
Causes and management of intraoperative complications in robot-assisted anatomical pulmonary resection for lung cancer
Introduction
The advantages of robot-assisted thoracic surgery (RATS) include good visibility with a three-dimensional camera and precise operability with robotic arms (1-3). However, RATS lacks the tactile feedback that is available with conventional video-assisted thoracic surgery (VATS) and open thoracotomy. The intraoperative complications specific to RATS were reported (4-6); however, only limited data are available regarding the details of intraoperative complications during RATS.
Conversion to thoracotomy should not be hesitated in the event of catastrophic intraoperative complications during RATS. Several studies of large database analyses showed that RATS lobectomy has a lower rate of conversion than that of VATS (7,8). Vascular injuries are more commonly the reason for conversion from RATS than for conversion from VATS. Emergency conversion is also more common during RATS (8). However, the details of actual surgical procedures for individual cases are unknown.
To perform safe RATS, it is important to share the details of causes and management about intraoperative complications among thoracic surgeons. Thus, we examined the causes, management, and outcomes of intraoperative complications encountered during robot-assisted anatomical lung resection at our institution. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-553/rc).
Methods
Patients
We conducted a retrospective, single-institutional study of RATS at our institution beginning in April 2018. We reviewed the data of the first 134 consecutive patients who underwent robot-assisted anatomical pulmonary resection (lobectomy or segmentectomy) at our institution between April 2018 and June 2021. We selected the initial 20 patients who had non-fused fissures, clinical N0 stage, and no other critical comorbidities. The initial 20 patients were also included in our study. After the initial 20 patients, we continued to perform RATS without considering the above criteria. Moreover, patients with combined pulmonary fibrosis, emphysema, or interstitial pneumonia were not rejected for RATS. In effort to decrease bias, we included the first consecutive cases. This retrospective study was approved by the Institutional Review Board of Sapporo Medical University (Approval No. 322-265). Individual informed consent was waived because of the retrospective nature of the study. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Introduction of RATS
Our two console surgeons switched from thoracoscopic surgery to robotic surgery gradually. Until April 2018, neither of them had any experience performing RATS. One of the console surgeons had experience with approximately 2,000 anatomical lung resections using VATS. The other console surgeon had experience with approximately 600 anatomical lung resections. The first console surgeon with the most VATS experience performed RATS for the initial 30 cases. Thereafter, the second console surgeon started performing RATS. Only surgeons who have passed the training program (e-learning, demonstration, e-learning test, case observation, and wet laboratory training) supervised by The Japanese Association for Chest Surgery are allowed to become console surgeons and perform RATS. A proctor surgeon was invited to perform the first case.
Surgical procedure
We used the da Vinci Xi Surgical System (Intuitive Surgical, Sunnyvale, CA, USA) for all robot-assisted anatomical pulmonary resections. A 30-mm mini-thoracotomy with a LAP PROTECTOR®️ mini (Hakko Co., Ltd. Medical Device Division, Tokyo, Japan) at the level of the fourth intercostal space was used as the assistant window. In right-sided RATS, the first, second, third, and fourth arms were connected to the Cadiere forceps, fenestrated bipolar forceps, camera, Maryland bipolar forceps or vessel sealer extend (VSE) (Figure 1A). In left-sided RATS, the first, second, third, and fourth arms were connected to the fenestrated bipolar forceps, camera, Maryland bipolar forceps or VSE, Cadiere forceps. At first, the port was placed in the same intercostal space as described by Cerfolio et al. (9). However, this port placement sometimes caused interferences with the robotic instruments in patients with a small physique. Therefore, we later changed the port placement to that shown in Figure 1. We prefer to use mini-thoracotomy in the fourth intercostal space for the assistant window because it can be used for tissue removal and also allows for safe and rapid conversion to thoracotomy during an emergency (Figure 1B).
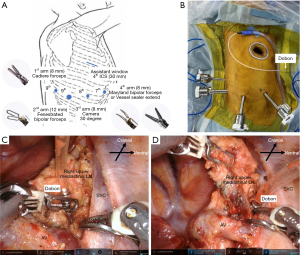
Dobon®︎ (Senko Medical Instrument Mfg., Tokyo, Japan) was placed in the thoracic cavity through the assistant window. The device is connected to a 10-Fr silicone tube, and the tube is connected to the wall suction unit. The console surgeon can easily grasp the Dobon using the robotic forceps. This enables simultaneous lymph node (LN) retraction and blood suction (Figure 1C). Dobon continuously suctions blood and fluids from the thoracic cavity (Figure 1D). This is a convenient suction device in RATS for maintaining a bloodless surgical view (10). CO2 insufflation was used at a pressure of 8–10 mmHg only when the patient’s physique was small and proper port placement was difficult. EZ access®️ (Hakko Co., Ltd., Medical Device Division, Tokyo, Japan) allows CO2 insufflation so that the AirSeal®︎ (CONMED, CO, USA) trocar can be inserted without air leak. EZ access is a silicon cap for the LAP Protector wound retractor. Mediastinal LN dissection was performed at the lobar-specific station for primary lung cancer. Both upper and lower mediastinal LN dissections were performed in cases of N1 node metastasis confirmed by frozen section analysis. During subcarinal LN dissection, we used a bronchial traction method to ensure a good field of view in a small area surrounded by vital organs (11).
Intraoperative complications
We retrospectively reviewed the causes, frequencies, and management of intraoperative complications from medical records. Intraoperative complications included unexpected events occurred during surgery and requiring surgical repair. In this report, adverse events graded ≥1 according to the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 were considered intraoperative complications.
Definition of intraoperative complications
We defined the intraoperative complications as follows: major vascular injury—any bleeding from the major vessels such as the pulmonary artery (PA), pulmonary vein (PV), azygos vein (AV) and the superior vena cava (SVC); bronchial injury and lung parenchymal injury—injuries which required surgical treatment such as suturing, sealing with biological glue, and stapling.
The AV is not usually defined as a great vessel. However, surgery (especially superior mediastinal LN dissection) is often performed near the AV. Because the injury sometimes leads to major bleeding, we included the AV injury as a major vascular injury. We defined a vascular injury as any bleeding caused by disruption of the vessel wall, regardless of the extent of the injury and the hemostatic time.
Criteria for intraoperative blood transfusion
Intraoperative transfusion was performed if any one of the following three conditions was met: abnormal vital signs; blood loss of more than 1,000 mL; or hemoglobin less than 7.0 g/dL.
Recent criteria for conversion to thoracotomy
Initially, we did not have definitive criteria for conversion to thoracotomy. We established the two criteria after approximately 30 cases and several intraoperative complications. Our criteria are as follows: (I) in case of bleeding, temporary hemostasis is not possible with the robotic or assistant’s instrument and the bleeding cannot be controlled or treated with RATS; (II) non-vascular injuries cannot be treated with RATS. The degree of pleural adhesion and interlobar fusion or the extension of the operation time are not considered as indications for conversion.
Statistical analyses
The summarized data are shown as median with range or interquartile range for continuous variables, and as number and percentage for categorical variables. The Chi-square test was used to evaluate the relationship between categorical variables, whereas the one-way analysis of variance and Wilcoxon signed-rank test were used for continuous variables. All P values were two-sided, and significance was set at ˂0.05. Statistical analyses were performed using the JMP Pro version 16 (SAS Institute Inc., Cary, NC, USA).
Results
Patient characteristics
The patient characteristics are presented in Table 1. In total, 134 patients with lung cancer underwent anatomical pulmonary resection with LN dissection by two qualified surgeons at our department between April 2018 and June 2021. Among those patients, 118 underwent lobectomy and 16 underwent segmentectomy. Among the 130 patients with primary lung cancer, clinical stage IA was the most common (95 patients, 73.1%). Adenocarcinoma was the most common histological type (103 patients, 79.2%). Metastatic lung tumor was found in four patients (3%). Almost all patients with primary lung cancer (96.2%) underwent hilar and mediastinal LN dissection (Table 1).
Table 1
| Characteristics | Numbers |
|---|---|
| Sex | |
| Male | 72 |
| Female | 62 |
| Age (years), median [range] | 69 [34–85] |
| Side | |
| Right | 71 |
| Left | 63 |
| Clinical stage for PLC (n=130) | |
| 0 | 1 |
| IA | 95 |
| IB | 16 |
| II | 15 |
| III | 3 |
| Histology | |
| Adenocarcinoma | 103 |
| Squamous cell carcinoma | 25 |
| Small cell carcinoma | 2 |
| Metastatic lung tumor | 4 |
| Others | 0 |
| Extent of lymph node dissection | |
| None | 4 |
| Hilar | 5 |
| Hilar + mediastinal | 125 |
PLC, primary lung cancer.
Details of the surgical procedure
Data on the resected lobe and segment, console time, and blood loss are presented in Table 2. In lobectomy, the most frequently resected lobe was the right upper lobe. The median console time was less than three hours for most lobectomies and segmentectomies, except for right middle lobectomy, right upper lobectomy + S6 and left upper division cases. The median blood loss in all patients was less than ≤30 mL (Table 2). Intraoperative transfusion was not performed for any patients.
Table 2
| Resected lobe or segment | n | Console time, min, median [range] | Operation time, min, median [range] | Blood loss, mL, median [range] |
|---|---|---|---|---|
| Lobectomy (n=118) | ||||
| RUL | 39 | 144 [86–360] | 205 [136–394] | 20 [0–350] |
| RML | 9 | 192 [112–230] | 260 [148–310] | 20 [50–150] |
| RLL | 24 | 133 [83–198] | 196 [135–289] | 10 [5–350] |
| LUL | 27 | 145 [77–270] | 206 [149–325] | 10 [5–530] |
| LLL | 19 | 139 [75–280] | 200 [124–444] | 30 [0–500] |
| Segmentectomy (n=16) | 136 [75–310] | 199 [134–353] | 8 [0–80] | |
| RUL + RS6 | 2 | |||
| RS6 | 1 | |||
| LUD | 2 | |||
| LLD | 1 | |||
| RS2 | 1 | |||
| RS3b | 1 | |||
| LS6 | 6 | |||
| LS8 | 2 |
RUL, right upper lobe; RML, right middle lobe; RLL, right lower lobe; LUL, left upper lobe; LLL, left lower lobe; RS6, right superior segment; LUD, left upper division; LLD, left lingular division; RS2, right posterior segment; RS3, right anterior segment; LS6, left superior segment; LS8, left anterior basal segment.
Types, causes, and management of intraoperative complications
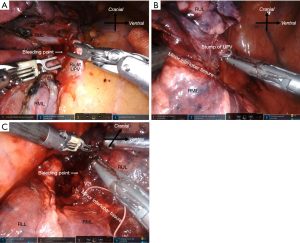
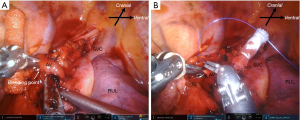
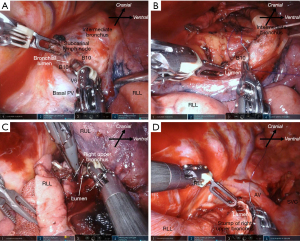
The characteristics of patients who experienced intraoperative complications during robot-assisted anatomical lung resection are shown in Table 3. Intraoperative complications occurred in 17 (12.7%) patients. Among the initial 30 patients that underwent RATS, seven patients encountered intraoperative complications. Three intercostal artery injuries were excluded because they were easy to treat with soft coagulation. Most intraoperative complications were grade 1 according to the CTCAE, except for one death. There was no conversion to thoracotomy. Intraoperative complications included seven PA injuries, three PV injuries, one AV injury, one SVC injury, three bronchial injuries, and four lung injuries requiring pulmonary sutures (Table 3). Details of management for intraoperative complications are shown in Video 1. Most PA injuries were distal side. The PA bleeding was controlled by pressure, fibrin sealant to the bleeding site, or stapling of the proximal side of the injured PA following temporal pressure hemostasis. The PA injuries were observed in seven patients, including two patients (No. 9, No. 17 in Table 3) who had their bleeding controlled by pressure within one minute. In addition, there was one patient (No. 5 in Table 3) of the PA injuries in the distal side of PA which had already been divided proximally by stapler. PV injuries were observed in three patients. In the first patient, the right upper pulmonary vein (UPV) was sandwiched and injured by the fenestrated bipolar forceps and Cadiere forceps during taping of the UPV (Figure 2A). To address this, we first applied pressure on the bleeding point using a roll gauze and applied a fibrin sealant sheet patch (TachoSil®; CSL Behring K.K., Tokyo, Japan). After hemostasis, we divided the proximal side of the UPV using a robotic stapler. In the second patient, the tip of the Maryland bipolar forceps punctured the V6 segment during the V6 dissection. We divided the proximal side of the V6 using VSE. In the third patient, the distal side of V2t was injured by fenestrated bipolar forceps during tunneling of a minor interlobar fissure after stapling UPV (Figure 2B,2C). We sealed the proximal side of V2t using VSE. Only one AV injury was observed during right upper lobectomies. The AV injury was caused by the Maryland bipolar forceps during LN (#10) dissection (Figure 3A). We grasped the bleeding point using the fenestrated bipolar forceps. The bleeding was controlled with sutures (Figure 3B). We experienced an SVC injury in our third right upper lobectomy patient (No. 2 in Table 3). This was caused by interference of the robotic forceps and assistant surgeon’s suction tube outside of the field of view during the upper mediastinal LN dissection. The SVC was injured by the suction tube tip pushed by the Maryland bipolar forceps (Figure 4A). We applied oxidized regenerated cellulose (SURGICEL® absorbable hemostat; Johnson & Johnson, New Brunswick, NJ, USA) over the bleeding point and applied pressure with roll gauze using fenestrated bipolar forceps. However, the pressure hemostasis was inadequate. We exchanged the roll gauze for an endoscopic sponge (SECUREA®︎; HOGY Medical. Co., Ltd., Tokyo, Japan) to facilitate blood suction (Figure 4B). Then, we layered polyglycolic acid sheets (NEOVEIL®︎; GUNZE Ltd., Osaka, Japan) and fibrin glue (BOLHEAL®︎; KM Biologics, Kumamoto, Japan) over the bleeding point (Figure 4C), thus finally controlling the bleeding. We then closed the right upper mediastinal pleura. Hemostasis for the SVC injury required approximately 30 minutes. Bronchial injures were observed in three patients. In the first case, B10 was injured by Maryland bipolar forceps during subcarinal LN dissection in right middle lobectomy (Figure 5A). The bronchial injury was repaired by suturing (Figure 5B). In second case, the membranous portion of the right upper bronchus was injured by Maryland bipolar forceps during a right upper lobectomy (Figure 5C). This was repaired by suturing (Figure 5D). In the third case, stapling of the bronchus with strong traction caused stapling failure in a left upper lobectomy. This was repaired by suturing. Lung parenchymal injuries were observed in four patients. In the first case, lung parenchyma of S3 was injured by robotic forceps penetration outside of the field of view and under poor visibility owing to the congestive lung after left lower PV division during left lower lobectomy. The patient developed prolonged air leakage and pneumonia postoperatively and died within 30 days of the surgery. In the second case, the right middle lobe was injured by robotic forceps outside of the field of view during the sealing test. In the third case, S3 was injured by robotic forceps during the sealing test. In the fourth case, S6 was injured by Cadiere forceps during traction of S6 during right upper lobectomy. The lung injuries were repaired by suturing with polytetrafluoroethylene felt or pericardial fat pledgets.
Table 3
| No. | Injured organ | Grade | Surgery | Causes | Management | Blood loss (mL) | Console time (min) |
|---|---|---|---|---|---|---|---|
| 1 | PA | 1 | RUL + ND2a-1 | Distal side of A3 was injured by Maryland forceps | Stapling A1+3 → PH + TachoSil | 350 | 145 |
| 2 | SVC | 1 | RUL + ND2a-1 | Assistant suction tip pushed by robotic forceps | PH + surgicel cotton → PGA sheet + Fibrin glue + PH with endoscopic sponge | 350 | 161 |
| 3 | Br | 1 | RML + ND2a-2 | B10 was injured by Maryland forceps during subcarinal LN dissection | Suture with pericardial fat | 5 | 230 |
| 4 | AV | 1 | RML + ND2a-2 | Azygos vein was injured by Maryland forceps during LN (#10) dissection | Suture | 5 | 167 |
| 5 | PA | 1 | RUL + ND2a-1 | Recurrent A2 stapled was injured by Maryland forceps during taping RUB | PH to A2 | 250 | 360 |
| 6 | Br | 1 | RUL + ND2a-1 | Right upper bronchus was injured by Maryland forceps during dissection of RUB | Suture with pericardial fat | 30 | 204 |
| 7 | PA | 1 | LUL + ND2a-1 | Mediastinal lingular PA was injured by interference between robotic arms | PH + surgicel cotton | 5 | 219 |
| Br | Stapling failure due to strong traction to the LUB during stapling | Suture with pericardial fat | |||||
| 8 | PV | 1 | RUL + ND2a-1 | UPV was sandwiched and injured by Maryland and Fenestrated forceps | PH + TachoSil → stapling UPV | 5 | 142 |
| 9 | PA† | 1 | LUL + ND2a-1 | Distal side of small A5 was injured by Maryland forceps | PH + sealing proximal A5 by vessel sealer | 50 | 174 |
| 10 | Lung | 5‡ | LLL + ND2a-1 | S8 was injured by penetrating with robotic forceps under poor visibility due to the congestive lower lobe after dividing LPV | Hemostasis by electrocautery, suture with pericardial fat | 500 | 280 |
| PA | Distal side of A8 was injured by strong traction with robotic forceps for the congestive lower lobe | Stapling LPA | |||||
| 11 | PV | 1 | RLL + ND2a-1 | Distal side of V6 was injured by Maryland forceps during dissection of V6 | PH + stapling LPV | 75 | 88 |
| 12 | Lung | 1 | RUL + ND2a-1 | Right middle lobe was injured by fenestrated forceps during sealing test | Suture with pericardial fat | 120 | 201 |
| 13 | Lung | 1 | LLL + ND2a-2 | S3 was injured by Fenestrated forceps during sealing test | Suture with pericardial fat | 0 | 112 |
| 14 | PV | 1 | RUL + ND2a-1 | Distal side of V2t was injured by Fenestrated forceps during tunneling of minor interlobar fissure after stapling UPV | Sealing with vessel sealer | 0 | 142 |
| 15 | PA | 1 | LS6 seg + ND2a-1 | Distal side of A6b was injured by tunneling dorsal interlobar fissure | PH + surgicel + sealing A6b by vessel sealer | 20 | 139 |
| 16 | Lung | 1 | RUL + ND2a-1 | S6 was injured by grasping with Cadiere forceps | Suture with pericardial fat | 5 | 126 |
| 17 | PA† | 1 | RLL + ND2a-1 | Basal PA was injured by interference between robotic forceps | PH + surgicel cotton | 5 | 151 |
†, the bleeding was controlled by pressure within one minute; ‡, one death attributed to postoperative pneumonia caused by air leakage after intraoperative lung parenchymal injury. PA, pulmonary artery; SVC, superior vena cava; Br, bronchus; AV, azygos vein; PV, pulmonary vein; RUL, right upper lobectomy; RML, right middle lobectomy; LUL, left upper lobectomy; LLL, left lower lobectomy; RLL, right lower lobectomy; LS6, left superior segment; seg, segmentectomy; ND, lymph node dissection; LN, lymph node; RUB, right upper bronchus; LUB, left upper bronchus; UPV, upper pulmonary vein; LPV, lower pulmonary vein; PH, pressure hemostasis; PGA, polyglycolic acid; LPA, lower pulmonary artery; TachoSil, Fibrin sealant sheet patch; Surgicel, oxidized regenerated cellulose.

Surgical outcomes and postoperative complications
Patients in the injury group had significantly longer operative times than those in the non-injury group (161 vs. 133 min) (Table 4). There were no significant differences in blood loss, drainage length, hospital stay duration, and overall postoperative complications (grade ≥1). The postoperative complication rate was higher in the injury group, but there were no significant differences.
Table 4
| Characteristics | Injury group (n=17) | Non-injury group (n=117) | P value |
|---|---|---|---|
| Blood loss (mL), median [IQR] | 20 [5–185] | 10 [5–50] | 0.59 |
| Console time (min), median [IQR] | 161 [105–212] | 133 [103–161] | 0.007 |
| Length of drainage (days), median [IQR] | 1 [1–4] | 1 [1–2] | 0.089 |
| Length of hospital stays (days), median [IQR] | 10 [7–11] | 9 [7–11] | 0.46 |
| Postoperative complications, overall n (%) | 3 (17.6) | 9 (7.7) | 0.18 |
| Prolonged air leak | 1 | 6 | |
| Cerebral infarction | 1 | 0 | |
| Atrial fibrillation | 1 | 1 | |
| Recurrent nerve paralysis | 0 | 2 |
IQR, interquartile range.
Discussion
In the present study, we examined the causes, management, and outcomes of all intraoperative complications during robot-assisted anatomical lung resections for lung cancer. The key findings are as follows. First, the intraoperative complications occurred in 17 patients. They were almost grade 1 according to the CTCAE. The intraoperative complications included PA injuries in seven patients, PV injuries in three, AV injury in one, SVC injury in one, bronchial injuries in three, and lung injuries in four patients. Second, most intraoperative complications can be safely managed robotically without conversion to thoracotomy. Most major vascular injuries were treated by pressure, fibrin sealant to the bleeding site, or stapling of the proximal side. All non-vascular injuries were treated by suturing robotically. Third, the 30-day mortality rate was 0.7%. The cause of mortality was pneumonia due to prolonged air leakage.
A previous study showed that conversion to thoracotomy was required in 4.7% (13 of 632) of patients who underwent robot-assisted anatomical lung resection. Major vascular injuries were observed in 2.4% (15 of 632), and they all required conversion to thoracotomy (4). A recent study showed that 1.6% (3 of 192) of patients who underwent robot-assisted anatomical lung resection were converted to thoracotomy. Of these bleeding from PA was caused by conversion to thoracotomy in two patients (6). Conversely, we did not experience any conversions to thoracotomy in our study. This result could be attributed to our relatively small sample size and different conversion criteria for thoracotomy among institutions. Cerfolio et al. set a time limit for conversion, which might have increased the conversion rate in their study (9). Conversely, the extension of operation time was not considered in our study. The differences in criteria may affect conversion rate.
Cerfolio et al. described the definition of a major vascular injury as bleeding that cloud not be controlled with minor pressure alone (one sponge held for one minute or less) (4). In contrast, we defined any bleeding from the major vessels (PA, PV, AV, SVC) as a major vascular injury. In our PA injuries, the bleeding was controlled within one minute in two cases. The PA injury site was distal side of the stapled PA in one case. Apart from these three cases, the frequency of PA injury in our study was not particularly higher than in previous reports. PV injuries were observed in three cases. There are no previous reports of a PV injury in RATS. As with the PA injuries in our study, all PV injuries occurred in the distal side. Therefore, the damage was not severe. Noteworthy is the case of the injured V2t. The console surgeons should be careful not to injure the PV in the blind side during the tunneling of the minor interlobar fissure.
We experienced difficulties in stopping hemorrhage from great vessels, in our case, the SVC. This was caused by the robotic instruments pushing into the suction tube of the assistant surgeon. To prevent major bleeding such as that which comes from an SVC injury, we believe the following three things can be useful. First, Dobon should be used instead of the assistant’s suction tube. This case prompted us to introduce the easy suction technique using Dobon. Second, adequate communication between the console surgeon and assistant surgeon is crucial. Dobon is useful for bloodless view only when small amounts of blood are present. In major bleeding, Dobon is not effective and an assistant surgeon’s suction tube is required. The assistant surgeon should announce to the console surgeon that the suction tube should be inserted into the pleural cavity. Although the console surgeon has a lot of flexibility, the assistant surgeon is restricted due to the robotic arms. Both surgeons need to be fully aware of this. Third, the console surgeon should move the camera to enlarge the field of view and check the position of the assistant surgeon’s suction tube and robotic instruments when necessary. The console surgeon should be conscious of the presence of the assistant surgeon’s suction tube outside of the field of view.
A previous study showed that four out of 1,810 (0.2%) patients who underwent robotic anatomical lung resection developed airway injuries. In the study, three of the four bronchial injuries required conversion to thoracotomy and either airway repair or reconstruction (5). Robotic instruments have a higher grasping, dissecting and pushing power than VATS instruments. This may result in injuries of the lung parenchyma, pulmonary vessels and bronchus when the console surgeon handles the controller improperly. The Maryland bipolar forceps can be used to perform delicate and sharp dissections. Conversely, this approach comes with a large dissection and penetration force. Most of the bronchial injuries occurred in the dorsal side of the bronchus in our study. Console surgeons should understand the characteristics of the bronchial wall during dissection. The bronchial injuries were repaired without conversion to thoracotomy since it was relatively easy to suture them with RATS.
In RATS, it is difficult to move the lung extensively at a time using robotic instruments. One example is dividing the interlobar fissure using a robotic stapler. This requires extensive movement of the lung. Some reports demonstrated the method of dividing the interlobar fissure instead of using a stapler. A previous report showed that LigaSure®️ (Medtronic, Dublin, Ireland) is a safe tissue sealing system for lung resection and can be a valid alternative to staplers (12). Our recent report demonstrated the safety of using VSE as an alternative to the robotic staplers for the division of interlobar fissures in RATS (13). We frequently divide the low-grade incomplete fissure using VSE. To prevent air leakage, the sealing line should not be placed on the interlobar fissure line but 2–3 mm from the fissure line on the side of the resected lung.
Most lung parenchymal injuries were caused by the robotic arm in the blind side or outside of the field of view. In our first case of parenchymal injury, the lower lobe became congested due to the dividing of the lower PV in advance, where the field of view could not be secured. In such situations, the lack of tactile sensation increases the risk of injury to the lung parenchyma. The patient developed prolonged air leakage and pneumonia postoperatively and died within 30 days of surgery. The 30-day mortality rate in our study was 0.7%. This was nearly identical to the postoperative mortality rate (0.6%) reported in a previous study (14). In the second and third patient, lung parenchymal injuries occurred during the sealing test. To avoid injuring the lung parenchyma, it is important to grasp the roll gauze or to hold down the lung using the shaft of robotic instruments during the sealing test, as opposed to holding the wrist or tip.
Among the initial 30 cases, seven intraoperative complications occurred. In the subsequent 104 cases, eight intraoperative complications occurred, excluding two minor PA injuries. Although the complication rate decreased with experience, the overall complication rate was higher than that of other studies. We believe that the high incidence of injuries had three causes. The first cause is the lack of tactile sensation, especially for the initial 30 cases. The console surgeon was still learning. It is important to recognize the absence of tactile sensation and strong force of the robotic forceps. During the early period of RATS, it may be better to use a blunt and long bipolar grasper rather than Maryland forceps. The second cause is insufficient communication with assistant surgeons. The initial 30 cases of RATS were performed by only one certified console surgeon. The assistant surgeons were surgical residents. There were no surgeons available to provide appropriate advice, which may have led to a lack of communication. Thereafter, RATS was performed by two certified console surgeons. Therefore, it was possible to perform RATS with better communication, and more appropriate advice was available. The assistant surgeon should be a senior surgeon if possible. The third cause is different surgical views and organ positioning between conventional VATS and RATS. We performed most anatomic lung resections using VATS. It took time to adjust the differences. Therefore, many misinterpretations of the anatomy (especially bronchial injury) occurred among the initial 30 cases. No bronchial injuries were observed since then. Although both RATS and VATS are classified as minimally invasive, the experience of performing VATS is not always applicable to RATS, especially during the early period of RATS. Because patient safety is the top priority, we believe that there should be no hesitation when conversion is necessary. The primary purpose of our study is to share our experience with RATS among thoracic surgeons so that such problems will not be repeated.
Our study had several limitations. This was a retrospective, single-institution study. Our cohort of patients who underwent RATS anatomical lung resection was relatively small, which could have led to selection bias. We tended to select patients with non-fused fissures and few comorbidities, especially during our early experiences with RATS. In the future, multi-institutional studies are needed to determine the generalizability of our findings.
Conclusions
Most major intraoperative complications can be safely managed during RATS without conversion to thoracotomy.
Acknowledgments
We thank Editage (www.editage.jp) for English language editing.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-553/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-553/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-553/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-553/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Institutional Review Board of Sapporo Medical University (Approval No. 322-265). The individual informed consent was waived because of the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ma J, Li X, Zhao S, et al. Robot-assisted thoracic surgery versus video-assisted thoracic surgery for lung lobectomy or segmentectomy in patients with non-small cell lung cancer: a meta-analysis. BMC Cancer 2021;21:498. [Crossref] [PubMed]
- Wei S, Chen M, Chen N, et al. Feasibility and safety of robot-assisted thoracic surgery for lung lobectomy in patients with non-small cell lung cancer: a systematic review and meta-analysis. World J Surg Oncol 2017;15:98. [Crossref] [PubMed]
- Emmert A, Straube C, Buentzel J, et al. Robotic versus thoracoscopic lung resection: A systematic review and meta-analysis. Medicine (Baltimore) 2017;96:e7633. [Crossref] [PubMed]
- Cerfolio RJ, Bess KM, Wei B, et al. Incidence, Results, and Our Current Intraoperative Technique to Control Major Vascular Injuries During Minimally Invasive Robotic Thoracic Surgery. Ann Thorac Surg 2016;102:394-9. [Crossref] [PubMed]
- Cao C, Cerfolio RJ, Louie BE, et al. Incidence, Management, and Outcomes of Intraoperative Catastrophes During Robotic Pulmonary Resection. Ann Thorac Surg 2019;108:1498-504. [Crossref] [PubMed]
- Ueno H, Watanabe Y, Hirayama S, et al. Intraoperative complications and troubles in robot-assisted anatomical pulmonary resection. Gen Thorac Cardiovasc Surg 2021;69:51-8. [Crossref] [PubMed]
- Oh DS, Reddy RM, Gorrepati ML, et al. Robotic-Assisted, Video-Assisted Thoracoscopic and Open Lobectomy: Propensity-Matched Analysis of Recent Premier Data. Ann Thorac Surg 2017;104:1733-40. [Crossref] [PubMed]
- Servais EL, Miller DL, Thibault D, et al. Conversion to Thoracotomy During Thoracoscopic vs Robotic Lobectomy: Predictors and Outcomes. Ann Thorac Surg 2022;114:409-17. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Skylizard L, et al. Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J Thorac Cardiovasc Surg 2011;142:740-6. [Crossref] [PubMed]
- Tsuruta K, Maki R, Miyajima M, et al. Easy Suction Technique During Robotic-Assisted Thoracoscopic Lobectomy. Ann Thorac Surg 2021;112:e381-2. [Crossref] [PubMed]
- Maki R, Miyajima M, Tsuruta K, et al. Subcarinal Lymph Node Dissection in Solo Robot-assisted Thoracic Surgery. Ann Thorac Surg 2022;113:e235-7. [Crossref] [PubMed]
- Bertolaccini L, Viti A, Cavallo A, et al. Results of Li-Tho trial: a prospective randomized study on effectiveness of LigaSure® in lung resections. Eur J Cardiothorac Surg 2014;45:693-8; discussion 698. [Crossref] [PubMed]
- Miyajima M, Shindo Y, Tsuruta K, et al. Interlobar division using vessel-sealing system in robot-assisted pulmonary lobectomy. JTCVS Tech 2022;13:211-6. [Crossref] [PubMed]
- Cao C, Louie BE, Melfi F, et al. Outcomes of major complications after robotic anatomic pulmonary resection. J Thorac Cardiovasc Surg 2020;159:681-6. [Crossref] [PubMed]


