
Effects of 24-hour postoperative intravenous fluid on postoperative outcomes after lobectomy: a retrospective observational study
Introduction
Lobectomy with systematic mediastinal lymph node dissection is the gold standard surgical treatment for early non-small cell lung cancer (NSCLC) (1). Lobectomy performed by video-assisted thoracoscopic surgery (VATS) has been shown to reduce postoperative pain, inflammatory response, and length of hospital stay (2). Although the overall mortality from lobectomy has declined to approximately 0.5–1% with the improvement of surgical techniques and perioperative management (3), the morbidity after lobectomy for lung cancer remains between 26.0% and 54.7% (3-5). The main causes of morbidity following pulmonary resections are of pulmonary origin and mainly include pneumonia, acute lung injury, and acute respiratory distress syndrome (ARDS) (3-5). These postoperative complications are, in part, related to intra- and postoperative fluid management (6).
Healthy people have a certain preload reserve capacity, and they have the ability to self-regulate on the ascending limb of the Frank-Starling curve without the need for additional fluid supplementation (7). Insufficient intravascular volume to patients who are fluid responsive can be adapted by self-adjustment, and excessive perioperative fluid administration to these patients may increase the risks of fluid overload without conferring additional benefit (8,9). Liberal fluid management often leads to a variety of negative outcomes, but restrictive fluid management also carries important risks, such as organ hypoperfusion leading to organ dysfunction and failure (8). Among them, the kidney is an organ that is particularly susceptible to changes in volume status, and hypovolemia often leads to renal impairment (8). During the perioperative period of a lobectomy, maintenance of intravascular volume via intravenous fluid is necessary to mitigate the hypovolemia caused by osmotic loss and bleeding (4). However, the upper bound and the lower limit of safe intravenous fluid in this setting remain unclear (8). For these reasons, the dosing of perioperative intravenous fluid varies widely among medical centers, and physicians may adopt a restrictive or liberal fluid management strategy based on their habits. Therefore, more evidence-based medical evidence is needed to guide clinical fluid management in lung surgery patients.
Postoperative fluid management plays a key role in ensuring adequate tissue perfusion, stabilizing hemodynamics, and reducing morbidities related to hemodynamics (10,11). We hypothesized that variation in 24-hour postoperative fluid administration within the range of standard clinical practice would be associated with differences in postoperative pulmonary complications, acute kidney injury (AKI), in-hospital mortality, and readmission within 30 days in patients with NSCLC who underwent VATS lobectomy. We sought to quantify the dose-response relationship between the 24-hour postoperative fluid volume and each of these outcomes across three groups of practice that spanned the range of restrictive to liberal fluid dosing. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-707/rc).
Methods
Patients
During a period of 1 year between May 2016 and April 2017, 592 consecutive adult patients with NSCLC who underwent VATS lobectomy were included for this observational retrospective study. The patients were treated on a routine basis in the First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, China. VATS lobectomy was conducted through 3 ports, and systematic mediastinal lymph node dissection was performed. The patient fasted for 8 hours before surgery, and a lung protective strategy (low tidal volume) was implemented. Patients with preoperative renal dysfunction, history of thoracic surgery, congestive heart failure, sleeve lobectomy, a simultaneous resection of more than 2 lobes, and second surgery were excluded (Figure 1). All patients received prophylactic first- or second-generation cephalosporins before chest tube removal. If postoperative pulmonary infection is diagnosed, sulbactam ampicillin or ciprofloxacin is administered. All patients started eating 6 to 8 hours after resuscitation from anesthesia unless they were intubated or at risk of aspiration. The TNM classification (seventh edition) proposed by the International Union Against Cancer was applied in this cohort. Demographic, intraoperative, postoperative, and outcome data were extracted from medical records (summarized in Table 1 and available online in Table S1). The amount of intraoperative total fluids was defined as the sum of crystalloids, colloids, and blood products administered between the onset of anesthesia care and resuscitation from anesthesia (12). The infusion rate of intraoperative total fluids was an average fluid administration rate, defined as the intraoperative total fluid volume per kilogram of weight divided by the operation duration (hours).
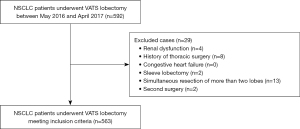
Table 1
| Characteristics | Total intravenous crystalloid infusion 24 h postoperatively | P value | |||
|---|---|---|---|---|---|
| Total (n=563) | Restrictive group (n=136), <1,080 mL | Moderate group (n=272), 1,080–<1,410 mL | Liberal group (n=155), ≥1,410 mL | ||
| ASA score, n (%) | 0.001 | ||||
| I | 472 (83.8) | 100 (73.5) | 244 (89.7) | 128 (82.6) | |
| II | 68 (12.1) | 29 (21.3) | 21 (7.7) | 18 (11.6) | |
| III | 23 (4.1) | 7 (5.1) | 7 (2.6) | 9 (5.8) | |
| Age (year) | 63.2±10.7 | 64.1±11.3 | 62.7±10.5 | 63.4±10.5 | 0.439 |
| Gender (female/male) | 336/227 | 76/60 | 164/108 | 96/59 | 0.553 |
| Weight, kg | 61.4±9.8 | 62.3±10.3 | 60.6±9.6 | 61.9±9.7 | 0.198 |
| Smoking, n (%) | 156 (27.7) | 35 (25.7) | 83 (30.5) | 38 (24.5) | 0.346 |
| Diabetes mellitus, n (%) | 48 (8.5) | 25 (18.4) | 15 (5.5) | 8 (5.2) | <0.001 |
| Coronary heart disease, n (%) | 9 (1.6) | 3 (2.2) | 4 (1.5) | 2 (1.3) | 0.813 |
| FEV1, L | 2.3±0.6 | 2.3±0.6 | 2.3±0.6 | 2.3±0.6 | 0.799 |
| FVC, L | 2.9±0.7 | 2.9±0.8 | 2.9±0.7 | 2.9±0.8 | 0.994 |
| PEF, L/s | 4.7±1.9 | 4.9±1.8 | 4.6±1.9 | 4.7±1.9 | 0.190 |
| FEV1% | (91.1±17.2)% | (90.9±18.5)% | (91.3±17.3)% | (90.8±16.1)% | 0.932 |
| FVC% | (91.3±15.7)% | (89.8±15.8)% | (91.5±15.7)% | (92.1±15.6)% | 0.417 |
| PEF% | (67.6±24.0)% | (70.4±20.4)% | (66.1±25.3)% | (67.9±24.3)% | 0.056 |
| Intraoperative bleeding, mL | 48.8±45.1 | 49.4±69.1 | 47.4±35.5 | 50.6±31.9 | 0.764 |
| Intraoperative blood transfusion, mL | 0 | 0 | 0 | 0 | – |
| Length of operation, min | 133.3±36.4 | 125.6±33.0 | 136.1±39.7 | 135.1±32.2 | 0.010 |
| Amount of intraoperative fluids, mL | 1,545.3±415.2 | 1,500.7±386.1 | 1,578.4±433.6 | 1,526.0±404.4 | 0.164 |
| Infusion rate of intraoperative total fluids, mL/kg/h | 11.6±3.8 | 12.2±3.7 | 12.3±4.0 | 11.6±3.8 | 0.158 |
| Total intravenous crystalloid infusion 24 h postoperatively, mL | 1,254.7±278.0 | 877.4±120.2 | 1,259.3±82.2 | 1,577.8±163.5 | <0.001 |
| Total intravenous colloid infusion 24 h postoperatively, mL | 0 | 0 | 0 | 0 | – |
| NSCLC staging, n (%) | 0.245 | ||||
| IA | 455 (80.8) | 107 (78.7) | 224 (82.4) | 124 (80.0) | |
| IB | 39 (6.9) | 8 (5.9) | 21 (7.7) | 10 (6.5) | |
| IIA | 34 (6.0) | 9 (6.6) | 10 (3.7) | 15 (9.7) | |
| IIB | 2 (0.4) | 1 (0.7) | 1 (0.4) | 0 (0.0) | |
| IIIA | 33 (5.9) | 11 (8.1) | 16 (5.9) | 6 (3.9) | |
| Postoperative pathology, n (%) | 0.976 | ||||
| Adenocarcinoma | 513 (91.1) | 125 (91.9) | 247 (90.8) | 141 (91.0) | |
| Squamous cell carcinoma | 43 (7.6) | 10 (7.4) | 21 (7.7) | 12 (7.7) | |
| Adenosquamous carcinoma | 7 (1.2) | 1 (0.7) | 4 (1.5) | 2 (1.3) | |
Values are presented as mean ± standard deviation, n or n (%). ASA, American Society of Anesthesiologists; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; PEF, peak expiratory flow; FEV1%, FEV1 as percentage of predicted; FVC%, FVC as percentage of predicted; PEF%, PEF as percentage of predicted; NSCLC, non-small cell lung cancer.
The Medical Ethics Committee of the First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China, approved the study (No. 2017-58). Due to the retrospective research method used in this study, no intervention was taken, and the Medical Ethics Committee agreed to waive the patients' informed consent. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Exposure variable
Total intravenous crystalloid infusion in the 24-hour postoperative period was derived from the medical record and defined as the volume of crystalloid administered in the 24-hour postoperative period. Initially, patients were classified into four groups representing incremental quartiles of the exposure variable of the total intravenous crystalloid infusion in the 24-hour postoperative period (8,12). The results eventually showed that the middle two groups were very similar in terms of the incidence of postoperative pulmonary complications. Therefore, we merged the middle two groups into a single group. Finally, patients were classified into three groups: quartile 1 (restrictive group: R group), quintile 2+3 (moderate group: M group), and quartile 4 (liberal group: L group).
Outcome measures
The primary outcomes were postoperative pulmonary complications and AKI. Postoperative pulmonary complications included ARDS, reintubation, pulmonary embolism, need for bedside bronchoscopy, prolonged air leak, failure of the lung to expand, atelectasis, and pneumonia within the period of postoperative hospitalization (4,8). Pulmonary embolism was confirmed by pulmonary computed tomography angiography (4,12). A prolonged air leak was defined as a leak lasting >1 week (4,12). The failure to expand was defined as an inability of the remaining lung to fill the pleural cavity on a chest X-ray, with or without air leak (4,12). Atelectasis was diagnosed by chest radiograph documentation (4,12). The diagnosis of postoperative pneumonia was made if a new pulmonary infiltrate, with leukocytosis and fever (ear temperature >38.0 ℃), was evident on chest X-ray (4,12). AKI refers to the increase of serum creatinine level by at least 0.3 mg/dL or 50% compared with the preoperative level within 48 hours postoperatively (8).
Secondary outcomes included in-hospital mortality, readmission within 30 days, prolonged hospital stay, postoperative length of stay, and total hospital care costs (yuan renminbi) (8). A prolonged hospital stay was defined as hospitalization for more than 7 days after surgery.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation and were compared using 1-way analysis of variance. In the case of uneven variance, a nonparametric test (Kruskal-Wallis H test for multiple independent samples) was used. Categorical variables are expressed as percentages, which were compared using an R×C chi-square test. When the theoretical frequency was <5, Fisher’s exact test was used. Binary logistic regression analyses were performed to determine the relationship between total intravenous crystalloid infusion in the 24-hour postoperative period with postoperative pulmonary complications, AKI, in-hospital mortality, readmission within 30 days, and prolonged hospital stay, respectively. Multiple linear regression analyses were conducted to determine the relationship between total intravenous crystalloid infusion in the 24-hour postoperative period with postoperative length of stay and total hospital care costs. Confounders were included according to univariate analysis (12). Odds ratios (ORs) were calculated from these models, together with their 95% CIs. The assignment of the variables in multivariate analysis is shown in the online Table S2. For all tests, a 2-tailed P value ≤0.05 was considered statistically significant. All analyses were performed using SPSS 25.0 (IBM Corp., Armonk, NY, USA) (8,12).
Results
Patient selection and comparative univariate analysis
A total of 563 patients were deemed eligible for analysis after our inclusion and exclusion criteria were applied (Figure 1). Of these, 136 (24.2%) patients with pulmonary complications and 13 (2.3%) patients with AKI were observed (Table S1). No patient died during the postoperative hospitalization (Table S1). Table 1 and Table S1 list the baseline characteristics of the cohort and the results of comparative univariate analysis.
Comparative multivariate analysis of three groups of different total intravenous crystalloid infusion in the 24-hour postoperative period
We included statistically significant factors in univariate analysis in our multivariate regression model in order to determine the degree of contribution of total intravenous crystalloid infusion 24 hours postoperatively on postoperative outcomes (Table S3).
Binary logistics regression analysis demonstrated the incidence of postoperative pulmonary complications was lowest in the moderate group (Table 2 and Table S3). In comparison, the risk for postoperative pulmonary complications was significantly increased in the restrictive group (OR 1.815, 95% CI: 1.083–3.043; P=0.024) and liberal group (OR 2.692, 95% CI: 1.684–4.305; P<0.001; Table 2). AKI incidence among the three groups was similar (P=0.464; Table 2).
Table 2
| Postoperative outcome | Total intravenous crystalloid infusion 24 h postoperatively | Total P value | ||
|---|---|---|---|---|
| Restrictive group (n=136), <1,080 mL | Moderate group (n=272), 1,080–<1,410 mL | Liberal group (n=155), ≥1,410 mL | ||
| Postoperative pulmonary complications | ||||
| Acute respiratory distress syndrome, n (%) | 2 (1.5) | 1 (0.4) | 1 (0.6) | 0.291 |
| Reintubation, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | – |
| Pulmonary embolism, n (%) | 2 (1.5) | 1 (0.4) | 0 (0.0) | 0.344 |
| Need for bedside bronchoscopy, n (%) | 4 (2.9) | 0 (0.0) | 0 (0.0) | 1.000 |
| Prolonged air leak, n (%) | 5 (3.7) | 6 (2.2) | 2 (1.3) | 0.529 |
| Failure to expand, n (%) | 1 (0.7) | 6 (2.2) | 6 (3.9) | 0.261 |
| Atelectasis, n (%) | 6 (4.4) | 2 (0.7) | 5 (3.2) | 0.083 |
| Pneumonia | <0.001 | |||
| N (%) | 24 (17.6) | 37 (13.6) | 49 (31.6) | |
| OR (95% CI) | 1.390 (0.779–2.478) | 1 | 2.869 (1.750–4.703) | |
| P value | 0.265 | – | <0.001 | |
| Patients with pulmonary complications | <0.001 | |||
| N (%) | 36 (26.5) | 45 (16.5) | 55 (35.5) | |
| OR (95% CI) | 1.815 (1.083–3.043) | 1 | 2.692 (1.684–4.305) | |
| P value | 0.024 | – | <0.001 | |
| Acute kidney injury, n (%) | 3 (2.2) | 8 (2.9) | 2 (1.3) | 0.464 |
| In-hospital mortality, n (%) | 0 | 0 | 0 | – |
| Readmission within 30 days, n (%) | 0 (0.0) | 1 (0.4) | 5 (3.2) | 0.114 |
| Prolonged hospital stay, n (%) | 25 (18.4) | 31 (11.4) | 14 (9.0) | 0.077 |
| Postoperative length of stay, days | 5.9±1.9 | 5.8±2.1 | 5.9±2.3 | 0.572 |
| Total hospital care costs (yuan renminbi) | 58,388.4±8,998.8 | 57,830.2±10,036.1 | 59,133.9±9,808.9 | 0.262 |
Results of binary logistics regression are presented as adjusted OR, 95% CI, and P value. The best-performing moderate group served as the reference group. OR, odds ratio; CI, confidence interval.
No deaths occurred in the three groups during hospitalization. Binary logistics regression analysis demonstrated that the incidences of readmission within 30 days (P=0.114) and prolonged hospital stay (P=0.077) were similar among the three groups (Table 2). There were also no statistical differences in postoperative length of stay (P=0.572) or total hospital care costs (P=0.262) among the three groups according to the multiple linear regression model (Table 2).
A separate binary logistics regression analysis was performed for each of the postoperative pulmonary complications. The results showed that the incidence of pneumonia was lowest in the moderate group (Table 2 and Table S3). Compared with that in the moderate group, the risk for pneumonia in the liberal group was significantly increased (OR 2.869, 95% CI: 1.750–4.703; P<0.001) while that in the restrictive group was increased but not significantly so (P=0.265; Table 2). The incidences of other postoperative pulmonary complications (including ARDS, reintubation, pulmonary embolism, need for bedside bronchoscopy, prolonged air leak, failure of the lung to expand, and atelectasis) were similar among the three groups (Table 2).
In addition, we also classified patients into four groups representing the incremental quartiles of the exposure variable of the total intravenous crystalloid infusion in the 24-hour postoperative period. The baseline characteristics and comparative univariate results of the 4 groups are presented in Table S4 and Table S5 while the results of multivariate analysis are presented in Tables S6-S8.
Discussion
In an analysis of 563 patients with NSCLC who underwent VATS lobectomy, we observed a robust association between total intravenous crystalloid infusion in the 24-hour postoperative period and postoperative pulmonary complications (Table 2). The major finding of this study was that for patients with NSCLC who underwent VATS lobectomy, restrictive (<1,080 mL) or liberal (≥1,410 mL) 24-hour postoperative crystalloid infusion led to an increased incidence of postoperative pulmonary complications, while moderate 24-hour postoperative crystalloid infusion (1,080–<1,410 mL) led to optimal outcomes (Table 2).
In clinical practice, patients undergoing surgery display large differences in postoperative fluid volume. This variability results from the diversity in habits of clinicians and the occurrence of some emergencies that require increased infusions (e.g., postoperative bleeding and hypotension). As there is currently no clinically applicable standard for the amount of 24-hour postoperative fluid to be administered after VATS lobectomy, clinicians use a relatively random amount of 24-hour postoperative fluid volume (6). Institution-specific protocols for postoperative fluid therapy should be developed, and our present study provides some references for clinical 24-hour postoperative fluid management.
Perioperative intravenous-fluid therapy serves to restore and maintain body water, electrolytes, and organ perfusion to achieve homeostasis (13). Avoiding excessive intravenous fluid is commonly recommended in programs for enhanced recovery after surgery (14). The harmful effects of fluid excess frequently manifest in the lungs, especially after pulmonary resections (4,8). Fluid overload can precipitate pulmonary edema and impair gas exchange, leading to postoperative pulmonary complications such as pneumonia, respiratory failure, and reintubation (8). Arslantas et al. reported that excessive perioperative infusion fluid during pulmonary resection results in an increased incidence of postoperative pulmonary complications (4). Pang et al. conducted a meta-analysis of perioperative fluid administration, and the results showed that perioperative liberal fluid administration could increase postoperative pulmonary and cardiac complications compared with perioperative restrictive fluid administration (15). However, excessive perioperative fluid restriction can be harmful (11,16); for instance, fluid restriction can cause hypovolemia and postoperative organ dysfunction (17). Shin et al. found that both the restrictive and liberal types of perioperative fluid administration are associated with an increased risk of postoperative pulmonary complications and increased 30-day mortality (8). Our previous study also indicated both restrictive and liberal intraoperative fluid administration to be related to adverse effects on postoperative outcomes in patients undergoing minimally invasive lobectomy (12). In our previous study, postoperative pneumonia was defined as a new pulmonary infiltrate on chest X-ray with leukocytosis and fever (ear temperature >37.5 ℃) (12). However, after the article was published, some readers pointed out that the incidence of postoperative pneumonia (37.9%) was too high. Therefore, in this study, the diagnosis of postoperative pneumonia was made if a new pulmonary infiltrate, with leukocytosis and fever (ear temperature >38.0 ℃), was evident on chest X-ray. This change led to the absence of a significant correlation between the infusion rates of intraoperative total fluid and postoperative pulmonary complications in multivariate analysis (Table S8), but we did find that the total intravenous crystalloid infusion in the 24-hour postoperative period was significantly associated with postoperative pulmonary complications. Our current study further suggests that moderation rather than extremes of fluid balance in the 24-hour postoperative period produces optimal outcomes.
Another finding of this study was that postoperative pneumonia, atelectasis, prolonged air leak, and failure of the lung to expand were the main 4 postoperative pulmonary complications in patients undergoing minimally invasive lobectomy, with incidences of 19.5%, 2.3%, 2.3%, and 2.3%, respectively (Table S1). Binary logistics regression analysis showed significant differences in the incidence of postoperative pneumonia across the three groups, with the result of the moderate group being the best (Table 2). This statistical difference regarding postoperative pneumonia was similar to the difference regarding the total postoperative pulmonary complications among the three groups of different total intravenous crystalloid infusion in the 24-hour postoperative period.
In recent years, the safety of perioperative fluid restriction has been questioned by many clinicians (15), with AKI being considered as the main adverse outcome of perioperative fluid restriction (16,18). AKI can be induced by renal hypoperfusion, and inadequate perioperative fluid administration is detrimental to kidney function (19,20). However, in our study, there were no differences in postoperative AKI incidence among the groups treated with the restrictive, moderate, and liberal 24-hour postoperative fluid therapies. This may be related to our research population. For one, we excluded patients with preoperative renal dysfunction, so the included cases had a good reserve of renal function. For another, all patients began to eat freely after 6–8 hours of postoperative recovery and therefore were unlikely to experience excessive organ hypoperfusion.
There were no deaths during hospitalization in this study, and this result is consistent with a previous report (3). Differences in the incidence of postoperative pulmonary complications did not result in significant differences in readmission within 30 days, prolonged hospital stay, postoperative length of stay, or total hospital care costs among the groups treated with the restrictive, moderate, or liberal 24-hour postoperative fluid therapy (Table 2). This may be because, among the pulmonary complications, pneumonia has the highest incidence. Most of the patients with postoperative pneumonia recovered quickly after short-term antibiotic treatment, which did not affect the postoperative length of stay. However, the diagnosis of postoperative pneumonia, especially the occurrence of postoperative fever >38.0 ℃, may cause discomfort and anxiety in the patient and should be paid attention to by the surgeon.
Limitations
Several limitations in our study should be noted. First, due to the retrospective nature of our current analyses, our findings should be regarded as step toward hypothesis generation, and a causal relationship between the postoperative fluid therapy and risk of postoperative complications could not be determined. Theoretically, the amount of fluid therapy can be greatly affected by postoperative complications. Therefore, we only selected 24-hour postoperative fluid therapy as an exposure variable, and most of the postoperative complications did not occur during the 24 hours postoperatively. Second, the American Society of Anesthesiologists (ASA) score, diabetes mellitus, and length of operation among groups were uneven. This imbalance can only be resolved through a prospective randomized trial. Therefore, we included all the above variables as potential confounders in the multivariate regression model to obtain reliable results. Third, we did not count the total fluid intake in the 24-hour postoperative period because the amount of oral fluid was not recorded in the medical records. However, the most important influence on cardiopulmonary function is the intravenous fluid infusion, and the effect of oral fluid is limited. Finally, we could not control the perioperative administration of drugs. The crystalloid used after surgery includes 5% glucose or 0.9% NaCl. In addition to these fluids, each patient was treated with antibiotics and the antiphlegmatic drug Mucosolvan until the removal of chest tubes; these actions were closely related to postoperative pulmonary complications. Although our study has these drawbacks, because the patients are a relatively homogeneous population and were treated uniformly at a single center, we believe that our data and the analysis results are reliable. To address these limitations, a subsequent, well-designed, randomized controlled study is necessary.
Conclusions
In conclusion, total intravenous crystalloid infusion in the 24-hour postoperative period was associated with postoperative pulmonary complications in patients with NSCLC undergoing VATS lobectomy. We have found both the restrictive and liberal 24-hour postoperative crystalloid infusions to be related to adverse effects on postoperative outcomes. In patients undergoing VATS lobectomy, the optimal volume of 24-hour postoperative intravenous crystalloid infusion is 1,080–<1,410 mL. Our current study provides evidence for clinicians to reconsider the management of postoperative fluid during VATS lobectomy.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (No. 31700690), the Zhejiang Provincial Natural Science Foundation (No. LQ19H160023), and the Project of Clinical Scientific Research of Zhejiang Medical Association (No. 2018ZYC-A19).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-707/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-707/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-707/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The Medical Ethics Committee of the First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China, approved the study (No. 2017-58). Due to the retrospective research method used in this study, no intervention was taken, and the Medical Ethics Committee agreed to waive the patients' informed consent. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gooseman MR, Brunelli A. Intraoperative Lymph Node Management During Non-small Cell Lung Cancer Surgery. Ann Surg Oncol 2021;28:6925-6. [Crossref] [PubMed]
- Xu J, Ni H, Wu Y, et al. Perioperative comparison of video-assisted thoracic surgery and open lobectomy for pT1-stage non-small cell lung cancer patients in China: a multi-center propensity score-matched analysis. Transl Lung Cancer Res 2021;10:402-14. [Crossref] [PubMed]
- Suzuki K, Saji H, Aokage K, et al. Comparison of pulmonary segmentectomy and lobectomy: Safety results of a randomized trial. J Thorac Cardiovasc Surg 2019;158:895-907. [Crossref] [PubMed]
- Arslantas MK, Kara HV, Tuncer BB, et al. Effect of the amount of intraoperative fluid administration on postoperative pulmonary complications following anatomic lung resections. J Thorac Cardiovasc Surg 2015;149:314-20, 321.e1.
- Tsukazan MTR, Terra RM, Vigo Á, et al. Video-assisted thoracoscopic surgery yields better outcomes than thoracotomy for anatomical lung resection in Brazil: a propensity score-matching analysis using the Brazilian Society of Thoracic Surgery database. Eur J Cardiothorac Surg 2018;53:993-8. [Crossref] [PubMed]
- Messina A, Robba C, Calabrò L, et al. Association between perioperative fluid administration and postoperative outcomes: a 20-year systematic review and a meta-analysis of randomized goal-directed trials in major visceral/noncardiac surgery. Crit Care 2021;25:43. [Crossref] [PubMed]
- Teixeira-Neto FJ, Valverde A. Clinical Application of the Fluid Challenge Approach in Goal-Directed Fluid Therapy: What Can We Learn From Human Studies? Front Vet Sci 2021;8:701377. [Crossref] [PubMed]
- Shin CH, Long DR, McLean D, et al. Effects of Intraoperative Fluid Management on Postoperative Outcomes: A Hospital Registry Study. Ann Surg 2018;267:1084-92. [Crossref] [PubMed]
- Hansen B. Fluid Overload. Front Vet Sci 2021;8:668688. [Crossref] [PubMed]
- Kayilioglu SI, Dinc T, Sozen I, et al. Postoperative fluid management. World J Crit Care Med 2015;4:192-201. [Crossref] [PubMed]
- Miller TE, Mythen M, Shaw AD, et al. Association between perioperative fluid management and patient outcomes: a multicentre retrospective study. Br J Anaesth 2021;126:720-9. [Crossref] [PubMed]
- Wu Y, Yang R, Xu J, et al. Effects of intraoperative fluid management on postoperative outcomes after lobectomy. Ann Thorac Surg 2019;107:1663-9. [Crossref] [PubMed]
- Mitrosz-Gołębiewska K, Rydzewska-Rosołowska A, Kakareko K, et al. Water - A life-giving toxin - A nephrological oxymoron. Health consequences of water and sodium balance disorders. A review article. Adv Med Sci 2022;67:55-65. [Crossref] [PubMed]
- Noba L, Rodgers S, Chandler C, et al. Enhanced Recovery After Surgery (ERAS) Reduces Hospital Costs and Improve Clinical Outcomes in Liver Surgery: a Systematic Review and Meta-Analysis. J Gastrointest Surg 2020;24:918-32. [Crossref] [PubMed]
- Pang Q, Liu H, Chen B, et al. Restrictive and liberal fluid administration in major abdominal surgery. Saudi Med J 2017;38:123-31. [Crossref] [PubMed]
- Messina A, Robba C, Calabrò L, et al. Perioperative liberal versus restrictive fluid strategies and postoperative outcomes: a systematic review and metanalysis on randomised-controlled trials in major abdominal elective surgery. Crit Care 2021;25:205. [Crossref] [PubMed]
- Li M, Peng M. Prospective comparison of the effects of intraoperative goal-directed fluid therapy and restrictive fluid therapy on complications in thoracoscopic lobectomy. J Int Med Res 2021;49:3000605211062787. [Crossref] [PubMed]
- Bundgaard-Nielsen M, Secher NH, Kehlet H. 'Liberal' vs. 'restrictive' perioperative fluid therapy--a critical assessment of the evidence. Acta Anaesthesiol Scand 2009;53:843-51. [Crossref] [PubMed]
- Zhao BC, Lei SH, Yang X, et al. Assessment of prognostic value of intraoperative oliguria for postoperative acute kidney injury: a retrospective cohort study. Br J Anaesth 2021;126:799-807. [Crossref] [PubMed]
- Ghoreifi A, Basin MF, Ghodoussipour S, et al. Perioperative outcomes of goal-directed versus conventional fluid therapy in radical cystectomy with enhanced recovery protocol. Int Urol Nephrol 2021;53:1827-33. [Crossref] [PubMed]
(English Language Editor: J. Grey)


