
Stratified treatment of localized cervical esophageal squamous cell carcinoma induced by neoadjuvant immunotherapy plus chemotherapy (SCENIC)
Introduction
Cervical esophageal cancer (CEC) has a low incidence, accounting for approximately 5% of all esophageal cancers. These are predominantly squamous cell carcinomas (1). The majority of CEC present most cervical esophageal local invasiveness and invade its surrounding adjacent structures. Surgical resection has not been recommended as primary treatment for patients with CEC or the possibility of a positive margin and low quality of life (QoL) after surgery (2,3). Instead, definitive chemoradiation therapy (dCRT) has been recommended by National Comprehensive Cancer Network (NCCN) (4,5). However, recent studies have revealed that surgical resection for CEC achieves better survival outcomes than chemoradiotherapy alone (6,7). Complications, including esophageal stenosis and stiffness after dCRT, result in dysphagia that needs subsequent endoscopic dilation, leading to poor QoL for these patients (8). Therefore, the standard treatment for CEC continues to be debated. The ideal treatment would improve long-term survival, preserve organ function and good QoL.
However, related clinical studies with double endpoints have not been carried out, and some new, powerful, and less harmful clinical screening methods need to be developed. Unlike radiotherapy, these new methods will not increase the difficulty of salvage surgery. They should also help clinicians to identify which patients are sensitive to dCRT so that the insensitive areas could be operated on to improve survival. The clinical trial “Neoadjuvant Immunotherapy and Chemotherapy for local advanced Esophageal cancer” (NICE) led by our center has recently obtained some surprising phased results with a pathological complete remission rate of 45.4% for esophageal cancer, the results of which were reported at the 2021 American Society of Clinical Oncology (ASCO) meeting (9). Therefore, we believe that immunotherapy combined with chemotherapy could be an ideal method for screening appropriate CEC patients for the following surgical resection or dCRT. The aim of this study was to assess the performance of neoadjuvant immunotherapy and chemotherapy in screening local advanced CEC patients for the following dCRT or radical resection. We present the following article in accordance with the SPIRIT reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-402/rc).
Methods
Study design and recruitment
This is a multicenter, prospective, interventional phase II clinical study. The study is being conducted in 4 high-volume Chinese hospitals: the Shanghai Chest Hospital (coordinating center), Shanghai Huadong Hospital, Shanghai Changhai Hospital, and Shanghai Zhongshan Hospital. The study was planned to begin in April 2022, and the results are expected in April 2025. The flow chart of the SCENIC trial is shown in Figure 1. The trial was registered at Chictr.Org as ChiCTR2200057732.
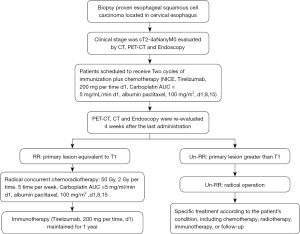
The study is being conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Shanghai Chest Hospital (approval number IS22005), and informed consent was obtained from all individual participants.
Eligibility criteria
Potentially curative CEC patients who are planning to undergo neoadjuvant therapy and who will undergo surgical resection will be recruited to this study. Patients will be considered eligible according to the following criteria.
The inclusion criteria are as follows: (I) histologically confirmed esophageal squamous cell carcinoma; (II) tumor located in the cervical esophagus; (III) clinical stage cT2-4NanyM0, according to the 8th Edition of the American Joint Committee on Cancer tumor-node-metastasis (AJCC TNM) classification for Esophageal Cancer; (IV) age: 18–75 years old at the date of informed consent; (V) Eastern Cooperative Oncology Group (ECOG) performance status: 0–1; and (VI) written informed consent is provided by the patient.
The exclusion criteria are as follows: (I) patients with a second primary malignant tumor; (II) previous history of chemotherapy and/or radiation therapy; (III) intolerance to surgery or chemoradiation; (IV) confirmation of distant metastasis; (V) having received attenuated vaccine within 4 weeks; and (VI) the presence of autoimmune disease.
Baseline examination and inclusion
At baseline, patients will undergo a gastrointestinal endoscopy with a routine tumor biopsy or endoscopic ultrasonography in case of a traversable tumor. Upper gastrointestinal barium meal or iodine angiography will be used to confirm the location of the tumor. Contrast enhanced computed tomography (CT) and whole-body positron emission tomography (PET)-CT will be conducted to stage the tumor and exclude distant dissemination. Written informed consent for study participation will be obtained from eligible patients after baseline diagnosis and staging.
Neoadjuvant immunotherapy and chemotherapy
All patients will receive 2 cycles of induction therapy comprising immunotherapy and chemotherapy, with a time interval of 3 weeks. The regimen will consist of tislelizumab intravenously (iv), 200 mg/time on day 1; nab-paclitaxel iv, 100 mg/m2 on days 1, 8, and 15, and Q3W and carboplatin intravenous drip at an area under the curve (AUC) of 5 mg/mL/min on day 1 (10).
Patient grouping
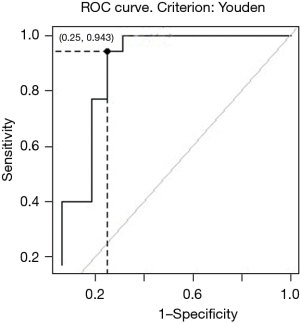
Tumors will be reevaluated with endoscopy and PET-CT 3 weeks after induction therapy is completed. Patients will be divided into 3 groups according to their response: a remarkable response (RR) group, limited partial response (LPR) group, and poor response (POR) group. Tumors in the RR group will meet the following criteria simultaneously: (I) characteristics of superficial esophageal cancer under endoscopy (Figure 2); and (II) percentage decrease of the maximum standardized uptake value (SUVmax) of PET-CT ≥71% (Figure 3). The remaining patients will be defined as LPR and POR.

Definitive chemoradiation
Patients in the RR group will receive dCRT, which contains nab-paclitaxel Intravenous drip in doses of 100 mg/m2 on days 1, 8, and 15; and carboplatin IV with an AUC of 5 mg/mL/min on day 1 for 2 cycles with an interval of 3 weeks. The radiotherapy dose will be 50 Gy given in 30 fractions, 5 fractions per week (11).
Surgery
Patients in the LPR group and the POR group will receive radical surgical resection, including resection of tumors, reconstruction of the upper digestive tract, and lymphadenectomy. Preservation of the larynx should be determined according to the location and T stage of the tumor. The gastric tube, jejunum, and colon will all be considered as a substitution. The protocol of lymph node dissection will be as follows: group 1 contains 101, and 106 rec; group 2 contains 102, 104, and 105; group 3 contains 106 tbL/106 tbR, 107, 108, and 109, and these lymph nodes should be removed when tumors involve the thoracic esophagus (12).
Maintenance treatment
Immunotherapy will be maintained for 4–6 weeks after radical chemoradiotherapy or surgery is finished. Then, patients in the RR groups will be administrated with tislelizumab alone for a further 1 year. Tislelizumab IV (200 mg on day 1) will be performed every 3 weeks until 1 year after enrollment or adverse events (AEs) occur, including tumor regression or recurrence, toxicity intolerance, initiation of a new anti-tumor therapy, termination of the trial, and so on. Imaging evaluation will be performed every 3 months after the end of radical chemoradiotherapy or surgery. Participant safety will be continuously assessed during the study. When patients terminate treatment, they will still need to receive a comprehensive examination, including laboratory tests and imaging examination. The treatment choice for patients in the LPR and POR groups will depend on the patient’s condition, including chemotherapy, radiotherapy, immunotherapy, or follow-up.
Follow-up
Safety follow-up and survival follow-up will be performed once the maintenance treatment is finished. The AEs will be assessed throughout the study and up to 30 days after the last dose of the study drug or the initiation of a new anticancer therapy, whichever occurs first, according to National Cancer Institute Common Terminology Criteria for Adverse Events v4.03 (https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm).
Safety follow-up will take place 30 days after the last dose of tislelizumab, or after the patient develops imaging progression, whichever occurs first. After the safety follow-up period, only all serious AEs (SAEs; including results in death/disability/incapacity/life-threatening/hospitalization/prolongation of existing hospitalization) considered to be related to the study drug will be collected and followed up every 30 days until 90 days by clinical follow-up or telephone follow-up. Data collection will include participants’ survival status, AEs, concomitant medications, and concomitant treatments. Investigators may add additional visits as needed for AEs follow-up with the aim of following up on the resolution of AEs.
Survival follow-up will be conducted via clinical follow-up or telephone follow-up every 3 months. Information including survival status, anti-tumor treatment, and disease progression will be collected until the participant’s death, loss to follow-up, or study termination.
Statistical analysis
Sample size calculation
According to our previous study, the 2-year progression-free survival (PFS) of concurrent neoadjuvant chemoradiotherapy for patients with CEC in our center is 51.7% (13). This study assumes 12 months of enrollment and 24 months of follow-up. It is expected that the 2-year EFS of the target population will be approximately 70% after joint therapy. The significance level ɑ was set at 0.05 (one-sided). Considering the power was 80% in addition to the 10% dropout, a total of 36 subjects were required for our study.
Data analysis
Statistical analysis in our study will be performed using SPSS 24.0 (IBM Corp., Armonk, NY, USA). Continuous variables will be recorded as mean ± standard deviation (M ± SD), median, minimum, and maximum, and the differences between the groups will be analyzed using a Mann-Whitney U test or a Student’s t-test according to the homoscedasticity of variance. Categorical variables in different groups will be assessed using the χ2 test or Fisher’s exact test. Survival curves will be estimated using the Kaplan-Meier method, and survival distribution will be compared using the log-rank test. Statistical significance will be considered when P≤0.05.
Results
Study endpoints
The primary endpoint is 2-year event-free survival (EFS) of the overall population, which is defined as the time from randomization to occurrence of any of the following events: (I) tumor progression according to imaging examination; (II) tumor recurrence, including local recurrence or distant metastasis assessed by imaging or biopsy evidence; or (III) death from any cause.
The key secondary endpoints are disease-free survival (DFS) and objective response rate (ORR). Other secondary endpoints include RR rate after induction therapy (downstaging to T1 N any M0), 1-year regression-free survival (RFS) of RR patients, 1-year RFS in non-RR patients; overall survival (OS), completion rate of induction therapy; completion rate of surgery for induction therapy, percentage of patients with disease progression after induction therapy, esophagus retention rate at time, and QoL assessment.
Discussion
The NCCN guidelines recommend dCRT as the only treatment for CEC, and the guidelines were based on the study conducted by Burmeister et al. (14). In their study, 34 patients with CEC were treated with chemoradiation to achieve local control while preserving organ function. Some 11 patients had mild stenosis that did not receive endoscopic dilation, and 4 patients experienced moderate stenosis and received repeated endoscopic dilation. However, 1 patient developed severe stenosis and died after endoscopic dilation failed, and long-term follow-up of laryngeal function was not analyzed. Nakata et al reported a 5-year survival of CEC patients after dCRT of over 50%. In the chemoradiotherapy group, 15 patients failed to acquire local control and 11 of then underwent salvage surgery, and the 5-year OS of the salvage surgery group was 64.8% which was significantly higher than that in conservative treatment group (44%) (15).
In the real world, local control rates achieved with dCRT remain poor, and are also associated with significant functional morbidity. In one study, 55 patients were included, with 3- and 5-year local control rates of 55% and 47%, and 3- and 5-year OS rates of 29% and 25%, respectively (4). In another study with 102 patients included, 3-year OS was 39.3% and locoregional recurrence-free survival was 35.3% with platinum-based chemoradiation (16). Another series of 92 patients using platinum doublets combined with radiation and 2–3 cycles after radiation revealed a 3-year OS of 49.8% and PFS of 42.1% (13). The only prospective study on concurrent chemoradiation in CEC was performed by Japanese scholars, which used three-dimensional (3D) techniques to treat 30 patients with 60 Gy in 30 fractions, along with 5FU and cisplatin. With a median follow-up of 40.8 months, 3-year OS was 66.5%, PFS was 36.6%, and laryngectomy-free survival was 52.5%. The 3-year survival rate was higher in complete responders (74.6%) than in non-responders (25.0%) (5).
Although dCRT appears to provide the best control for CEC with the most acceptable morbidity, there remains a need for improved local control, with 5-year failure rates of 50–70% and distant metastasis rates of approximately 40%. In contrast, in the NEOCRTEC5010 study, the number of these patients was much lower than in patients with thoracic esophageal squamous cell carcinoma (SCC), where OS and DFS were 69.1% and >70%, respectively (17).
Therefore, with the accumulated evidence of clinical trials, the NCCN guidelines for CEC might be updated for the following reasons: (I) the 5-year survival of surgery-centered comprehensive treatment for CEC has been significantly better than that of chemoradiotherapy alone; (II) large-scale population studies showed that the larynx is anatomically preserved, but the function of the larynx was impaired at different degree after dCRT; and (III) stenosis after dCRT would lead to a large number of patients being unable to eat normally, and repeated dilatation would be needed which seriously affects the life quality.
Previous studies, such as the NEOCRTEC5010 study and the CROSS study, have demonstrated that thoracic esophageal SCC achieves better long-term survival after neoadjuvant chemoradiation therapy (17-19). In our study, the concept of chemoselection has been studied and considered for various purposes, including organ preservation, improvement of OS, and reduction of distant metastasis in resectable hypopharyngeal cancer patients, which can achieve the goal of improving OS and QoL (20). Using selective induction chemotherapy, or “chemoselection”, a retrospective study reported a strategy to refer each individual chemotherapy responder to definitive chemoradiation compared to primary chemoradiation (15). Recognizing that this was an intrinsically biased study, 2-year OS was significantly improved in the induction arm (65.1%) compared with the standard chemoradiation arm (40%). The local control rate also increased from 25.0% to 68.0% at 2 years after treatment, translating into a better laryngeal preservation rate. Since these data are retrospective. It is difficult to determine whether induction therapy is the standard of care. Increasing data suggests that outcomes vary widely between treatment responders and non-responders disability, suggesting that an induction or ‘chemoselection’ approach might be a good strategy to intensify the treatment of patients with low response. In order to use a chemoselection strategy to stratify patients, the screening therapy must be effective and well-tolerated. The advent of immunotherapy has opened this door. The NICE treatment regimen led by our center has recently obtained a pathological complete remission rate of nearly 50% for esophageal cancer, which is currently the best therapeutic effect of non-radiotherapy worldwide (9). Therefore, we believe that the NICE regimen can be a pioneer weapon in dual-endpoint screening therapy for CEC.
To our knowledge, the SCENIC study is the first multicenter, prospective, exploratory study in CEC to investigate the role of the combination of immunotherapy and chemotherapy in CEC. Stratified screening after immuno-neoadjuvant therapy is expected to preserve organ function and improve long-term survival. The results of the SCENIC study may change the treatment mode of CEC and offer high-level evidence for future CEC treatments.
Acknowledgments
We would like to thank AME editing service for their help in polishing our paper.
Funding: This work was supported by the Shanghai Esophageal Cancer Cohort Database of Shanghai Hospital Development Center (No. SHDC2020CR6002 to ZL) and 2020 “Hospital New Star” Young Medical Talent Program of Shanghai to CL.
Footnote
Reporting Checklist: The authors have completed the SPIRIT reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-402/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-402/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-402/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study is being conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Shanghai Chest Hospital (approval number IS22005), and informed consent was obtained from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Buckstein M, Liu J. Cervical Esophageal Cancers: Challenges and Opportunities. Curr Oncol Rep 2019;21:46. [Crossref] [PubMed]
- Daiko H, Hayashi R, Saikawa M, et al. Surgical management of carcinoma of the cervical esophagus. J Surg Oncol 2007;96:166-72. [Crossref] [PubMed]
- Sun F, Li X, Lei D, et al. Surgical management of cervical esophageal carcinoma with larynx preservation and reconstruction. Int J Clin Exp Med 2014;7:2771-8. [PubMed]
- Gkika E, Gauler T, Eberhardt W, et al. Long-term results of definitive radiochemotherapy in locally advanced cancers of the cervical esophagus. Dis Esophagus 2014;27:678-84. [Crossref] [PubMed]
- Zenda S, Kojima T, Kato K, et al. Multicenter Phase 2 Study of Cisplatin and 5-Fluorouracil With Concurrent Radiation Therapy as an Organ Preservation Approach in Patients With Squamous Cell Carcinoma of the Cervical Esophagus. Int J Radiat Oncol Biol Phys 2016;96:976-84. [Crossref] [PubMed]
- Takebayashi K, Tsubosa Y, Matsuda S, et al. Comparison of curative surgery and definitive chemoradiotherapy as initial treatment for patients with cervical esophageal cancer. Dis Esophagus 2017;30:1-5. [PubMed]
- Xu L, Chen XK, Xie HN, et al. Treatment and Prognosis of Resectable Cervical Esophageal Cancer: A Population-Based Study. Ann Thorac Surg 2022;113:1873-81. [Crossref] [PubMed]
- Kojima T, Shah MA, Muro K, et al. Randomized Phase III KEYNOTE-181 Study of Pembrolizumab Versus Chemotherapy in Advanced Esophageal Cancer. J Clin Oncol 2020;38:4138-48. [Crossref] [PubMed]
- Liu J, Yang Y, Liu Z, et al. Multicenter, single-arm, phase II trial of camrelizumab and chemotherapy as neoadjuvant treatment for locally advanced esophageal squamous cell carcinoma. J Immunother Cancer 2022;10:e004291. [Crossref] [PubMed]
- Xu J, Bai Y, Xu N, et al. Tislelizumab Plus Chemotherapy as First-line Treatment for Advanced Esophageal Squamous Cell Carcinoma and Gastric/Gastroesophageal Junction Adenocarcinoma. Clin Cancer Res 2020;26:4542-50. [Crossref] [PubMed]
- Chen YH, Lu HI, Lo CM, et al. The Clinical Outcomes of Locally Advanced Cervical Esophageal Squamous Cell Carcinoma Patients Receiving Curative Concurrent Chemoradiotherapy: A Population-Based Propensity Score-Matched Analysis. Cancers (Basel) 2019;11:451. [Crossref] [PubMed]
- Japan Esophageal Society. Japanese Classification of Esophageal Cancer, 11th Edition: part I. Esophagus 2017;14:1-36.
- Li HX, Liu J, Cheng Y, et al. Concurrent chemoradiotherapy for cervical esophageal squamous cell carcinoma: treatment results from a prospective observational study. Dis Esophagus 2018; [Crossref] [PubMed]
- Burmeister BH, Dickie G, Smithers BM, et al. Thirty-four patients with carcinoma of the cervical esophagus treated with chemoradiation therapy. Arch Otolaryngol Head Neck Surg 2000;126:205-8. [Crossref] [PubMed]
- Nakata Y, Hanai N, Nishikawa D, et al. Comparison between chemoselection and definitive radiotherapy in patients with cervical esophageal squamous cell carcinoma. Int J Clin Oncol 2017;22:1034-41. [Crossref] [PubMed]
- Zhang P, Xi M, Zhao L, et al. Clinical efficacy and failure pattern in patients with cervical esophageal cancer treated with definitive chemoradiotherapy. Radiother Oncol 2015;116:257-61. [Crossref] [PubMed]
- Yang H, Liu H, Chen Y, et al. Neoadjuvant Chemoradiotherapy Followed by Surgery Versus Surgery Alone for Locally Advanced Squamous Cell Carcinoma of the Esophagus (NEOCRTEC5010): A Phase III Multicenter, Randomized, Open-Label Clinical Trial. J Clin Oncol 2018;36:2796-803. [Crossref] [PubMed]
- Eyck BM, van Lanschot JJB, Hulshof MCCM, et al. Ten-Year Outcome of Neoadjuvant Chemoradiotherapy Plus Surgery for Esophageal Cancer: The Randomized Controlled CROSS Trial. J Clin Oncol 2021;39:1995-2004. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Lefebvre JL, Andry G, Chevalier D, et al. Laryngeal preservation with induction chemotherapy for hypopharyngeal squamous cell carcinoma: 10-year results of EORTC trial 24891. Ann Oncol 2012;23:2708-14. [Crossref] [PubMed]


