm. Therefore, a narrow-band notch filter was installed in front of the input slit of the spectrometer to cut the signal off from the excitation light. Details regarding this system have been published previously (21-23). The probe (diameter =10 mm, length =350 mm) can be inserted through a 12 mm port, requiring only a small incision, and is long enough to explore a wide area within the thoracic cavity. Furthermore, it is sterilizable and can be used repeatedly (Figure 1). The monitor displays images from the ICG camera on the left and the image from spectral measurement on the right side (Video 1).
NIR camera
We used a Hyper Eye Medical System Handy camera (MIZUHO, Tokyo, Japan) for NIR imaging. This camera emits light in the range of 760–780 nm, which triggers ICG to emit NIR light.
Adjustment of ICG concentration
ICG is a relatively hydrophobic tricarbocyanine dye with a molecular weight of 751.4 Da, an absorption peak at 780 nm, and an emission peak at 830 nm. When ICG is administered intravascularly, it binds to serum α-lipoprotein, β-lipoprotein, and albumin almost immediately, with negligible leakage into the interstitium (24). Therefore, ICG was mixed with fetal bovine serum (FBS) (Gibco, USA), and the concentration of ICG was adjusted to 5.0×10−2 mg/mL in reference to a previous study (21).
Pseudo-tumor preparation
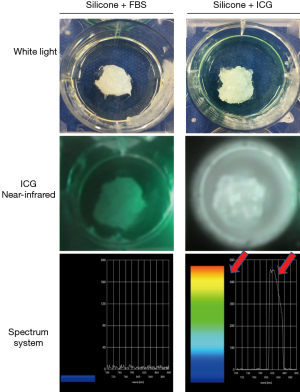
Pseudo-tumors were prepared using commercially available silicone resin (Konishi Co., Ltd., Osaka, Japan). A 20 mm, round pseudo-tumor was created using 2.0 mL of silicone resin. Studies have indicated that tumor accumulation of intravenously administered ICG occurs 24 hours or more after delivery (25,26). Therefore, assuming intravenous administration of ICG, 1.0 mL of ICG (5.0×10−2 mg/mL) adjusted at the above concentration was quickly mixed with the pseudo-tumor. A negative control was prepared in the same way but combined with 1.0 mL of FBS rather than the ICG solution (Figure 2).
Sponge preparation
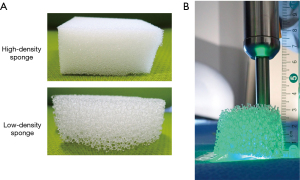
The lung tissue density in vivo depends on whether the lung is collapsed or expanded. To mimic this variability, we cut commercially available polyurethane foam sponges with different tissue densities (high- and low-density) to appropriate sizes for the experiments (Figure 3A).
Tracheal and lung blocks
Porcine tracheal and lung blocks were purchased from Tokyo Shibaura Organ Co. The lungs were frozen and thawed several hours before use.
Ethical considerations
The heart and both lungs were taken en bloc from freshly slaughtered porcine. Since the tissues were obtained only from animals slaughtered for nutritional purposes, ethical approval from the relevant authorities for animal studies was not required.
Dry lab experiments using sponges
First, we confirmed that ICG-mixed pseudo-tumors and negative control pseudo-tumors could be detected without intervening objects using a spectrophotometer and an ICG camera (Figure 2). The pseudo-tumors were wrapped in plastic to prevent ICG from adhering to the surrounding area. The high- and low-density sponges were sliced to the following thickness: 15, 25, and 30 mm. An additional 40 mm-thick slice of the low-density sponge was also prepared (Figure 3B). The sponges were placed on top of the pseudo-tumor, and the spectra were measured by pressing the spectroscope probe on top of the sponge. The probe of the NIR was not fixed; when it was difficult to confirm the intensity of the ICG emission, the distance between the target and probe was varied to assess whether the emission was indeed not measurable.
Wet lab experiments using porcine lungs (ex vivo)
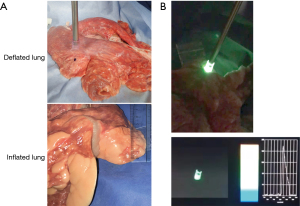
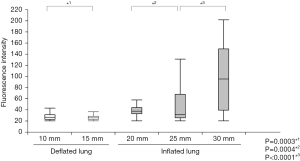
Similarly, the plastic-wrapped ICG-mixed pseudo-tumors were covered with deflated porcine lungs. The lungs were not sliced, but lung thickness was measured at different sites. The probe of the spectrometer was placed in contact with portions that were 10, 15, and 20 mm-thick (Figure 4A). Then, with the main bronchus clamped, the lungs were inflated, and measurements were taken at 20, 25, and 30 mm-thick portions. Spectra were measured before and after inflating the lungs (Figure 4B). As in the dry lab experiment, the NIR camera was placed at various distances.
Evaluation of experiments
All NIR camera images and spectral system wavelengths were evaluated by three surgeons who were aware of the nature of the pseudo-tumors they were assessing.
Statistical analysis
When the spectral probe was applied to sponges or porcine lungs of various thicknesses, the values when the system responded within the peak wavelength range for ICG fluorescence (approximately 805–845 nm) were recorded as continuous variables. Mann-Whitney U-tests were used to compare values between groups. Values were compared between the high- and low-density sponges for each sponge thickness. Values for inflated and deflated porcine lungs were analyzed separately. Statistical analysis was performed using JMP Pro 16® (SAS Institute, Cary, NC, USA). All P values were based on a two-tailed test, and statistical significance was set at P<0.05.
Results
Dry lab experiments
Before the pseudo-tumors solidified, they were quickly mixed with FBS or ICG adjusted for concentration in a well-plate and allowed to stand in a light-shielded condition until solidification. Then, the ICG camera and spectroscopy system were applied to the negative control and ICG-mixed pseudo-tumors. The control did not emit light with the ICG camera, and the spectroscopy system showed no increase in wavelength around 830 nm, the emission wavelength range of ICG. On the other hand, in the ICG-mixed pseudo-tumor, the ICG camera showed tumor luminescence, and spectral measurements detected an increase in wavelength in the wavelength range of ICG luminescence (Figure 2).
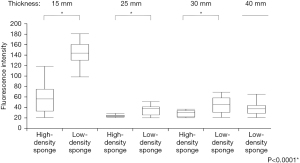
The ICG-mixed pseudo-tumor was then wrapped in plastic, and a sponge was placed on top of it for evaluation. The ICG camera had difficulty detecting the spectrum when covered by even the least thick (10 mm) high-density sponge. At the same time, the spectral system was still able to detect spectra covered by the 30 mm-thick high-density sponge. The ICG camera and spectral systems detected spectra when covered by low-density sponges up to a thickness of 20 and 40 mm, respectively. The spectrophotometer detected tumors deeper than the ICG camera in high-density and low-density sponges. Significantly more spectral attenuation was observed for the denser material across all thicknesses (Figure 5).
Wet lab experiments
The pseudo-tumors were mixed with ICG adjusted for concentration, as in the sponge experiment, and wrapped in plastic. The porcine lung was not sliced but was placed so that the thickness was measured, and the pseudo-tumor was positioned under the part of the desired thickness. The ICG camera and spectroscopy system were then applied and evaluated on it. In the deflated porcine lung, the ICG camera had difficulty detecting spectra covered by 10 mm-thick tissue. In contrast, the spectrophotometer detected spectra covered by 15 mm-thick tissue. Moreover, when the lungs were inflated, the ICG camera was still unable to detect the tumor at any thickness. In contrast, the spectrophotometer detected spectra covered by 20, 25, and 30 mm-thick tissue (Figure 6). Like the sponge experiments, the spectrophotometer was able to detect deeper tumors than the ICG camera in both the deflated and inflated lung. Moreover, in spectrophotometer, tumors in deeper locations could be detected when the lungs were inflated (Figure 6).
Discussion
We investigated the usefulness of a novel ICG fluorescence spectroscopy system for tumor localization during thoracoscopic surgery. Our findings indicate that this method can identify tumors deeper than the lung surface that are difficult to identify using conventional ICG cameras.
ICG accumulates in solid tumors due to the enhanced permeability and retention (EPR) effect. This EPR effect refers to the property of small molecules, such as ICG, to accumulate systemically and passively in tumors due to defective endothelial cells and wide fenestrations (600–800 nm) associated with tumor neovascularization (27). Once in the tumor microenvironment, these particles are retained solely based on global properties such as molecular size, shape, charge, and polarity, rather than tumor-specific targeting mechanisms, such as ligand-receptor interactions (28). This property allows more ICG to accumulate and be retained in tumors relative to the surrounding tissues. This difference in ICG concentration marks the tumor and distinguishes it from background and is called the tumor-to-background ratio (TBR), which is greatest approximately 24 hours after intravenous administration of ICG (25,26).
Up to 98% of ICG binds to proteins in the blood and accumulates in tumors. Therefore, ICG was diluted in FBS and mixed with the pseudo-tumors. The optimal dilution concentration was adjusted based on methods used in the previous study (21).
Identifying lung tumors under 5 mm in size and more than 20 mm in depth from the surface is difficult when using an ICG camera and the naked eye (20). However, the novel spectral system developed in the current study detects NIR light wavelengths using specialized instrumentation; hence, it may be able to detect lesions that cannot be identified by the conventional ICG camera. Our findings indicated that the conventional ICG camera had difficulty detecting pseudo-tumors when covered by tissue or tissue substitute thicker than 10 mm, while the spectral analysis device could detect spectra covered by thicker tissue or tissue substitute. These results suggest that the distance between the tumor and the probe, as well as the density of the intervening tissue between the tumor and the probe, are important factors in detecting ICG wavelength. The lung is an air-containing tissue, and the thickness and density of the tissue, even in the same area differ depending on whether the lung is inflated. The density of lung tissue also varies depending on the background lung condition. For example, in patients with emphysema, the lung tends to overexpand, resulting in low tissue density. The results of our wet-lab experiments indicated that spectral values for deflated lungs decreased significantly as the thickness of the lung increased. However, when lungs are inflated, the spectral values significantly increased as the thickness of the lung increased. These results indicate that tissue transmittance of NIR light is higher in air-filled lungs than in deflated lungs, even though the distance from the lung surface to the tumor is longer. This also suggests that the spectrum is easier to detect when the lung contains some air, which is a characteristic unique to the lung that is not observed in other tissues. According to a previous study, the detection limit of the spectrum in the stomach is approximately 13 mm (21). However, in the lungs, identification of deeper tumors may be possible depending on the conditions of measurement.
Factors affecting the detection of NIR light in biological tissues include scattering and absorption in the tissue. Of these, the effect of scattering is particularly strong (29). NIR light scatters through biological tissue at a distance of several tens of micrometers, and the average distance the light travels prior to absorption is reported to be several tens of millimeters (30). Thus, in normal biological tissues, the intensity gradually decreases with complex scattering in the range of several tens of micrometers. In the lungs, if increased air content results in decreased density at the alveolar level (i.e., a change in density from a few micrometers to a few tens of micrometers), both scattering and absorption are affected. In the medical field, the processes by which reflection and absorption of NIR light occur are complex and challenging to evaluate. However, in our study, significant differences in detection were noted even with subtle changes measured in millimeters. Naturally, the detection limit of the tumor may be affected by the condition of the lung, the density of tumor cells, and the location of blood vessels and bronchi in each case. However, ICG probes that detect this spectrum of NIR light allow for the detection of tumors located deeper from the surface than conventional ICG cameras used in clinical settings.
In addition, we would like to emphasize that the safety and versatility of this method are largely associated with the use of ICG, which is widely used in clinical practice today. ICG is commonly administered intravenously 24 hours before surgery and does not need complicated procedures. The intraoperative procedure used in this study solely involved applying the ICG probe to the lung to detect the NIR light spectrum. Thus, tumor identification can be performed both preoperatively and intraoperatively, regardless of the surgeon’s experience. Therefore, the procedure is less invasive, which reduces the risk of postprocedural complications, and it can be performed at any facility since it does not require the use of expensive equipment. Although we cannot present detailed equipment prices now, costs are presumed to be lower because there are no consumables and equipment costs may be lower than those of the ICG cameras currently in clinical use. In addition, the probe can be used in conjunction with a thoracoscopic camera, thus eliminating the need to purchase a new endoscopy system. Furthermore, although it has not yet been examined, it could be used for sentinel node biopsies, blood flow measurements, and other procedures performed by physicians from other departments and on different organs. Considering the potential versatility relative to the required investment, the overall cost is not expensive.
Limitations
The present study had several limitations, including the use of porcine lungs. In addition, the experiments were not performed using actual biological tissues, which is an important consideration given that the water content in tissues and organs with blood flow may affect the results. Furthermore, the experiments were conducted using pseudo-tumors of a fixed size, in which the optimal concentration of ICG was adjusted. In this study, ICG was mixed with 2 mL of silicone sealant, while actual tumors may be frosted or as small as a few millimeters. However, based on our promising results, we aim to conduct a clinical study in which ICG will be administered intravenously to measure spectra in human lung tumors.
Conclusions
Tumor identification using a system for analyzing ICG spectra may aid in the intraoperative localization of tumors during VATS, which is becoming increasingly popular. Our findings indicate that spectral detection using this system may be possible for deeply located tumors and in expanded lungs, highlighting the need for further analysis in clinical settings.
Acknowledgments
We would like to thank Editage (https://www.editage.jp/) for English language editing.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-244/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-244/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-244/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-244/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Ethical approval was not needed, as the lung tissue used for the experiments originated from porcine slaughtered at a slaughterhouse for nutritional purposes.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Duffy SW, Field JK. Mortality Reduction with Low-Dose CT Screening for Lung Cancer. N Engl J Med 2020;382:572-3. [Crossref] [PubMed]
- National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Hornbech K, Ravn J, Steinbrüchel DA. Outcome after pulmonary metastasectomy: analysis of 5 years consecutive surgical resections 2002-2006. J Thorac Oncol 2011;6:1733-40. [Crossref] [PubMed]
- Pastorino U, Buyse M, Friedel G, et al. Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg 1997;113:37-49. [Crossref] [PubMed]
- Melfi FM, Menconi GF, Mariani AM, et al. Early experience with robotic technology for thoracoscopic surgery. Eur J Cardiothorac Surg 2002;21:864-8. [Crossref] [PubMed]
- Veronesi G, Galetta D, Maisonneuve P, et al. Four-arm robotic lobectomy for the treatment of early-stage lung cancer. J Thorac Cardiovasc Surg 2010;140:19-25. [Crossref] [PubMed]
- Ng CS. Single-port thoracic surgery: a new direction. Korean J Thorac Cardiovasc Surg 2014;47:327-32. [Crossref] [PubMed]
- Sihoe AD. The evolution of minimally invasive thoracic surgery: implications for the practice of uniportal thoracoscopic surgery. J Thorac Dis 2014;6:S604-17. [PubMed]
- Li WW, Lee TW, Lam SS, et al. Quality of life following lung cancer resection: video-assisted thoracic surgery vs thoracotomy. Chest 2002;122:584-9. [Crossref] [PubMed]
- Liu B, Gu C. Expert consensus workshop report: Guidelines for preoperative assisted localization of small pulmonary nodules. J Cancer Res Ther 2020;16:967-73. [Crossref] [PubMed]
- Sato M, Kuwata T, Yamanashi K, et al. Safety and reproducibility of virtual-assisted lung mapping: a multicentre study in Japan. Eur J Cardiothorac Surg 2017;51:861-8. [Crossref] [PubMed]
- Kawada M, Okubo T, Poudel S, et al. A new marking technique for peripheral lung nodules avoiding pleural puncture: the intrathoracic stamping method. Interact Cardiovasc Thorac Surg 2013;16:381-3. [Crossref] [PubMed]
- Rouzé S, de Latour B, Flécher E, et al. Small pulmonary nodule localization with cone beam computed tomography during video-assisted thoracic surgery: a feasibility study. Interact Cardiovasc Thorac Surg 2016;22:705-11. [Crossref] [PubMed]
- Sortini D, Feo C, Maravegias K, et al. Intrathoracoscopic localization techniques. Review of literature. Surg Endosc 2006;20:1341-7. [Crossref] [PubMed]
- Yanagiya M, Amano Y, Hiyama N, et al. Initial experience of virtual-assisted lung mapping utilizing both indocyanine green and indigo carmine. Gen Thorac Cardiovasc Surg 2021;69:1035-9. [Crossref] [PubMed]
- Nex G, Schiavone M, De Palma A, et al. How to identify intersegmental planes in performing sublobar anatomical resections. J Thorac Dis 2020;12:3369-75. [Crossref] [PubMed]
- Yang YL, Li ZZ, Huang WC, et al. Electromagnetic navigation bronchoscopic localization versus percutaneous CT-guided localization for thoracoscopic resection of small pulmonary nodules. Thorac Cancer 2021;12:468-74. [Crossref] [PubMed]
- Yotsukura M, Okubo Y, Yoshida Y, et al. Indocyanine green imaging for pulmonary segmentectomy. JTCVS Tech 2021;6:151-8. [Crossref] [PubMed]
- Newton AD, Predina JD, Corbett CJ, et al. Optimization of Second Window Indocyanine Green for Intraoperative Near-Infrared Imaging of Thoracic Malignancy. J Am Coll Surg 2019;228:188-97. [Crossref] [PubMed]
- Predina JD, Newton AD, Corbett C, et al. Near-infrared intraoperative imaging for minimally invasive pulmonary metastasectomy for sarcomas. J Thorac Cardiovasc Surg 2019;157:2061-9. [Crossref] [PubMed]
- Ebihara Y, Li L, Noji T, et al. A novel laparoscopic near-infrared fluorescence spectrum system with indocyanine green fluorescence overcomes limitations of near-infrared fluorescence image-guided surgery. J Minim Access Surg 2022;18:125-8. [Crossref] [PubMed]
- Saito T, Ebihara Y, Li L, et al. A novel laparoscopic near-infrared fluorescence spectrum system for photodynamic diagnosis of peritoneal dissemination in pancreatic cancer. Photodiagnosis Photodyn Ther 2021;33:102157. [Crossref] [PubMed]
- Li L, Ebihara Y, Shirogane R, et al. Near infrared fluorescence imaging and spectrum of indocyanine green for laparoscopy diagnosis in gastric cancer. Chinese Opt Lett 2012;10:S21003. [Crossref]
- Alander JT, Kaartinen I, Laakso A, et al. A review of indocyanine green fluorescent imaging in surgery. Int J Biomed Imaging 2012;2012:940585. [Crossref] [PubMed]
- Okusanya OT, Holt D, Heitjan D, et al. Intraoperative near-infrared imaging can identify pulmonary nodules. Ann Thorac Surg 2014;98:1223-30. [Crossref] [PubMed]
- Keating J, Newton A, Venegas O, et al. Near-Infrared Intraoperative Molecular Imaging Can Locate Metastases to the Lung. Ann Thorac Surg 2017;103:390-8. [Crossref] [PubMed]
- Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res 1986;46:6387-92. [PubMed]
- Heneweer C, Holland JP, Divilov V, et al. Magnitude of enhanced permeability and retention effect in tumors with different phenotypes: 89Zr-albumin as a model system. J Nucl Med 2011;52:625-33. [Crossref] [PubMed]
- Frangioni JV. In vivo near-infrared fluorescence imaging. Curr Opin Chem Biol 2003;7:626-34. [Crossref] [PubMed]
- Khullar O, Frangioni JV, Grinstaff M, et al. Image-guided sentinel lymph node mapping and nanotechnology-based nodal treatment in lung cancer using invisible near-infrared fluorescent light. Semin Thorac Cardiovasc Surg 2009;21:309-15. [Crossref] [PubMed]



