
Surgical treatment of patients with aortic valve disease complicated with moderate functional mitral regurgitation and heart failure with midrange ejection fraction: a cohort study
Introduction
While evidence-based clinical guidelines recommend to perform mitral valve surgery during the aortic valve replacement (AVR) in patients with aortic valve disease combined with severe functional mitral regurgitation (FMR) (1), controversies exist on the treatment of moderate FMR (2,3). A substantial proportion of patients with severe aortic valve disease followed by moderate FMR complicates with heart failure, which is an important clinical syndrome that has been affecting human health.
During the last decade, there has been an increasingly interest on the classification of heart failure. Guidelines introduce a new concept—heart failure with borderline fraction ejection (EF) (4), which is renamed as heart failure with midrange EF (HFmrEF) later in the new guidelines (5). Since then, heart failure has been divided into three entities including heart failure with preserved, reduced and midrange EF. Specifically, HFmrEF is defined as an EF between 40% to 49% followed by symptoms and/or signs of heart failure (5).
According to previous studies, the prevalence of HFmrEF among patients ranges from 13% to 26% (6-9), and it shows important influence on the prognosis of the patients. He et al. reports in their study that HFmrEF increases the risk of deterioration of cardiac function after permanent pacemaker implantation (10). Elsewhere, Ovidiu and colleagues notice in their prospective observational study that patients with HFmrEF experience an intermediate rate of death when compared to those with heart failure with reduced or preserved EF (11). Furthermore, researchers also observe in their recent study that among patients undergoing coronary artery bypass grafting, HFmrEF increases the risk of mortality by 30% when compared to those with normal EF (12). As a consequence, patients with HFmrEF should be fully evaluated before determining the treatment strategy.
One of the most important causes of heart failure is the valvular heart disease, including FMR. As mentioned above, debates exist on whether to operate on mitral valve in patients with moderate FMR during the AVR procedure (2,13), while no data exists regarding the impact of concomitant mitral valve surgery on the postoperative outcome in this population of patients who are complicated with HFmrEF.
This study is aimed to investigate the prognostic difference between isolated AVR and AVR + mitral valve surgery in a population of severe aortic valve disease complicated with moderate FMR and HFmrEF during 2010 to 2019 at our institute, and to indirectly evaluate the value of HFmrEF in determining the surgical treatment strategy in this group of patients. We present the following article in accordance with the STROBE reporting checklist (14) (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-278/rc).
Methods
Study design
In this single-centered retrospective cohort study, we continuously recruited HFmrEF patients who underwent AVR and complicated with moderate FMR between January 2010 and December 2019 at Fuwai Hospital (Beijing, China), to compare the clinical outcomes of different surgical procedures, including isolated AVR and AVR + mitral valve surgery. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board at Fuwai Hospital (No.: 2021-1585) and individual consent for this retrospective analysis was waived.
Patient selection and grouping
As mentioned above, we enrolled adult patients who underwent AVR and complicated with moderate FMR as well as HFmrEF during the study period at our center. The exclusion criteria included the patients: (I) who were diagnosed with rheumatic valvular heart disease, (II) with a history of infective endocarditis, (III) with organic lesion of mitral leaflet and chordae tendineae according to previous echocardiography or other tests, or (IV) who were under the age of 18 years. All patients underwent AVR, with (treatment group) or without (control group) concomitant mitral valve surgery (repair or replacement). Since there’s no standard guideline recommendation for the treatment of moderate FMR during AVR, the decision on whether to operate on mitral valve in this group of patients was made according to comprehensive evaluation of the patient characteristics. For those with larger left ventricle or mitral annulus, eccentric mitral regurgitation and/or longstanding course of aortic valve disease, surgeons might tend to choose AVR + mitral valve intervention over isolated AVR. A right atrial incision followed by trans-septal approach was performed in patients who received concomitant mitral valve intervention.
The primary endpoint was the occurrence of major adverse cardiovascular and cerebrovascular events (MACCE, defined as the composite of all-cause death, non-fetal myocardial infarction, ischemic or hemorrhagic stroke, hospitalization for heart failure and repeat valvular surgery). The secondary endpoints were postoperative complications and the changes in echocardiographic characteristics, including the left ventricular EF, left ventricular end-diastolic diameter (LVEDD), left atrial diameter (LAD), as well as the level of mitral regurgitation.
Moderate FMR was determined using transthoracic echocardiography by examining the vena contracta and regurgitant jet area for at least two times by different sonographers before the surgery, and patients who were considered to have less or more than moderate level of mitral regurgitation were excluded. All patients received transesophageal echocardiography in the operating room prior to the surgical procedure to confirm the level of FMR again. Perioperative death was defined as death within 30 days postoperatively. Improvement of moderate FMR was defined as the decrease of regurgitation for at least one level based on transthoracic echocardiography, whereas complete improvement of FMR was defined as the disappearance of the mitral regurgitation.
Baseline and early postoperative characteristics of the patients were obtained from inpatient electronic records, while follow-up echocardiographic results were collected from outpatient visits. Phone call interview was used for patients who were unavailable for outpatient follow-up.
Statistical analysis
The normality of continuous variables was determined using Shapiro-Wilk test. Continuous variables were presented as mean ± standard deviation if they were normally distributed, and tested by student’s t test. Otherwise, they were presented using medians with the 25th and 75th percentiles and tested by Kruskal-Wallis H test. Categorical variables were presented as no (%) and tested by Chi-square test with or without Yates' continuity correction, or by Fisher exact test, as appropriate. The overall and MACCE-free survival rate was calculated using the Kaplan-Meier method and compared by the log-rank test, followed by multiple adjustment with inverse probability treatment weighting (IPTW) analysis. A standardized mean difference (SMD) <0.2 or P value >0.05 was considered to indicate adequate balance for between-group differences. In the follow-up echocardiography analysis, only patients who provided echocardiographic results were included. A P value <0.05 was considered statistically significant, and Bonferroni correction was applied, as appropriate. Statistical analyses were performed using R 4.0.2 (R Core Team, Vienna, Austria).
Results
Baseline and intraoperative characteristics
Of all the patients enrolled, 36 (46.2%) received isolated AVR, while 42 (53.8%) received AVR with mitral valve repair (24.4%) or replacement (29.5%). Age, contribution of sex and preoperative comorbidities were comparable between the two groups, and as was the same regarding preoperative echocardiographic parameters including the EF, LVEDD and LAD. However, patients in the treatment group had higher rates of receiving mechanical aortic valve (55.6% vs. 88.1%, P=0.001). In addition, since there’s the opportunity for further evaluation of tricuspid valve through direction observation, more patients in this group were likely to receive tricuspid valve repair than those who underwent isolated AVR (2.8% vs. 33.3%, P=0.001), although the severity of tricuspid regurgitation was comparable between the two groups (P=0.912). Baseline patient characteristics were summarized in Table 1. To increase the comparability of the two groups, IPTW analysis was performed. Variables listed in the Table 2 were included in the IPTW analysis, and the baseline and intraoperative characteristics were considered to be well balanced.
Table 1
| Variables | Control (n=36) | Treatment (n=42) | P value | SMD |
|---|---|---|---|---|
| Age, years | 60.2±11.3 | 56.4±11.8 | 0.152 | 0.329 |
| Male | 24 (66.7) | 32 (76.2) | 0.351 | 0.212 |
| Body mass index (kg/m2) | 22.5 [20.7, 24.7] | 22.5 [20.9, 25.6] | 0.703 | 0.112 |
| Body surface area (m2) | 1.7 [1.6, 1.8] | 1.8 [1.6, 1.9] | 0.557 | 0.046 |
| Atrial fibrillation | 5 (13.9) | 8 (19.0) | 0.542 | 0.139 |
| NYHA class III or IV | 18 (50.0) | 27 (64.3) | 0.203 | 0.292 |
| Hypertension | 14 (38.9) | 13 (31.0) | 0.463 | 0.167 |
| Dyslipidemia | 11 (30.6) | 9 (21.4) | 0.357 | 0.209 |
| Coronary artery disease | 8 (22.2) | 5 (11.9) | 0.223 | 0.277 |
| Diabetes mellitus | 5 (13.9) | 2 (4.8) | 0.160 | 0.318 |
| Renal failure | 2 (5.6) | 3 (7.1) | 0.775 | 0.065 |
| Stroke | 3 (8.3) | 2 (4.8) | 0.521 | 0.145 |
| Preoperative | ||||
| EF (%) | 44.2±2.8 | 43.7±2.7 | 0.428 | 0.181 |
| LVEDD (mm) | 66.8±10.0 | 70.4±11.5 | 0.147 | 0.335 |
| LAD (mm) | 45.5±7.1 | 47.9±7.5 | 0.151 | 0.330 |
| Aortic valve disease | 0.630 | 0.109 | ||
| Insufficiency | 23 (63.9) | 29 (69.1) | ||
| Stenosis | 13 (36.1) | 13 (31.0) | ||
| Tricuspid regurgitation | 0.912 | 0.097 | ||
| No | 14 (38.9) | 15 (35.7) | ||
| Mild | 18 (50.0) | 23 (54.8) | ||
| Moderate or more | 4 (11.1) | 4 (9.5) | ||
| Prosthetic valve type | 0.001* | 0.776 | ||
| Mechanical | 20 (55.6) | 37 (88.1) | ||
| Bioprosthetic | 16 (44.4) | 5 (11.9) | ||
| Concomitant surgery | ||||
| CABG | 8 (22.2) | 4 (9.5) | 0.121 | 0.353 |
| Tricuspid valve repair | 1 (2.8) | 14 (33.3) | 0.001* | 0.866 |
*, statistically significant. Data are presented as No. (%), Mean ± SD, or Median [Q1, Q3]. SMD, standardized mean difference; NYHA, New York Heart Association; EF, ejection fraction; LVEDD, left ventricular end-diastolic diameter; LAD, left atrial diameter; CABG, coronary artery bypass grafting; SD, standard deviation.
Table 2
| Variables | Control (n=78.98) | Treatment (n=84.33) | P value | SMD |
|---|---|---|---|---|
| Age, years | 57.8±10.4 | 59.6±11.6 | 0.496 | 0.186 |
| Sex, male | 43.0 (54.4) | 48.3 (57.2) | 0.893 | 0.057 |
| Body mass index (kg/m2) | 24.2 [21.4, 24.2] | 21.9 [21.1–24.4] | 0.183 | 0.209 |
| Body surface area (m2) | 1.8 [1.7, 1.8] | 1.7 [1.6, 1.8] | 0.409 | 0.225 |
| Atrial fibrillation | 10.0 (12.6) | 26.7 (31.6) | 0.196 | 0.470 |
| NYHA class III or IV | 47.3 (59.8) | 60.1 (71.3) | 0.467 | 0.244 |
| Hypertension | 20.9 (26.5) | 22.2 (26.4) | 0.993 | 0.003 |
| Dyslipidemia | 17.3 (21.9) | 14.5 (17.1) | 0.650 | 0.121 |
| Coronary artery disease | 9.8 (12.4) | 7.4 (8.7) | 0.599 | 0.119 |
| Diabetes mellitus | 5.9 (7.5) | 3.4 (4.0) | 0.494 | 0.149 |
| Renal failure | 2.6 (3.3) | 3.6 (4.3) | 0.804 | 0.048 |
| Stroke | 5.2 (6.5) | 18.9 (22.4) | 0.210 | 0.463 |
| Preoperative | ||||
| EF (%) | 43.98±2.54 | 43.33±3.15 | 0.531 | 0.227 |
| LVEDD (mm) | 65.22±8.80 | 64.39±12.58 | 0.838 | 0.077 |
| LAD (mm) | 46.02±6.26 | 45.80±6.99 | 0.901 | 0.033 |
| Aortic stenosis | 42.3 (53.5) | 44.2 (52.4) | 0.953 | 0.022 |
| Concomitant CABG | 9.8 (12.4) | 6.4 (7.6) | 0.477 | 0.162 |
| Concomitant TV surgery | 20.5 (26.0) | 15.1 (17.9) | 0.665 | 0.196 |
| Mechanical valve | 60.2 (76.3) | 57.9 (68.7) | 0.659 | 0.171 |
Data are presented as No. (%), Mean ± SD, or Median [Q1, Q3]. IPTW, inverse probability treatment weighting; SMD, standardized mean difference; NYHA, New York Heart Association; EF, ejection fraction; LVEDD, left ventricular end-diastolic diameter; LAD, left atrial diameter; CABG, coronary artery bypass grafting; TV, tricuspid valve; SD, standard deviation.
Postoperative results
Patients in the treatment group had longer duration of cardiopulmonary bypass time [95.5 (74.5, 131.0) min vs. 133.0 (119.3, 174.3) min, P=0.004], as well as cross-clamp time [67.5 (55.0, 93.5) min vs. 104.5 (87.8, 135.5) min, P<0.001], which was sustained in the IPTW analysis. There was no perioperative death. However, the rate of postoperative acute kidney failure of the treatment group was significantly higher than the control group (P=0.007) in the IPTW analysis. As for the postoperative echocardiographic results, patients in the treatment group showed larger LAD than control group (36.6±4.6 vs. 41.9±6.7 mm, P<0.001), while the EF and LVEDD were similar between the two groups (P=0.591, P=0.144, respectively). FMR was improved among all of the patients after the surgery. Echocardiographic results were sustained in the IPTW analysis (Table 3). No difference in the postoperative improvement of FMR (100% vs. 100%, P>0.99) as well as the EF (48.1%±9.8% vs. 52.1%±9.1%, P=0.291) in patients with aortic stenosis, while same results were observed regarding both the improvement of FMR (100% vs. 100%, P>0.99) and the EF (44.4%±9.5% vs. 41.1%±9.1%, P=0.209), all of which were in line with the IPTW analysis.
Table 3
| Variables | Unmatched | IPTW | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Control (n=36) | Treatment (n=42) | P value | SMD | Control (n=78.98) | Treatment (n=86) | P value | SMD | ||
| Postoperative results | |||||||||
| CPB duration, min | 95.5 [74.5, 131.0] | 133.0 [119.3, 174.3] | 0.004* | 0.517 | 94.0 [82.8, 124.6] | 155.0 [121.0, 173.4] | <0.001* | 0.790 | |
| Cross-clamp time, min | 67.5 [55.0, 93.5] | 104.5 [87.8, 135.5] | <0.001* | 0.821 | 62.8 [57.7, 82.7] | 113.2 [90.0, 143.3] | <0.001* | 1.211 | |
| Perioperative transfusion | 2 (5.6) | 6 (14.3) | 0.372 | 0.295 | 3.3 (4.1) | 14.3 (17.0) | 0.106 | 0.426 | |
| IABP usage | 2 (5.6) | 0 | 0.407 | 0.343 | 3.0 (3.7) | 0 | 0.203 | 0.279 | |
| Acute kidney injury | 2 (5.6) | 5 (11.9) | 0.561 | 0.226 | 2.5 (3.2) | 24.4 (28.9) | 0.007* | 0.750 | |
| New onset atrial fibrillation | 2 (5.6) | 3 (7.1) | >0.99 | 0.065 | 2.6 (3.3) | 3.7 (4.4) | 0.780 | 0.055 | |
| Secondary surgery | 0 | 1 (2.4) | >0.99 | 0.221 | 0 | 1.2 (1.4) | 0.367 | 0.168 | |
| Postoperative | |||||||||
| EF (%) | 45.8±9.6 | 44.5±10.3 | 0.591 | 0.123 | 46.62±8.77 | 48.76±10.26 | 0.494 | 0.224 | |
| LVEDD (mm) | 58.2±8.0 | 61.4±10.6 | 0.144 | 0.339 | 56.99±7.99 | 56.66±10.53 | 0.920 | 0.035 | |
| LAD (mm) | 36.6±4.6 | 41.9±6.7 | <0.001* | 0.897 | 35.30±4.87 | 41.00±5.73 | 0.002* | 1.072 | |
| Mitral regurgitation improved | 36 (100.0) | 42 (100.0) | >0.99 | <0.001 | 79.0 (100.0) | 84.3 (100.0) | >0.99 | <0.001 | |
| Follow-up results | |||||||||
| MACCE | 8 (22.2) | 15 (35.7) | 0.770** | 12.5 (15.8) | 38.7 (45.8) | 0.342** | |||
| Death | 4 (11.1) | 7 (16.7) | 0.645** | 7.6 (9.7) | 28.9 (34.2) | 0.246** | |||
| Heart failure | 2 (5.6) | 7 (16.7) | - | 2.6 (3.3) | 8.8 (10.4) | - | |||
| Stroke | 3 (8.3) | 1 (2.4) | - | 2.2 (2.8) | 1.0 (1.2) | - | |||
| Secondary surgery | 0 | 1 (2.4) | - | 0 | 1.0 (1.2) | - | |||
*, statistically significant; **, P value for log-rank test. Data are presented as No. (%), Mean ± SD, or Median [Q1, Q3]. IPTW, inverse probability treatment weighting; SMD, standardized mean difference; CPB, cardiopulmonary bypass; IABP, intra-aortic balloon pump; EF, ejection fraction; LVEDD, left ventricular end-diastolic diameter; LAD, left atrial diameter; MACCE, major adverse cardiovascular and cerebrovascular events.
Follow-up outcomes
The median follow-up time was 28.7 (14.3, 85.0) months. During the follow-up, 4 of the patients in the control group suffered from death (3 from cardiogenic reason and 1 from hemorrhagic stroke), while 7 patients in the treatment group died of different reasons including 5 from cardiac death, 1 from electric accident, and 1 from pancreatic disease (unmatched: Plogrank=0.645, IPTW: Plogrank=0.246). MACCE-free survival of patients in the control group at 1-, 3- and 5-year were 100%, 89.1% and 69.4%, while they were 100%, 79.4% and 74.7% in the treatment group, respectively. There was no significant difference in the rate of MACCE between the two groups (Plogrank=0.770), which was in line with the IPTW analysis (Plogrank=0.342) (Table 3, Figures 1,2).
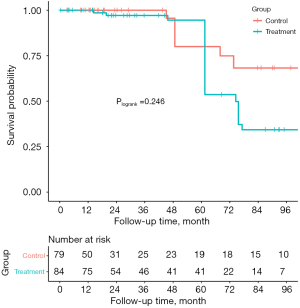
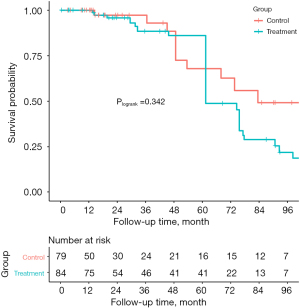
Follow-up echocardiographic results
Follow-up echocardiographic results were also collected, most of which were performed during 3 to 12 months postoperatively. Twenty-three patients (71.9%) in the control group and 28 (66.7%) in the treatment group provided the follow-up echocardiography. No difference was observed in the EF (53.7%±8.5% vs. 50.0%±14.4%, P=0.276), LVEDD (51.1±7.5 vs. 55.5±13.6 mm, P=0.171), and LAD (39.9±7.3 vs. 43.3±7.7 mm, P=0.113) between the groups in the unmatched cohort, all of which were sustained in IPTW analysis. Nevertheless, while the improvement of FMR was comparable (97.2% vs. 95.2%, P=0.650), there was difference in the distribution of mitral regurgitant level between the groups (P=0.026), indicating that concomitant mitral valve surgery improved the FMR more thoroughly (Table 4).
Table 4
| Variables | Unmatched | IPTW | |||||
|---|---|---|---|---|---|---|---|
| Control (n=23) | Treatment (n=28) | P value | Control (n=39.2) | Treatment (n=61.4) | P value | ||
| Echocardiography | |||||||
| EF (%) | 53.7±8.5 | 50.0±14.4 | 0.276 | 52.3±9.10 | 53.0±11.67 | 0.738 | |
| EF level† | 0.605 | 0.732 | |||||
| Deteriorated | 2 (8.7) | 4 (14.3) | 4.7 (12.0) | 4.1 (6.6) | |||
| Stable | 4 (17.4) | 7 (25.0) | 8.0 (20.5) | 10.1 (16.4) | |||
| Improved | 17 (73.9) | 17 (60.7) | 26.4 (67.4) | 47.2 (76.9) | |||
| LVEDD (mm) | 51.1±7.5 | 55.5±13.6 | 0.171 | 51.5±8.0 | 50.8±11.4 | 0.719 | |
| LAD (mm) | 39.9±7.3 | 43.3±7.7 | 0.113 | 39.5±7.2 | 40.7±7.0 | 0.414 | |
| FMR improved | 35 (97.2) | 40 (95.2) | 0.650 | ||||
| FMR | 0.093 | 0.026* | |||||
| No | 15 (65.2) | 24 (85.7) | 27.7 (70.7) | 56.0 (91.2) | |||
| Mild | 7 (30.4) | 2 (7.1) | 10.4 (26.5) | 2.5 (4.1) | |||
| Moderate | 1 (4.4) | 2 (7.1) | 1.1 (2.7) | 2.9 (4.7) | |||
†, compared to preoperative EF level; *, statistically significant. Data are presented as No. (%) or Mean ± SD. IPTW, inverse probability treatment weighting; EF, ejection fraction; LVEDD, left ventricular end-diastolic diameter; LAD, left atrial diameter; FMR, functional mitral regurgitation; SD, standard deviation.
Discussion
In this study, we observed that in HFmrEF patients with aortic valve disease complicated with moderate FMR, there was no difference in both the overall and MACCE-free survival between isolated AVR and AVR + mitral valve surgery, and the improvement of EF was also similar between the two groups, even after multiple adjustment for the potential confounders using IPTW analysis. However, we also noticed that while concomitant mitral valve surgery improved mitral regurgitation more completely, it increased the duration of cardiopulmonary bypass and cross-clamp time. Furthermore, mitral valve surgery also increased the risk of postoperative acute kidney injury, which was more significant after multiple adjustment with IPTW analysis.
The heart valve surgery is often the only way to improve the long-term survival of a patients with severe valvular heart disease. However, it is associated with the risk of serious postoperative complications, including death or postoperative acute kidney injury requiring renal replacement therapy (15,16). Therefore, it is of critical importance to evaluate the effect of heart valve surgeries.
Whether to proceed concomitant mitral valve surgery during AVR in patients with aortic valve disease complicated with moderate FMR is controversial (2,3,17). Sarah and colleagues report in their study that although mitral regurgitation improves immediately after AVR, 17% of the patients experience the recurrence (18). Later in their study, Sorabella et al. also observes that patients with moderate FMR undergoing AVR experience poorer long-term survival, indicating that concomitant mitral valve intervention is needed during the AVR (13). However, in a study comparing AVR with or without concomitant mitral valve surgery in the population of aortic valve disease with moderate FMR, researchers notice that despite the benefit in reducing mitral regurgitation, mitral valve surgery did not improve the survival outcome (19). Nevertheless, evidence on this topic is limited, and there is no study evaluating the treatment strategies on these group of patients who are also complicated with HFmrEF. In this study, we observed that concomitant mitral valve surgery did not improve the survival outcome, which was consistent with the prior study findings. However, concomitant mitral valve surgery increased the risk of postoperative acute kidney injury than isolated AVR. This might be due to the prolongation of cardiopulmonary bypass and cross-clamp time. Therefore, from this aspect, it might be more reasonable not to operate on mitral valve during the AVR procedure in patients with aortic valve disease followed by moderate FMR and HFmrEF.
HFmrEF is a relatively new concept which has been taking the interest of physicians and researchers in recent years. According to prior studies, HFmrEF significantly affects the patient outcome, and the mortality as well as the rate of adverse events are intermediate between heart failure with preserved and reduced EF (20-23). Furthermore, other studies report that HFmrEF is composed of three subsets, including deteriorated, stable and improved (according to the prior EF level), and the prognosis of these three groups of patients were differed (9,24), indicating that the improvement of EF is crucial to the improvement of patient outcome. There is also evidence on the impact of HFmrEF in patients receiving coronary artery bypass grafting, suggesting that HFmrEF negatively impacts patient outcomes including survival, myocardial infarction and hospitalization for heart failure (12). However, the impact of HFmrEF on patients undergoing heart valve surgery, especially on patients with aortic valve disease complicated with moderate FMR, is unknown. Here, we evaluated the change of EF after surgery in these patients enrolled in this study. We noticed that there was no difference regarding the component of deteriorated, improved or unchanged EF between the isolated AVR and AVR + mitral valve surgery groups. And the same was observed in LVEDD and LAD. It is worth mentioning that mitral valve surgery improved mitral regurgitation more completely than the control group, even though this did not benefit in the improvement of survival outcome. Hence, although concomitant mitral valve surgery might have priority on improving mitral regurgitation more thoroughly, it might not bring extra benefit in the improvement of HFmrEF than isolated AVR. More studies are needed.
This study has several unneglectable limitations. First of all, this was a retrospective cohort study from a single center. Thus, the bias caused by the study design was unavoidable. Secondly, this study was also limited with smaller sample size and relatively shorter follow-up duration, which might have compromised the power of tests. In addition, although IPTW analysis has priority on improving the between-group imbalances, potential unmeasured confounders might still exist, and it might result in over-fitting of the available data, especially in situations with small sample sizes. Furthermore, we failed to collect echocardiography for all of the patients who have survived during the follow-up. As a consequence, the echocardiographic results might be not enough to fully represent all of the population, to some extent. Last but by no means the least, EF of the patients at least three months prior to this study was not available, thus failing to further analyze the subset of HFmrEF on the patient outcome. Prospective studies with larger sample sizes are needed.
Conclusions
In patients with aortic valve disease followed by moderate FMR and HFmrEF, mitral valve surgery concomitant to AVR may not bring extra benefit in the MACCE-free survival and the improvement of HFmrEF. However, while concomitant mitral valve surgery might have priority on the complete improvement of mitral regurgitation, it might also increase the risk of postoperative acute kidney injury.
Acknowledgments
The authors thank Runzhen Chen, MD, from the Fuwai Hospital, Chinese Academy of Medical Sciences, and Peking Union Medical College for assistance with the statistical analysis.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-278/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-278/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-278/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board at Fuwai Hospital (No.: 2021-1585) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2017;70:252-89. [Crossref] [PubMed]
- Kowalówka AR, Onyszczuk M, Wańha W, et al. Do we have to operate on moderate functional mitral regurgitation during aortic valve replacement for aortic stenosis? Interact Cardiovasc Thorac Surg 2016;23:806-9. [Crossref] [PubMed]
- Alghamdi AA, Elmistekawy EM, Singh SK, et al. Is concomitant surgery for moderate functional mitral regurgitation indicated during aortic valve replacement for aortic stenosis? A systematic review and evidence-based recommendations. J Card Surg 2010;25:182-7. [Crossref] [PubMed]
- WRITING COMMITTEE MEMBERS. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013;128:e240-327. [PubMed]
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891-975. [Crossref] [PubMed]
- Coles AH, Tisminetzky M, Yarzebski J, et al. Magnitude of and Prognostic Factors Associated With 1-Year Mortality After Hospital Discharge for Acute Decompensated Heart Failure Based on Ejection Fraction Findings. J Am Heart Assoc 2015;4:002303. [Crossref] [PubMed]
- Kapoor JR, Kapoor R, Ju C, et al. Precipitating Clinical Factors, Heart Failure Characterization, and Outcomes in Patients Hospitalized With Heart Failure With Reduced, Borderline, and Preserved Ejection Fraction. JACC Heart Fail 2016;4:464-72. [Crossref] [PubMed]
- Tsuji K, Sakata Y, Nochioka K, et al. Characterization of heart failure patients with mid-range left ventricular ejection fraction-a report from the CHART-2 Study. Eur J Heart Fail 2017;19:1258-69. [Crossref] [PubMed]
- Zhang X, Sun Y, Zhang Y, et al. Heart Failure With Midrange Ejection Fraction: Prior Left Ventricular Ejection Fraction and Prognosis. Front Cardiovasc Med 2021;8:697221. [Crossref] [PubMed]
- He H, Li X, Ke B, et al. Midrange ejection fraction as a risk factor for deterioration of cardiofunction after permanent pacemaker implantation. J Interv Card Electrophysiol 2019;55:213-24. [Crossref] [PubMed]
- Chioncel O, Lainscak M, Seferovic PM, et al. Epidemiology and one-year outcomes in patients with chronic heart failure and preserved, mid-range and reduced ejection fraction: an analysis of the ESC Heart Failure Long-Term Registry. Eur J Heart Fail 2017;19:1574-85. [Crossref] [PubMed]
- Deo SV, Sundaram V, Sahadevan J, et al. Outcomes of coronary artery bypass grafting in patients with heart failure with a midrange ejection fraction. J Thorac Cardiovasc Surg 2021; [Epub ahead of print]. [Crossref] [PubMed]
- Sorabella RA, Olds A, Yerebakan H, et al. Is isolated aortic valve replacement sufficient to treat concomitant moderate functional mitral regurgitation? A propensity-matched analysis. J Cardiothorac Surg 2018;13:72. [Crossref] [PubMed]
- Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLoS Med 2007;4:e297. [Crossref] [PubMed]
- Duchnowski P, Hryniewiecki T, Kuśmierczyk M, et al. Performance of the EuroSCORE II and the Society of Thoracic Surgeons score in patients undergoing aortic valve replacement for aortic stenosis. J Thorac Dis 2019;11:2076-81. [Crossref] [PubMed]
- Duchnowski P, Hryniewiecki T, Kuśmierczyk M, et al. Anisocytosis predicts postoperative renal replacement therapy in patients undergoing heart valve surgery. Cardiol J 2020;27:362-7. [Crossref] [PubMed]
- Wan CK, Suri RM, Li Z, et al. Management of moderate functional mitral regurgitation at the time of aortic valve replacement: is concomitant mitral valve repair necessary? J Thorac Cardiovasc Surg 2009;137:635-640.e1. [Crossref] [PubMed]
- Schubert SA, Yarboro LT, Madala S, et al. Natural history of coexistent mitral regurgitation after aortic valve replacement. J Thorac Cardiovasc Surg 2016;151:1032-9, 1042.e1.
- Coutinho GF, Correia PM, Pancas R, et al. Management of moderate secondary mitral regurgitation at the time of aortic valve surgery. Eur J Cardiothorac Surg 2013;44:32-40. [Crossref] [PubMed]
- Lyu S, Yu L, Tan H, et al. Clinical characteristics and prognosis of heart failure with mid-range ejection fraction: insights from a multi-centre registry study in China. BMC Cardiovasc Disord 2019;19:209. [Crossref] [PubMed]
- Martone R, Marchionni N, Cappelli F. Heart failure with mid-range ejection fraction: Current evidence and uncertainties. Monaldi Arch Chest Dis 2019; [Crossref] [PubMed]
- Mesquita ET, Barbetta LMDS, Correia ETO. Heart Failure with Mid-Range Ejection Fraction - State of the Art. Arq Bras Cardiol 2019;112:784-90. [Crossref] [PubMed]
- Guo P, Dai JF, Feng C, et al. Special prognostic phenomenon for patients with mid-range ejection fraction heart failure: a systematic review and meta-analysis. Chin Med J (Engl) 2020;133:452-61. [Crossref] [PubMed]
- Brann A, Janvanishstaporn S, Greenberg B. Association of Prior Left Ventricular Ejection Fraction With Clinical Outcomes in Patients With Heart Failure With Midrange Ejection Fraction. JAMA Cardiol 2020;5:1027-35. [Crossref] [PubMed]


