Idiopathic laryngotracheal stenosis
Introduction
Idiopathic laryngotracheal stenosis (ILTS) is a rare inflammatory disease. The first three patients reported in the literature were described by Brandenburg in 1972 (1). There have since been more case reports and series describing this condition, the pathology, management and long term outcomes regarding conservative and definitive surgical intervention. Importantly, it is thought of as a diagnosis of exclusion given after other immunologic, infectious or traumatic etiologies have been excluded. A recent body of research focuses on hormonal factors influencing the development of this disease given the predominance of females affected, however there have yet to be any new causal links identified and the etiology remains unknown (2).
Pathophysiology
ILTS is characterized by the percent of luminal stenosis, the distance of involved airway from the vocal cords, and the overall length of the stenotic segment. Idiopathic disease characteristically is comprised of circumferential lesions of varying length from 1–3 cm, the majority of which demonstrate maximal stenosis at the level of the cricoid (3). On pathologic examination, affected tissue demonstrates replacement of the tracheal lamina propria with dense keloidal, collagenous fibrosis which characterizes the stenotic segment (4). This disease entity is termed idiopathic given the etiology of pathologic transformation remains elusive.
Presentation
Nearly every case study in the literature, including the largest series of patients reported by Wang et al., describes greater than 98% of patients with idiopathic disease being females, with a median age of afflicted patients being 47 years old (5). Clinical presentation includes symptoms of upper airway obstruction including progressive dyspnea on exertion, wheezing, or stridor. Thirty-seven percent of patients report abnormality in their voice upon presentation (5). Patients typically become symptomatic when their airway is reduced by more than 50% (2). After infectious, neoplastic, immunologic or traumatic etiologies are ruled out, a diagnosis of idiopathic disease is given, which guides initial management. Exclusion of Wegener’s granulomatosis is confirmed by a negative ANCA. Patients should be screened for connective tissue disorders by ANA status. It can be elevated without specific diagnosis.
Historically, many patients have undergone conservative medical and endoluminal therapies with reasonable short-term results. These include laser ablation, mechanical dilation using rigid bronchoscopy, or balloon dilatation with flexible bronchoscopy (6). Medical treatment with agents such as mitomycin C (MMC) and steroids are used as adjunctive treatments. However, the rate of recurrence with these therapies has been reported as high as 87% at 5 years (3). Wang et al. recommends based on their experience, at most 3 dilations, before referral for surgical intervention, as these interventions may cause further airway damage without long-term improvement. Collectively, these measures are all palliative and temporizing, with diminishing returns, as surgical intervention must be considered.
Pre-operative evaluation
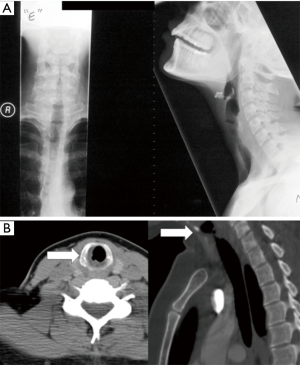
Preoperative planning and evaluation includes appropriate exclusion of underlying immunologic processes, in addition to antecedent events, such as infection, trauma, or prior irradiation. Simple soft tissue X-rays and thin slice spiral CT imaging are used to better characterize the stenotic segments of the airway (Figure 1) (5). It is preferred to electively defer surgical management 2–3 months in the setting of active inflammation and perform dilations prior to resection, however, this is not uniformly practiced. Wang et al. reports 20% of patients were dilated only at initial evaluation and surgical correction deferred.
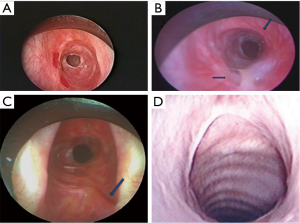
All surgical candidates should undergo bronchoscopic evaluation of the airway to identify proximal and distal extent of involvement, as well as degree of active inflammation (Figure 2) (5). If the stenosis abuts the vocal cords, or vocal cord mobility is impaired, a single stage repair may not be possible (2). Otolaryngologists should be consulted prior to operative intervention in these patients so as to optimize the ability to extubate the patient post operatively. However, significant stenosis that begins within 5 mm of the glottis is challenging and conditions must be optimal. In those patients deferred because of inflammation, we recommend saline nebulizers twice per day to clear the inflammatory exudate.
Operative management
ILTS has become an increasingly common indication for laryngotracheal resection in our institution over the past 25 years (2). Definitive resection can be most often optimally achieved via a single stage repair, with or without a posterior tracheal membranous wall flap. Close collaboration with an excellent anesthesia team proves to be the most important intraoperative variable to successful outcomes (2). Extubation at the completion of the surgery is sought and should be achieved in nearly all patients.
Careful endoscopic examination of the airway and identification of extent of diseased trachea is essential. Given the proximity to the subglottic airway and vocal cords in idiopathic disease, most require resection of the anterior half of the cricoid and reinforcement of the posterior cricoid (Figure 3) (8). Excessive anastomotic tension and preservation of the blood supply are vitally important. The first two tracheal rings are typically part of the resection, falling approximately 2 cm below the cricoid (9). One must be careful to ensure that circumferential dissection is maintained at the level of the diseased trachea, as tracheal vasculature is segmental and enters laterally (2). Dissection is kept on the trachea at all times to avoid injury to the recurrent nerves.
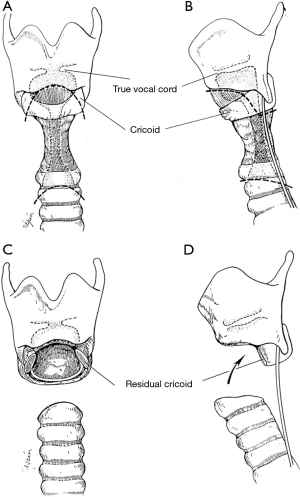
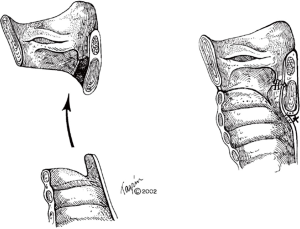
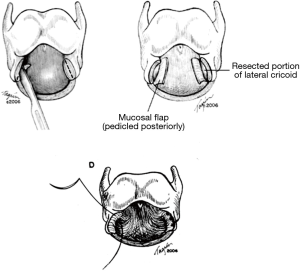
Most commonly observed with ILTS disease, residual scar will remain on the posterior cricoid plate, given its characteristic circumferential disease pattern. This should be resected and resurfaced with a tracheal membranous wall flap (Figure 4) (2). One is able to enlarge the lateral dimensions of the subglottic airway by approximately 4 to 5 mm by meticulously excising small segments of thickened cricoid cartilage on either side and resection of the thickened submucosal scar, while careful to maintain the cricoid structural integrity (7). The mucosa overlying the resected cartilage is importantly preserved and resurfaced as a pedicled flap (Figure 5) (8). A single 5-0 Vicryl suture is used to advance the flap and secure it to the cricoid. Previously multiple sutures were used. The anastomotic sutures now are used to secure the flap to the cricoid. This is done to prevent exposed cartilage in the airway and minimize granulation tissue formation.
Once reconstruction commences, reduction of anastomotic tension is important. Interrupted sutures are placed between the inferior aspects of the posterior cricoid cartilage and the membranous wall flap (8). The membranous wall flap sutures are placed as vertical mattress sutures so as to have the knots end up outside the lumen of the airway. The laryngeal mucosa is then sutured to the membranous wall flap (2). These sutures can be placed so knots are inside or outside the lumen. Next, working posterior to anterior, the anastomotic sutures approximating the laryngeal mucosa and lateral cricoid lamina sutures are placed first, followed by the tracheal mucosa and cartilage (Figure 6) (7).
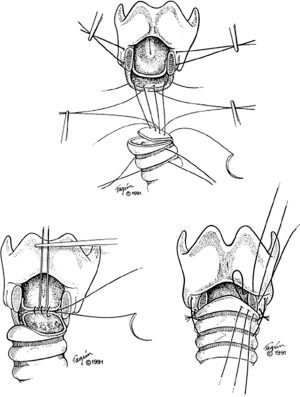
All sutures are placed first prior to tying down each throw. The traction sutures are then tied down first, followed by the posterior membranous flap and inferior edge of the posterior cricoid plate, followed by the sutures of the laryngeal mucosa and membranous wall flap (2). Proceeding posterior to anterior, the remaining sutures are tied. The ET tube is advanced past the anastomosis into appropriate position. The thyroid bag is then deflated, the neck is flexed, and the stay sutures are tied first on either side. Then working lateral to medial, the anterior sutures are tied down, finishing in the same fashion with the posterior sutures (2). Thyroid isthmus or strap muscles are buttressed over the anastomosis upon closure.
The integrity of the anastomosis is tested by submerging it under saline and delivering positive pressure via the endotracheal tube. Any defects are approximated with simple interrupted sutures. Approximately 6–7 days later, the patient will undergo surveillance bronchoscopy. Satisfactory examination allows for removal of the guardian chin stitch and diet advancement (2).
Outcomes
Both short and long term results for patients undergoing definitive surgical intervention for ILTS are excellent. Wang et al. recently published the largest series in the literature, consisting of 263 patients with ILTS who underwent single stage reconstructive surgery. Just 9% of patients experienced recurrent disease, the majority of which were managed with occasional dilations (5%) and 4% have been recalcitrant requiring repeated dilations. Ninety percent of patients had good results (5).
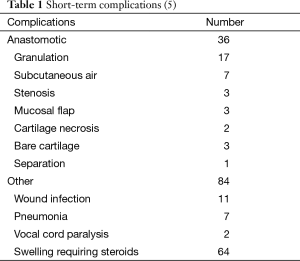
Post-operative complications were low (Table 1). Granulation tissue at the anastomosis and glottic edema are the most common short-term complications (5). Edema typically presents 2 to 5 days postoperatively. Post-operative edema or stridor may be treated with 24–48 h of dexamethasone (2). Diuresis, elevation of the head of the bed, and use of Heliox can be helpful. Granulations usually were successfully debrided with a single bronchoscopy.
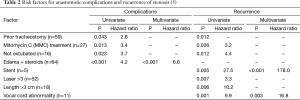
Anastomotic complications in this cohort were found to be the most closely associated with previous tracheostomy, stent placement, laser treatment greater than three times, or endobronchial MMC injection (Table 2) (5). Further, vocal cord involvement, length of resection greater than 3 cm, and inability to extubate in the OR were risk factors for recurrent disease (5).
A change in voice is experienced by many patients (53%) postoperatively (5). Patients notice a slightly deeper voice or inability to reach high notes if they sing. Their most common problem is a diminished ability to project their voice (67%) (5).
Despite the fact that the etiology of ILTS remains elusive, laryngotracheal resection as a definitive surgical treatment has demonstrated long-term benefits in this patient cohort. This surgical option should be considered early in order to lessen complications and optimize outcomes.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Brandenburg JH. Idiopathic subglottic stenosis. Trans Am Acad Ophthalmol Otolaryngol 1972;76:1402-6. [PubMed]
- Mathisen DJ, Morse C. Master Techniques in Surgery: Thoracic Surgery: Transplantation, Tracheal Resections, Mediastinal Tumors, Extended Thoracic Resections, 1st edition. Philadelphia: LWW, 2014.
- Kanarek DJ. Infectious, inflammatory, infiltrative, idiopathic, and miscellaneous tracheal lesions. In: Grillo HC, editor. Surgery of the Trachea and Bronchi. Hamilton/London: BC Decker, 2004;363-77.
- Grillo HC, Mark EJ, Mathisen DJ, et al. Idiopathic laryngotracheal stenosis and its management. Ann Thorac Surg 1993;56:80-7. [PubMed]
- Wang H, Wright CD, Wain JC, et al. Idiopathic Subglottic Stenosis: Factors Affecting Outcome After Single-Stage Repair. Ann Thorac Surg 2015;100:1804-11. [PubMed]
- Ashiku SK, Kuzucu A, Grillo HC, et al. Idiopathic laryngotracheal stenosis: effective definitive treatment with laryngotracheal resection. J Thorac Cardiovasc Surg 2004;127:99-107. [PubMed]
- Liberman M, Mathisen DJ. Treatment of idiopathic laryngotracheal stenosis. Semin Thorac Cardiovasc Surg 2009;21:278-83. [PubMed]
- Liberman M, Mathisen DJ. Tailored cricoplasty: an improved modification for reconstruction in subglottic tracheal stenosis. J Thorac Cardiovasc Surg 2009;137:573-8; discussion 578-9. [PubMed]
- Wright CD. Surgical management of subglottic stenosis. Oper Tech Thorac Cardiovasc Surg 2008;13:53-65.