
Report on lung cancer surgery during COVID-19 pandemic at a high volume US institution
Introduction
COVID-19 has disrupted every aspect of medical care. Thoracic oncology has been particularly affected given the virus’s effects on lung tissue and the pulmonary-disease-prone patient population that lung cancer typically affects (1,2). Importantly, delays in the treatment of non-small cell lung cancer (NSCLC) have been associated with upstaging on pathology with worsened long-term outcomes (3-5).
Our aim was to discover how COVID-19 affected lung cancer care at our institution by evaluation of key oncology care metrics. We hypothesized specifically that patients would wait longer for surgery, have more advanced tumors, and experience more complications during the COVID-19 crisis. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-5/rc).
Methods
This study was approved by the Mass General Brigham Human Research Protection Committee (Protocol number: 2006P002482) and informed consent for this retrospective analysis was waived. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
A cohort study included all patients with pathologically confirmed NSCLC after surgery at our institution from January 1, 2019, to December 31, 2019 (pre-COVID-19 group) and March 1, 2020 to June 2, 2020 (COVID-19 group). Surgeries performed included wedge resections, segmentectomies, lobectomies, bilobectomies, and pneumonectomies for curative intent. Morbidity was graded on the Clavien-Dindo system of surgical complications; Grade II and higher were considered significant. Time to first follow-up was collected for the COVID-19 group.
Statistical analysis
Values were compared between groups using Chi-square test, Wilcoxon rank-sum test, and Fisher’s exact test where appropriate. Clinical stage was compared to pathologic stage to determine up- or down-staging. All statistical tests were performed with STATA version 14 (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP).
All pandemic patients had negative COVID tests preoperatively and were directed to self-quarantine from the time of their COVID test until day of surgery.
The Massachusetts Governor issued some guidance on surgery and prohibited elective surgery during portions of the pandemic. At this institution, the surgical division was asked to triage case selection due to actual and predicted bed space secondary to limited availability from staffing and inpatient beds used for COVID-19 patients. For thoracic surgery cases, presumed diagnosis was listed and then triage was done by rating multiple variables on a 0–100 scale based on urgency, with 0 being not urgent and 100 being extremely urgent. Scores were based on documentation and discussion with the treating physician; a list of these variables is available in Table S1. An overall score was tabulated and then an overall Urgency Assessment value was created. Case selection was then done on an institutional level by a multidisciplinary group, including members of the thoracic surgery faculty, and cases proceeded based on fluctuating available bed space.
Hospital protocols in place in the early phase of the pandemic were evolving but primarily consisted of limiting visitor and non-essential personnel access to the hospital, daily symptom attestation, preoperative COVID-19 testing, individual rooms for patients, and dedicated intensive care units and hospital wards for COVID-19 positive patients.
Results
In 2019, 375 operations for NSCLC were completed vs. 58 during the height of the COVID-19 crisis (Table 1). Median clinical tumor size was minimally larger during the COVID-19 era vs. pre-COVID-19 and though not significant, a higher proportion of clinical Stage II-IV cases were encountered during COVID-19. Median time from final clinic visit to surgery was no different between groups or divided by early stage (I and II) vs. late stage (III and IV); and our group found no difference in the pathologic stage distribution. Overall rate of complications and grade of complications were no different between the pre-COVID-19 group and the COVID-19 group. There were no mortalities within 90 days among the COVID-19 group (Table 2). COVID-19 group median first follow-up was 15 days from surgery, and no patients reported symptoms or were diagnosed with COVID-19 disease by follow-up visit.
Table 1
| Characteristic | Pre-COVID-19, n=375 | COVID-19, n=58 | P value |
|---|---|---|---|
| Clinical tumor size (cm) | 1.9 [1.2, 2.8] | 2.2 [1.5, 3.2] | 0.048 |
| Clinical stage* | 0.148 | ||
| Stage 1 | 307 (81.9) | 41 (70.7) | |
| Stage 2 | 31 (8.3) | 8 (13.8) | |
| Stage 3 | 32 (8.5) | 7 (12.1) | |
| Stage 4 | 5 (1.3) | 2 (3.4) | |
| Time to surgery, all (days) | 17 [9, 27] | 16.5 [9, 34] | 0.542 |
| Time to surgery | |||
| Early stage I+II (days) | 18 [10, 27] | 17 [9, 34] | 0.562 |
| Late stage III+IV (days) | 11 [6, 20] | 12 [10, 27] | 0.345 |
| Pathological stage | 0.058 | ||
| Stage 0 | 17 (4.5) | 4 (6.9) | |
| Stage 1 | 272 (72.5) | 40 (69.0) | |
| Stage 2 | 46 (12.3) | 6 (10.3) | |
| Stage 3 | 35 (9.3) | 6 (10.3) | |
| Stage 4 | 5 (1.3) | 2 (3.4) | |
| Change in stage | |||
| Early stage I+II | 0.040 | ||
| Decrease | 14 (4.1) | 6 (12.2) | |
| No change | 272 (80.5) | 39 (79.6) | |
| Increase | 52 (15.4) | 4 (8.2) | |
| Late stage III+IV | 0.718 | ||
| Decrease | 20 (54.1) | 4 (44.4) | |
| No change | 17 (45.9) | 5 (55.6) | |
| Increase | 0 (0.0) | 0 (0.0) |
Data were presented in mean [IQR] or n (%). *, staging under the American Joint Committee on Cancer (AJCC) TNM Staging System for Lung Cancer, 8th Edition. IQR, interquartile range.
Table 2
| Characteristic | Pre-COVID-19, n=375 | COVID-19, n=58 | P value |
|---|---|---|---|
| Any postop complication | 116 (30.9) | 18 (31.0) | 1.000 |
| Grade II* | 101 (26.9) | 15 (25.9) | 1.000 |
| Grade III | 31 (8.3) | 8 (13.8) | 0.213 |
| Grade IV | 9 (2.4) | 1 (1.7) | 1.000 |
| Grade V | 1 (0.3) | 0 (0.0) | 1.000 |
| 30-day mortality | 1 (0.3) | 0 (0.0) | 0.213 |
| 60-day mortality | 3 (0.8) | 0 (0.0) | 1.000 |
| 90-day mortality | 4 (1.1) | 0 (0.0) | 1.000 |
Data were presented in n (%). *, grade under the Clavien-Dindo classification of surgical complications.
Upon direct comparison of the peak of the COVID-19 outbreak at our institution, March through May 2020, 53 cases were done vs. 107 in the same time period of 2019 (Figure 1). A significant deceleration was only seen in April 2020. By May 2020, case volume began to return to pre-pandemic levels, but was still half that of the same time period in 2019. No significant differences were seen in staging change for March through May 2020 vs. the same period the year prior (Table 3).
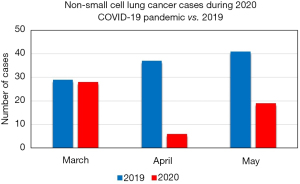
Table 3
| Characteristic | March–May 2019, n=107. | March–May 2020, n=53. | P value |
|---|---|---|---|
| Clinical tumor size (cm) | 2.1 [1.2, 3.0] | 2.2 [1.5, 3.4] | 0.185 |
| Clinical stage* | 0.051 | ||
| Stage 1 | 87 (81.3) | 36 (67.9) | |
| Stage 2 | 7 (6.5) | 8 (15.1) | |
| Stage 3 | 13 (12.1) | 7 (13.2) | |
| Stage 4 | 0 (0.0) | 2 (3.8) | |
| Time to surgery, all (days) | 19 [9, 29] | 14 [9, 30] | 0.595 |
| Time to surgery | |||
| Early stage I+II (days) | 19 [12, 30] | 16.5 [8.5, 30] | 0.463 |
| Late stage III+IV (days) | 9 [6, 26] | 12 [10, 27] | 0.228 |
| Pathological stage | 0.377 | ||
| Stage 0 | 8 (7.5) | 3 (5.7) | |
| Stage 1 | 75 (70.1) | 36 (67.9) | |
| Stage 2 | 15 (14.0) | 6 (11.3) | |
| Stage 3 | 9 (8.4) | 6 (11.3) | |
| Stage 4 | 0 (0.0) | 2 (3.8) | |
| Change in stage | |||
| Early stage I+II | 0.320 | ||
| Decrease | 7 (7.4) | 5 (11.4) | |
| No change | 70 (74.5) | 35 (79.5) | |
| Increase | 17 (18.1) | 4 (9.1) | |
| Late stage III+IV | 0.666 | ||
| Decrease | 8 (61.5) | 4 (44.4) | |
| No change | 5 (38.5) | 5 (55.6) | |
| Increase | 0 (0.0) | 0 (0.0) |
Data were presented in mean [IQR] or n (%).*, staging under the American Joint Committee on Cancer (AJCC) TNM Staging System for Lung Cancer, 8th Edition. IQR, interquartile range.
Discussion
This analysis covering COVID-19 and its effects on thoracic surgery found neither longer wait times nor an increase in upstaging on final pathology. However, the volume of surgery was much lower during the worst month of the crisis than in previous years. There is a clinically important difference in the numbers of patients who received operations of each stage of lung cancer despite lack of statistical significance. This study was not able to capture patients whose initial diagnosis of NSCLC was delayed, who delayed treatment due to the pandemic, or who chose against surgery as initial treatment for their NSCLC. As the pandemic continues, the decrease in operations for surgically resectable NSCLC is concerning for untreated cancers that may progress without treatment, the consequences of which we may not see for 3–5 years. The early impact of these issues is already being seen with decreased screening for multiple cancers and especially for lung cancer (6,7).
Our institution benefitted from a novel triaging system that may limit the generalizability of this work, similar to other strategies reported (8,9). The system was developed to ensure timely delivery of care to our patients based on clinical stage, neoadjuvant treatment status, and need for surgery. This ensured low wait times between last clinic visit and surgery so that patients proceeded appropriately to resection. This avoidance of surgical delay is likely why our downstaging, tumor size, complications, and overall distribution of cases was no different from the year before. Most importantly, none of the resected patients experienced symptoms or received a diagnosis of COVID-19 during their hospitalization or by their first follow-up.
There was a clinical—but not statistical—difference toward more advanced stages in the COVID era. This may be due to the triage system favoring advanced cases; perhaps the result of symptomatic patients being more likely to visit the physician than asymptomatic patients, who were avoiding hospitals during this precarious time. However, this connection was not able to be ascertained due to the low case numbers, and specific outpatient details were not available in the database and were a limitation of the study.
Additional limitations to this work involve the lower number of patients, that while anticipated, obfuscate a high degree of precision in statistical comparisons of demographics and outcomes. This work was done at one institution; therefore, applicability may be limited outside similar environments.
In summary, a novel triage/scoring system for lung cancer patients may help to eliminate delays in lung cancer surgery when hospitals are forced to make system-wide adjustments to operating room availability. There was a clinical—but not statistical—difference toward more advanced stages in the COVID era. With proper precautions, definitive lung cancer treatment can be safely accomplished without incurring additional risk. However, the lower rate of surgery and the potential progression of patients who have not been assessed on time is concerning for the future.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-5/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-5/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-5/coif). SJS is a consultant for Ethicon and Covidien. The other authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- COVID-19: The US Perspective. The Society of Thoracic Surgeons. Accessed June 26, 2020. Available online: https://www.sts.org/publications/news-surgeons-view/covid-19-us-perspective
- Dingemans AC, Soo RA, Jazieh AR, et al. Treatment Guidance for Patients With Lung Cancer During the Coronavirus 2019 Pandemic. J Thorac Oncol 2020;15:1119-36. [Crossref] [PubMed]
- Mohammed N, Kestin LL, Grills IS, et al. Rapid disease progression with delay in treatment of non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2011;79:466-72. [Crossref] [PubMed]
- Samson P, Patel A, Garrett T, et al. Effects of Delayed Surgical Resection on Short-Term and Long-Term Outcomes in Clinical Stage I Non-Small Cell Lung Cancer. Ann Thorac Surg 2015;99:1906-12; discussion 1913. [Crossref] [PubMed]
- Gao SJ, Corso CD, Wang EH, et al. Timing of Surgery after Neoadjuvant Chemoradiation in Locally Advanced Non-Small Cell Lung Cancer. J Thorac Oncol 2017;12:314-22. [Crossref] [PubMed]
- Bakouny Z, Paciotti M, Schmidt AL, et al. Cancer Screening Tests and Cancer Diagnoses During the COVID-19 Pandemic. JAMA Oncol 2021;7:458-60. [Crossref] [PubMed]
- Van Haren RM, Delman AM, Turner KM, et al. Impact of the COVID-19 Pandemic on Lung Cancer Screening Program and Subsequent Lung Cancer. J Am Coll Surg 2021;232:600-5. [Crossref] [PubMed]
- Depypere LP, Daddi N, Gooseman MR, et al. The impact of coronavirus disease 2019 on the practice of thoracic oncology surgery: a survey of members of the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothorac Surg 2020;58:752-62. [Crossref] [PubMed]
- Martínez-Hernández NJ, Caballero Silva U, Cabañero Sánchez A, et al. Effect of COVID-19 on Thoracic Oncology Surgery in Spain: A Spanish Thoracic Surgery Society (SECT) Survey. Cancers (Basel) 2021;13:2897. [Crossref] [PubMed]


