
Sarcopenia defined by skeletal muscle mass index at the third lumbar vertebra is a prognostic factor for extensive-stage small cell lung cancer patients: a retrospective study
Introduction
Lung cancer has the highest mortality among all malignancies (1), and small cell lung cancer (SCLC) is considered to be the most aggressive type of lung cancer, accounting for approximately 14% of all cases (2). It is characterized by low differentiation, great invasiveness, rapid resistance after chemo-/radiotherapy, and poor prognosis. The median survival for patients with limited-stage SCLC is 25 to 30 months, with a 5-year survival of 30% (3). The median survival for extensive-stage patients is only 7 to 10 months, with less than 10–20% of patients alive at 2 years (4). Thus, there is an urgent need to find reliable prognostic factors for SCLC to facilitate improved patient management and previous studies have reported that some clinical indicators such as sex, age, tumor-node-metastasis (TNM) phase, received platinum-based chemotherapy, chest radiation, preventive radiotherapy of whole brain, have prognostic importance (5,6).
Sarcopenia, defined as loss of muscle mass and function, has been shown to be related to patient prognosis in several types of cancers (7,8). In general, the evaluation of skeletal muscle mass is based on computed tomography (CT). CT imaging of the cross-sectional areas at the third lumbar vertebra (L3) is considered to be a reference for the diagnosis of sarcopenia because there is a linear relationship between the skeletal muscle mass index at L3 (L3MI) and whole-body muscle mass (9). The prognostic value of L3MI in gastrointestinal tumors (10), head and neck tumors (11), breast cancers, and non-small cell lung cancer (NSCLC) has been well-demonstrated (12,13), with low L3MI typically predictive of poor prognosis. However, the value of L3MI in extensive-stage SLCL remains controversial, Korean researchers reported that L3MI was independent prognostic factors of shorter OS in SCLC patients (14), but study from Japanese did not find significant associations of sarcopenia defined by muscle quantity with Overall survival (OS) in extensive-stage SCLC patients (15). The possible reason for this inconsistency seems to be that in Japanese study, researchers used a regional standard rather than international standard to identify sarcopenia. Considering sarcopenia is recognized as a component of cancer cachexia syndrome and influences patients’ prognosis among kinds of cancers, we think it is necessary to carry out further study to explore the effect of L3MI on the prognosis of patients with extensive-stage SCLC.
Herein, a retrospective study was conducted to compared clinicopathological characteristics and survival outcomes between groups divided by L3MI status to investigate whether sarcopenia evaluated by L3MI is a prognostic marker for extensive-stage SCLC. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-782/rc).
Methods
Patients
This retrospective analysis included extensive-stage SCLC patients diagnosed at the Sun Yat-sen University Cancer Center from January 2009 to March 2017, who were treated with platinum-based chemotherapy and excluded patients if therapeutic courses were not fully available, extensive-stage SCLC was defined using the American Joint Committee on Cancer (AJCC) staging guidelines and finally, there were totally 139 patients included in our research. The end-point of our study was OS, defined as the interval between the date of treatment started and the date of death or censoring. Follow-up data were collected by contacting patients or their families through telephone or obtained from records of our hospital and OS was calculated in months. The following clinicopathological factors were collated: gender, age, smoking history, pathological stage, thoracic radiotherapy, preventive radiotherapy of whole brain, and progression after first line treatment. The patient’s height, weight, and CT or PET/CT datasets measured at the time of diagnosis were examined to assess the body mass index (BMI) and the muscle index of the third lumbar vertebra (L3MI). The BMI was defined as weight divided by height squared (kg/m2) and patients were categorized as underweight (<18.5 kg/m2), normal weight (18.5–25 kg/m2), or overweight (≥25.0 kg/m2). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the Institutional Review Board of Sun Yat-sen University Cancer Center (B2022-187), individual consent for this retrospective analysis was waived.
Inflammatory markers
White blood cell (WBC), neutrophil (NEU), lymphocyte (LY), serum albumin (ALB), lactate dehydrogenase (LDH), and C-reactive protein (CRP) in venous blood samples collected at the first visit to our hospital were measured.
Computed tomography analysis and definition of sarcopenia
The L3MI (cm2/m2) was defined as the cross-sectional area of the muscle at the L3 level normalized by BMI, in accordance with the research by Kim et al. (14). Quantitative assessments of the muscle area were performed using commercially available software (Terarecon 3.4.2.11, San Mateo, CA, USA) by a trained chest radiologist. Sarcopenia defined by L3MI was based on the international consensus for cancer cachexia. Specifically, L3MI <55 cm2/m2 for men and <39 cm2/m2 for women was regarded as the low L3MI group, with all others regarded as the high L3MI group (16).
Statistical analysis
Descriptive statistics are reported as proportions, means with standard deviations (SDs), or medians and interquartile ranges. Comparisons of categorical variables between the high and low L3MII groups were performed using Pearson’s chi-squared test or Fisher’s exact test. Continuous variables were compared using the independent two-sample t-test for variables with normal distributions; otherwise, the nonparametric Mann-Whitney U test was used.
Univariable and multivariable Cox proportional hazard models were used to identify prognostic factors of OS. Variables with a P value <0.1 by the log-rank test were included in the multivariable analysis, two-sided P values of less than 0.05 were considered statistically significant. The Kaplan-Meier product limit method and log-rank tests were used to show the difference in OS between the high L3MI and low L3MI groups, and two-sided P value less than 0.05 was considered statistically significant. All analyses were performed using SPSS for Windows ver. 25.0 and GraphPad prism 8.0 for Windows.
Results
Clinical characteristics
In total, 139 advanced SCLC patients with complete clinical information and follow-up were included in our analysis. The patient clinical characteristics are described in Table 1. The median age was 59 years old (range, 24 to 82 years). Males were the predominant sex (n=133, 95.7%). A total of 36 patients (25.9%) were allocated into the high L3MI group and 103 patients (74.1%) were assigned into the low L3MI group. The median follow-up time was 26.1 months (range, 0.4 to 79.4 months). The median OS was 9.5 months. At the time of analysis, 19 (13.6%) patients were alive and 120 (86.4%) had died.
Table 1
| Clinical characteristics | Values |
|---|---|
| Gender, n (%) | |
| Female | 6 (4.3) |
| Male | 133 (95.7) |
| Age (years), n (%) | |
| ≥60 | 66 (47.48) |
| <60 | 73 (52.52) |
| Median age (25%, 75%) (years) | 59 (25, 63) |
| Smoking history, n (%) | |
| Yes | 115 (82.73) |
| No | 24 (17.27) |
| BMI (kg/m2), n (%) | |
| ≥24 | 38 (27.34) |
| 18.5–24 | 83 (59.71) |
| <18.5 | 18 (12.95) |
| T stage, n (%) | |
| T4 | 46 (33.09) |
| T3 | 43 (30.94) |
| T2 | 42 (30.22) |
| T1 | 8 (5.75) |
| N stage, n (%) | |
| N3 | 69 (49.64) |
| N2 | 56 (40.29) |
| N1 | 6 (4.31) |
| N0 | 8 (5.76) |
| Thoracic radiotherapy, n (%) | |
| Yes | 34 (24.46) |
| No | 105 (75.54) |
| Preventive radiotherapy of whole brain, n (%) | |
| Yes | 26 (18.71) |
| No | 113 (81.29) |
| Progression after first line treatment, n (%) | |
| Yes | 63 (45.32) |
| No | 76 (54.68) |
| L3MI group, n (%) | |
| High group | 36 (25.9) |
| Low group | 103 (74.1) |
SCLC, small cell lung cancer; BMI, body mass index; L3MI, muscle index of the third lumbar vertebra
The association between L3MI and clinical characteristics
There were no significant differences in age, T stage, N stage, inflammatory factors (WBC, NEU, LYM, CRP, ALB, and LDH), nor progression after first-line treatment between the high L3MI and low L3MI groups. However, the BMI was significantly different, with the high L3MI group showing a higher BMI compared to the low L3MI group (24.1±2.9 vs. 21.4±3.0; P<0.01; Table 2).
Table 2
| Clinical characteristics | L3MI group | P value | |
|---|---|---|---|
| High L3MI | Low L3MI | ||
| Age (years) | 0.230 | ||
| ≥60 | 14 | 52 | |
| <60 | 22 | 51 | |
| BMI (kg/m2) | <0.001 | ||
| ≥24 | 19 | 19 | |
| 18.5–24 | 17 | 66 | |
| <18.5 | 0 | 18 | |
| T stage | 0.636 | ||
| T4 | 7 | 39 | |
| T3 | 18 | 24 | |
| T2 | 8 | 34 | |
| T1 | 2 | 6 | |
| N stage | 0.593 | ||
| N3 | 17 | 52 | |
| N2 | 14 | 42 | |
| N1 | 3 | 3 | |
| N0 | 2 | 6 | |
| Progression after first line treatment | 0.297 | ||
| Yes | 19 | 44 | |
| No | 17 | 59 | |
| Inflammatory factors | |||
| WBC (109/L, mean ± SD) | 8.40±1.73 | 8.27±2.51 | 0.782 |
| NEU (109/L, mean ± SD) | 5.53±1.64 | 5.68±2.20 | 0.708 |
| LY (109/L, mean ± SD) | 1.95±0.66 | 1.70±0.69 | 0.068 |
| ALB (g/L, mean ± SD) | 41.13±3.46 | 40.20±4.42 | 0.254 |
| CRP [mg/L, M (25%, 75%)] | 6.34 (3.16, 42.46) | 13.47 (2.82, 46.05) | 0.781 |
| LDH [U/L, M (25%, 75%)] | 245.45 (206.20, 308.28) | 252.05 (197.88, 335.33) | 0.521 |
| NLR [M (25%, 75%)] | 3.25 (2.54, 4.77) | 3.14 (2.16, 4.25) | 0.372 |
L3MI, muscle index of the third lumbar vertebra; SCLC, small cell lung cancer; BMI, body mass index; WBC, white blood cell; NEU, neutrophil; LY, lymphocyte; ALB, serum albumin; CRP, C-reactive protein; LDH, lactate dehydrogenase; NLR, neutrophil-to-lymphocyte ratio.
Univariate and multivariate analyses of overall survival among the 139 advanced SCLC patients
Univariate analysis involving the 139 patients revealed that L3MI and receiving radiotherapy were significantly associated with OS. Multivariate analysis identified that high L3MI (HR, 0.623; 95% CI, 0.405–0.960; P=0.032) and treatment with radiotherapy (HR, 0.410; 95% CI, 0.255–0.660; P<0.001) were independent prognostic factors for longer OS (Table 3). In addition, Kaplan-Meier analysis (Figure 1) showed that the OS of the high L3MI group was significantly longer than that of the low L3MI group (14.045 vs. 9.985 months; P=0.007).
Table 3
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| P value | HR (95% CI) | P value | ||
| Age (years) | ||||
| ≥60 | 0.283 | – | – | |
| <60 | ||||
| Smoking history | ||||
| Yes | 0.414 | – | – | |
| No | ||||
| T stage | ||||
| T1 | Reference | – | – | |
| T2 | 0.838 | |||
| T3 | 0.873 | |||
| T4 | 0.724 | |||
| N stage | – | – | ||
| N0 | Reference | |||
| N1 | 0.39 | |||
| N2 | 0.439 | |||
| N3 | 0.464 | |||
| Thoracic radiotherapy | ||||
| No | <0.001 | Reference | <0.001 | |
| Yes | 0.410 (0.255–0.660) | |||
| Preventive radiotherapy of whole brain | ||||
| No | 0.065 | Reference | 0.362 | |
| Yes | 0.795 (0.486–1.301) | |||
| Progression after first line treatment | – | – | ||
| No | 0.382 | |||
| Yes | ||||
| BMI (kg/m2) | ||||
| <18.5 | Reference | – | – | |
| 18.5–24 | 0.516 | |||
| ≥24 | 0.174 | |||
| L3MI group | ||||
| Low group | 0.007 | Reference | 0.032 | |
| High group | 0.623 (0.405–0.960) | |||
SCLC, small cell lung cancer; HR, hazard ratio; CI, confidence interval; BMI, body mass index; L3MI, muscle index of the third lumbar vertebra.
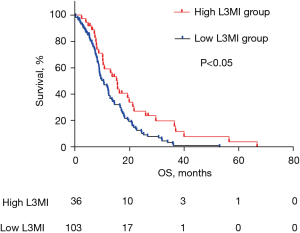
Discussion
This study demonstrated that sarcopenia defined by L3MI was significantly associated with OS in extensive-stage SCLC patients and L3MI is an independent prognostic factor that influences OS in extensive-stage SCLC. Indeed, the OS of the high L3MI group was longer than that of the low L3MI group (14.045 vs. 9.985 months; P=0.007).
Although the prognostic value of L3MI has been shown in other types of malignancies, its value in SCLC remains controversial. The earliest research regarding L3MI and SCLC can be found in a Korean study led by Kim et al. (14), which demonstrated that sarcopenia defined by L3MI is an independent prognostic factor for SCLC patients when using the international consensus of cancer cachexia (L3MI <55 cm2/m2 for men and <39 cm2/m2 for women). Furthermore, the latter study (17) showed that the presence of sarcopenia evaluated by L3MI at the time of diagnosis predicts poor outcomes (median OS of 5.0 months for patients with sarcopenia and adipopenia; 8.9 months for patients with sarcopenia; and 18.3 months for patients without sarcopenia or adipopenia). However, a Japanese study by Minami and colleagues found that sarcopenia evaluated by psoas muscle mass is not a prognostic marker for extensive-stage SCLC patients treated with a standard regimen of platinum-based chemotherapy (15).
This discrepancy is interesting because Kim’s study (14) also found that the prognostic significance disappeared when using the Korean standard to define sarcopenia by L3MI (49 cm2/m2 for men and 31 cm2/m2 for women). Minami’s research used the Japanese standard instead of the international consensus of cancer cachexia to define sarcopenia, and they did not find an association between sarcopenia and prognosis of extensive-stage SCLC patients. Our current study used the international consensus of cancer cachexia to define sarcopenia through L3MI because no Chinese consensus of sarcopenia has been reached, and our results are consistent with Kim’s study (14).
Considering our study and previous reports (14,15), we hypothesize that sarcopenia defined by L3MI has prognostic value for SCLC patients, including those with extensive-stage SCLC. However, the clinical methods and standards of sarcopenia evaluation may vary among countries or occasions, leading to inconsistent results. Therefore, further studies are warranted to investigate the relationship between sarcopenia and SCLC, so as to improve the management of SCLC patients. Furthermore, the standard of sarcopenia defined by L3MI requires more consideration if it is to be used to predict prognosis of cancer patients.
A previous study by Kim et al. (17) reported that in SCLC patients, sarcopenia defined by L3MI was significantly associated with inflammatory markers, specifically, a high Neutrophil-to-lymphocyte ratio(NLR) and CRP, and a low lymphocyte count and serum albumin levels. However, in our current research, there were no differences in inflammatory markers (WBC, NEU, LY, ALB, LDH, and CRP) between the high and low L3MI groups. Our results also suggested that BMI is not a prognostic factor for extensive-stage SCLC patients and this result is consistent with previous study (18), although in NSCLC, a lower BMI is associated with poor OS (19,20).
There were some limitations to this investigation. First, the sample size was small and it may not be possible to extend our results to all extensive-stage SCLC patients. Second, the nutritional status of patients and the retrospective nature of the study may have caused bias. Third, in our study, the international consensus of cancer cachexia was used to define sarcopenia. Future studies should develop ethnic-specific cutoffs for sarcopenia defined by L3MI.
Conclusions
In conclusion, this study demonstrated that sarcopenia defined by L3MI is a prognostic factor for extensive-stage SCLC patients and early intervention for muscle mass may result in a better prognosis for such patients.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-782/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-782/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-782/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by The Institutional Review Board of Sun Yat-sen University Cancer Center (B2022-187), individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7-30. [Crossref] [PubMed]
- Tariq S, Kim SY, Monteiro de Oliveira Novaes J, et al. Update 2021: Management of Small Cell Lung Cancer. Lung 2021;199:579-87. [Crossref] [PubMed]
- Bogart JA, Waqar SN, Mix MD. Radiation and Systemic Therapy for Limited-Stage Small-Cell Lung Cancer. J Clin Oncol 2022;40:661-70. [Crossref] [PubMed]
- Saltos A, Shafique M, Chiappori A. Update on the Biology, Management, and Treatment of Small Cell Lung Cancer (SCLC). Front Oncol 2020;10:1074. [Crossref] [PubMed]
- Huang LL, Hu XS, Wang Y, et al. Survival and pretreatment prognostic factors for extensive-stage small cell lung cancer: A comprehensive analysis of 358 patients. Thorac Cancer 2021;12:1943-51. [Crossref] [PubMed]
- Buddharaju LNR, Ganti AK. Immunotherapy in lung cancer: the chemotherapy conundrum. Chin Clin Oncol 2020;9:59. [Crossref] [PubMed]
- Ruan GT, Ge YZ, Xie HL, et al. Association Between Systemic Inflammation and Malnutrition With Survival in Patients With Cancer Sarcopenia-A Prospective Multicenter Study. Front Nutr 2021;8:811288. [Crossref] [PubMed]
- Clemente-Suárez VJ, Redondo-Flórez L, Rubio-Zarapuz A, et al. Nutritional and Exercise Interventions in Cancer-Related Cachexia: An Extensive Narrative Review. Int J Environ Res Public Health 2022;19:4604. [Crossref] [PubMed]
- Tagliafico AS, Bignotti B, Torri L, et al. Sarcopenia: how to measure, when and why. Radiol Med 2022;127:228-37. [Crossref] [PubMed]
- Horie K, Matsuda T, Yamashita K, et al. Sarcopenia assessed by skeletal muscle mass volume is a prognostic factor for oncological outcomes of rectal cancer patients undergoing neoadjuvant chemoradiotherapy followed by surgery. Eur J Surg Oncol 2022;48:850-6. [Crossref] [PubMed]
- Findlay M, White K, Brown C, et al. Nutritional status and skeletal muscle status in patients with head and neck cancer: Impact on outcomes. J Cachexia Sarcopenia Muscle 2021;12:2187-98. [Crossref] [PubMed]
- Tang R, Deng JP, Zhang L, et al. Prognostic significance of the skeletal muscle index and systemic inflammatory index in patients with lymph node-positive breast cancer after radical mastectomy. BMC Cancer 2022;22:234. [Crossref] [PubMed]
- Mallet R, Decazes P, Modzelewski R, et al. Prognostic value of low skeletal muscle mass in patient treated by exclusive curative radiochemotherapy for a NSCLC. Sci Rep 2021;11:10628. [Crossref] [PubMed]
- Kim EY, Kim YS, Park I, et al. Prognostic Significance of CT-Determined Sarcopenia in Patients with Small-Cell Lung Cancer. J Thorac Oncol 2015;10:1795-9. [Crossref] [PubMed]
- Minami S, Ihara S, Komuta K. Sarcopenia and Visceral Adiposity Are Not Independent Prognostic Markers for Extensive Disease of Small-Cell Lung Cancer: A Single-Centered Retrospective Cohort Study. World J Oncol 2020;11:139-49. [Crossref] [PubMed]
- Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489-95. [Crossref] [PubMed]
- Kim EY, Kim YS, Seo JY, et al. The Relationship between Sarcopenia and Systemic Inflammatory Response for Cancer Cachexia in Small Cell Lung Cancer. PLoS One 2016;11:e0161125. [Crossref] [PubMed]
- Shepshelovich D, Xu W, Lu L, et al. Body Mass Index (BMI), BMI Change, and Overall Survival in Patients With SCLC and NSCLC: A Pooled Analysis of the International Lung Cancer Consortium. J Thorac Oncol 2019;14:1594-607. [Crossref] [PubMed]
- Yuan Q, Du M, Loehrer E, et al. Postdiagnosis BMI Change Is Associated with Non-Small Cell Lung Cancer Survival. Cancer Epidemiol Biomarkers Prev 2022;31:262-8. [Crossref] [PubMed]
- Chen YM, Lai CH, Lin CY, et al. Body Mass Index, Weight Loss, and Mortality Risk in Advanced-Stage Non-Small Cell Lung Cancer Patients: A Focus on EGFR Mutation. Nutrients 2021;13:3761. [Crossref] [PubMed]
(English Language Editor: J. Teoh)


