Comparison of ticagrelor and clopidogrel in the treatment of patients with coronary heart disease carrying CYP2C19 loss of function allele
Introduction
Coronary heart disease (CHD) is a pathological process of myocardial ischemia, hypoxia or necrosis, ranking first in the global causes of death (1). Age, smoking, blood pressure and total cholesterol are the traditional risk factors of CHD, with lipid-related components, metabolism-related factors, inflammatory factors, gene polymorphism and psychological factors as new risk factors of CHD (2).
Clopidogrel is a traditional P2Y12 inhibitor that can inhibit platelet activation and aggregation (3). However, clinical study of platelet function show that 16–50% of patients have either clopidogrel resistance or a low response, and these patients are still prone to adverse cardiovascular events even if they are treated with a standard dose of clopidogrel (4). Clopidogrel resistance is affected by many genetic and non-genetic factors, and CYP2C19 gene polymorphism is considered to be an independent predictor of this phenomenon (5). It was found that CYP2C19 gene expression and activation decreased, clopidogrel active metabolites decreased, and platelet aggregation inhibition was weakened (6). Patients carrying the loss of function (LOF) allele (CYP2C19*2–*8) have an increased risk of adverse cardiovascular events (e.g., cardiovascular death, myocardial infarction, and stent thrombosis) compared with normal metabolizers (7). However, patients with CHD who underwent percutaneous coronary intervention (PCI) and clopidogrel antithrombotic therapy had a higher risk of major bleeding events when carrying one or more CYP2C19*17 gene mutations (8). In the US Food and Drug Administration clopidogrel drug description updated in September 2016, it was again suggested that the effectiveness of clopidogrel may decrease in patients with poor CYP2C19 metabolism compared with patients with normal CYP2C19 function, suggesting that a different platelet P2Y12 inhibitor should be used for such patients (9).
Prasugrel is also a P2Y12 receptor inhibitor that inhibits platelet activation and aggregation faster and more strongly than clopidogrel. Pragrel was used in the early stage of TROPICAL study, followed by clopidogrel, which confirmed the feasibility and safety of individualized antiplatelet therapy by detecting platelet function in patients with CHD after PCI. The Triton-Timi38 study showed that prasugrel significantly reduced cardiovascular adverse events up to 15 months compared to clopidogrel. However, prasugrel is also a precursor drug, which is affected by CYP2C19 gene polymorphism and has a 4-fold increase in bleeding events compared with clopidogrel. Patients over 75 years of age and weight less than 60 kg received prasugrel and had no significant benefit compared with clopidogrel. Currently, it is not listed in China (10).
Ticagrelor is a novel antiplatelet agent of the cyclopentyl triazole pyrimidines, and has strong and reversible inhibitory effects on platelet activation and aggregation. Compared with clopidogrel, ticagrelor has the advantages of faster onset of action and faster recovery of platelet function after withdrawal. Except for the cautious use of ticagrelor in people with higher blood risk, the main adverse reactions are dyspnea caused by bronchospasm, which is more common in the early stage of medication. Because discontinuation of oral antiplatelet drugs or conversion to other antiplatelet drugs will increase the risk of recurrence of adverse cardiovascular events, clinical use of ticagrelor should be cautious (1).
CUHRENT-OASIS 7 showed that patients receiving a double dose of clopidogrel (600 mg on day 1, 150 mg on days 2–7, and then 75 mg/d) were significantly reduced the incidence of major adverse cardiovascular event (MACE) and the formation of stent thrombosis than patients receiving a standard dose of clopidogrel (300 mg on day 1, then 75 mg/d). However, the GRAVITAS trial has shown little benefit from increasing clopidogrel dose. It enrolled 2,214 patients who showed high platelet response after dual antiplatelet therapy (DAPT) after PCI and randomized them to either a high dose of clopidogrel (150 mg/d) or a standard dose of clopidogrel (75 mg/d). There was no statistically significant difference in the incidence of MACE between the two groups (11). Although the results of these genotype-guided clopidogrel dose-escalation studies are disappointing, the ineffectiveness of clopidogrel dose-escalation regiments cannot be fully demonstrated.
A systematic review and meta-analysis in 2020 involved 5,829 cardiovascular patients with CYP2C19 LOF allele suggested that alternative antiplatelet treatments instead of clopidogrel based on genotyping test can induce better clinical outcomes on LOF allele carriers; however, this medication should be tailored according to the balance between patients’ ischemic and bleeding risk (12).
Because different individuals have different responses to clopidogrel, according to their CYP2C19 gene type (13), patients carrying one or two CYP2C19 LOF alleles require an increased dose of clopidogrel (loading dose is 300 or 600 mg) or be changed to ticagrelor/prasugrel to achieve the effect of blocking platelet aggregation. In this study, the full length of CYP2C19 related to clopidogrel metabolism was analyzed by molecular labeling technology and 10XGenomics technology platform combined with gene capture and sequencing technology, and the CYP2C19 genotype of some CHD patients was detected to predict the efficacy of antithrombotic therapy. Most previous researchers will set the end point as MACE, may ignore the part who have a progression of disease but does not meet the end of MACE. In this study, symptoms occur repeatedly or the coronary arterial lesion aggravate is considered a poor prognosis, we try to identified more patients with CYP2C19 LOF alleles and slow drug metabolism. Providing genetic information support for subsequent personalized antiplatelet therapy programs will ultimately achieve effective inhibition of platelet activation and aggregation, reduce drug risk, and increase the effectiveness and safety of antithrombotic therapy. We present the following article in accordance with the MDAR reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-740/rc).
Methods
Patients
The study group comprised 170 patients diagnosed with CHD and regularly receiving oral clopidogrel or ticagrelor antiplatelet therapy in the Department of Cardiology of Wuxi Second People’s Hospital were screened between August and December 2019. The inclusion criteria were: (I) signed written informed consent to participate in the study; (II) male or female aged ≥18 years on the date of signing informed consent; (III) stable CHD or acute coronary syndrome (ACS) confirmed by coronary angiography (CAG) or coronary computed tomography angiography (CTA); and (IV) clopidogrel (Plavix 75 mg/day or Talcom 50 mg/day) or ticagrelor (Brilinta 180 mg/day) combined with or without aspirin according to the judgment of clinicians. Exclusion criteria were: (I) contraindications of clopidogrel and ticagrelor; (II) awaiting heart transplantation or continuous intravenous infusion of positive inotropic drugs, or have/plan to implant ventricular assist device; and (III) serious cardiac complications or non-cardiogenic heart disease with life expectancy <1 year. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the Wuxi Second People’s Hospital of Nanjing Medical University (No. Y-50). All subjects gave written informed consent.
Study methods
The baseline data of the 170 patients were collected and their percutaneous CAG or coronary CTA results were recorded. The type, dose, frequency and time of oral drugs were recorded.
A 2-mL fasting venous blood sample was taken into an EDTA Na2 anticoagulant tube, and genomic DNA was extracted. PCR amplification and hybridization were performed using a CYP2C19 gene detection kit (YZY Biopharma, Wuhan, China). After the reaction, gene chip image analysis software was used to conduct image scanning and data analysis of the gene chip, and the detection results were obtained. After discharge, the patients were followed up for 12 months by telephone/outpatient/inpatient for assessment of prognosis and outcome.
Statistical analysis
SPSS26.0 software was used for statistical analysis. Measurement data with a normal distribution are expressed as (), and t-test was used for comparison between groups. The chi-square test was used for comparison between groups of qualitative data. Measurement data with a non-normal distribution are represented by the median (P25, P75) and the rank sum test was used. Multivariate analysis was performed by logistic regression. P<0.05 was considered statistically significant.
Results
Of the 170 patients with CHD, 93 took clopidogrel orally as an antiplatelet drug. After 12 months of follow-up, 13 patients were lost to follow-up, and 80 patients completed the experimental observation. The other 77 patients took ticagrelor orally as the antiplatelet drug and after 12 months of follow-up, 4 patients were lost to follow-up, 1 patient ceased the study due to massive gastrointestinal bleeding, and 72 patients completed the experimental observation.
CYP2C19 genotyping
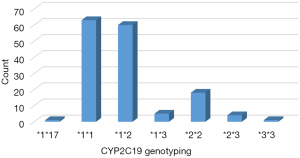
Among the 152 patients who completed the experimental observation, CYP2C19 genotyping revealed that 1 patient had the fast metabolic type (*1*17), accounting for 0.66%, and 63 had the normal metabolic type (*1*1), accounting for 41.45%; 42.76% had 1 LOF allele, namely the intermediate metabolic type (65 cases in total, 60 patients were *1*2, 5 patients were *1*3), and 15.13% patients carried 2 LOF alleles, namely the poor metabolic type (23 cases in total, 18 patients were *2*2, 4 patients were *2*3, 1 patient was *3*3) (Figures 1,2). Among the 89 patients with loci mutations, 82 had *2 mutation, 10 patients had *3 mutation, and 1 patient had *17 mutation. The *2 mutation accounted for 89.29% of all loci mutations, the *3 mutation accounted for 9.82%, and the *17 mutation accounted for only 0.89% (Figure 3).


CYP2C19 genotyping in the clopidogrel group
Among the 80 CHD patients who regularly took oral clopidogrel, the genotyping results were as follows: 1 patient had a mutation of the *17 gene, 41 patients had a mutation of the *2 gene, and 3 patients had a mutation of the *3 gene. Considering that the *2 and *3 alleles account for >99% of LOF alleles in the Asian population, the patient with the fast metabolic type (*1*17) was excluded from the study, and the remaining 79 patients were defined as the clopidogrel group. Based on the number of LOF alleles, they were divided into the normal metabolism (NM), intermediate metabolism (IM) and poor metabolism (PM) subgroups.
Comparison of the patients’ general information
The mean age of the 79 patients was 69.75±9.49 years (52 male, 27 female). There were no significant differences in age, body weight, body mass index (BMI), hypertension, diabetes, smoking history, family history of CHD, oral aspirin, lipid-lowering drugs, proton pump inhibitors (PPIs) and other indicators among three groups (P>0.05) (Table 1).
Table 1
| Items | Total (n=79) | NM (n=37) | IM (n=28) | PM (n=14) | P |
|---|---|---|---|---|---|
| Age (years), | 69.75±9.49 | 69.43±10.27 | 70.25±9.78 | 69.57±7.01 | 0.941 |
| Sex (male), n (%) | 52 (65.8) | 29 (78.4) | 16 (57.1) | 7 (50.0) | 0.079 |
| Weight (kg), | 65.96±10.31 | 67.61±10.24 | 67.71±10.67 | 64.11±9.85 | 0.411 |
| BMI (kg/m2), | 23.99±3.06 | 24.12±2.92 | 23.51±3.55 | 24.63±2.35 | 0.513 |
| Hypertension, n (%) | 63 (79.7) | 29 (78.4) | 22 (78.6) | 12 (85.7) | 0.829 |
| Diabetes, n (%) | 24 (30.4) | 14 (37.8) | 4 (14.3) | 6 (42.9) | 0.066 |
| Smoking history, n (%) | 28 (35.4) | 17 (45.9) | 8 (28.6) | 3 (21.4) | 0.168 |
| Family history, n (%) | 3 (3.8) | 1 (2.7) | 0 (0.0) | 2 (14.3) | 0.066 |
| Oral drugs taken concurrently during follow-up, n (%) | |||||
| Aspirin | 41 (51.9) | 17 (45.9) | 13 (53.6) | 5 (64.3) | 0.492 |
| Lipid-lowering drug | 76 (96.2) | 37 (100.0) | 26 (92.9) | 13 (92.9) | 0.253 |
| PPI | 30 (38.0) | 13 (35.1) | 10 (35.7) | 7 (50.0) | 0.592 |
NM, normal metabolism; IM, intermediate metabolism; BMI, body mass index; PM, poor metabolism; PPI, proton pump inhibitor.
Prognosis of clopidogrel patients with different CYP2C19 genotypes
Of the 79 patients, 37 (46.8%) were NM (*1*1), 28 (6.3%) were IM (*1*2, *1*3) and 14 (17.7%) were PM (*2*2, *2*3, *3*3). Chi-square test was used to compare the prognosis of patients with different genotypes, and the difference was statistically significant (P<0.05) (Table 2). There were significant differences between NM and IM (χ2=9.697; P=0.002), and between NM and PM (χ2=4.255; P=0.039). There was no significant difference between IM and PM (χ2=0.014; P=0.906) (Figure 4).
Table 2
| Group | N | Good prognosis, n (%) | Poor prognosis, n (%) | χ2 | P |
|---|---|---|---|---|---|
| NM | 37 | 25 (67.6) | 12 (32.4) | 10.755 | 0.005 |
| IM | 28 | 8 (28.6) | 20 (71.4) | ||
| PM | 14 | 5 (35.7) | 9 (64.3) |
NM, normal metabolism; IM, intermediate metabolism; PM, poor metabolism.

Multifactor logistic regression analysis
With the prognosis of the clopidogrel group as the dependent variable, logistic regression analysis was performed on the patients’ age, sex, BMI, hypertension, diabetes, smoking history, family history, CYP2C19 genotype and other factors as independent variables. It was found that CYP2C19 genotype correlated with the prognosis of patients (P<0.05), whereas age, sex, BMI, hypertension, diabetes, smoking history and family history negatively correlated with prognosis (Table 3).
Table 3
| Factors | Wald χ2 | P | OR | OR (95% CI) |
|---|---|---|---|---|
| Age | 1.509 | 0.219 | ||
| Sex | 1.987 | 0.159 | ||
| BMI | 0.808 | 0.369 | ||
| Hypertension | 0.498 | 0.48 | ||
| Diabetes | 0.897 | 0.343 | ||
| Smoking history | 2.173 | 0.14 | ||
| Family history | 0.613 | 0.434 | ||
| CYP2C19 genotype classification | ||||
| NM | 10.195 | |||
| IM | 9.129 | 0.003 | 5.208 | 1.786–15.192 |
| PM | 4.021 | 0.045 | 3.75 | 1.030–13.648 |
BMI, body mass index; NM, normal metabolism; IM, intermediate metabolism; PM, poor metabolism; OR, odds ratio; CI, confidence interval.
Comparison of ticagrelor and clopidogrel in the treatment of CHD
The 72 patients with CHD who took ticagrelor and completed the observation were defined as ticagrelor group and compared with the clopidogrel group.
Comparison of patient data
There were no significant differences between the clopidogrel and ticagrelor groups for body weight, BMI, hypertension, diabetes, family history, concurrent oral lipid-lowering agents, CYP2C19 genotype and other indicators (P>0.05). There were statistically significant differences in age, sex, smoking history, concurrent oral aspirin and PPI, and undergoing PCI (P<0.05) (Table 4).
Table 4
| Items | Total (n=151) | Clopidogrel group (n=79) | Ticagrelor group (n=72) | P |
|---|---|---|---|---|
| Age (years), | 66.73±10.81 | 69.75±9.49 | 63.42±11.26 | 0.001 |
| Sex (male), n (%) | 113 (74.8) | 52 (65.8) | 61 (84.7) | 0.008 |
| Weight (kg), | 67.11±10.26 | 65.96±10.31 | 68.36±10.13 | 0.152 |
| BMI (kg/m2), | 24.19±3.02 | 23.99±3.06 | 24.40±2.98 | 0.407 |
| Hypertension, n (%) | 117 (77.5) | 63 (79.7) | 54 (75.0) | 0.485 |
| Diabetes, n (%) | 51 (33.8) | 24 (30.4) | 27 (37.5) | 0.355 |
| Smoking history, n (%) | 76 (50.3) | 28 (35.4) | 48 (66.7) | 0.001 |
| Family history, n (%) | 6 (4.0) | 3 (3.8) | 3 (4.2) | 1.000 |
| Oral drugs taken concurrently during follow-up, n (%) | ||||
| Aspirin | 110 (72.8) | 41 (51.9) | 69 (95.8) | 0.000 |
| Lipid-lowering drug | 147 (97.4) | 76 (96.2) | 71 (98.6) | 0.679 |
| PPI | 69 (45.7) | 30 (38.0) | 39 (54.2) | 0.046 |
| Aspirin | 107 (71.8) | 40 (51.9) | 67 (93.1) | 0.000 |
| CYP2C19 genotype, n (%) | 0.140 | |||
| NM | 63 (41.7) | 37 (46.8) | 26 (36.1) | |
| IM | 65 (43.0) | 28 (35.4) | 37 (51.4) | |
| PM | 23 (15.2) | 14 (17.7) | 9 (12.5) |
BMI, body mass index; PPI, proton pump inhibitor; NM, normal metabolism; IM, intermediate metabolism; PM, poor metabolism.
Multifactor logistic regression analysis
Multivariate logistic regression analysis was performed using the patient’s prognosis as a dependent variable, and after removing the influence of age, sex, smoking history, and concurrent oral aspirin and PPI, there was no significant correlation between clopidogrel or ticagrelor antiplatelet therapy and the prognosis of patients with CHD (χ2=2.119; P=0.146>0.05) (Table 5).
Table 5
| Factors | Wald χ2 | P | OR | OR (95% CI) |
|---|---|---|---|---|
| Age | 6.249 | 0.012 | 1.042 | 1.009–1.076 |
| Sex | 0.399 | 0.527 | ||
| Smoking history | 0.778 | 0.378 | ||
| Aspirin | 1.694 | 0.193 | ||
| PPI | 1.422 | 0.233 | ||
| PCI operation | 2.117 | 0.146 | ||
| Ticagrelor/clopidogrel | 2.119 | 0.146 |
OR, odds ratio; CI, confidence interval; PPI, proton pump inhibitor; PCI, percutaneous coronary intervention.
Subgroup analysis
According to CYP2C19 genotype classification, the ticagrelor and clopidogrel groups were divided into three subgroups. There was no statistical difference in the prognosis of patients with CHD taking either ticagrelor regularly or regularly in the NM group or PM group. In the IM group, prognosis of CHD patients was significantly associated with regular use of ticagrelor or clopidogrel (Table 6).
Table 6
| Items | Sum, n (%) | Good prognosis, n (%) | Poor prognosis, n (%) | χ2 | P |
|---|---|---|---|---|---|
| NM | 0.244 | 0.621 | |||
| Ticagrelor group | 26 (41.3) | 16 (61.5) | 10 (38.5) | ||
| Clopidogrel group | 37 (58.7) | 25 (67.6) | 12 (32.4) | ||
| IM | 9.697 | 0.002 | |||
| Ticagrelor group | 37 (56.9) | 25 (67.6) | 12 (32.4) | ||
| Clopidogrel group | 28 (43.1) | 8 (28.6) | 20 (71.4) | ||
| PM | 0.878 | 0.417 | |||
| Ticagrelor group | 9 (39.1) | 5 (55.6) | 4 (44.4) | ||
| Clopidogrel group | 14 (60.9) | 5 (35.7) | 9 (64.3) |
NM, normal metabolism; IM, intermediate metabolism; PM, poor metabolism.
PCI was performed in 93.1% of patients in the ticagrelor group and in only 51.9% of patients in the clopidogrel group. The prognosis of patients who underwent PCI significantly correlated with regular use of ticagrelor or clopidogrel (Figure 5), whereas the prognosis of patients who did not undergo PCI did not correlate with regular use of ticagrelor or clopidogrel (Figure 6).


Discussion
Clopidogrel is thiophenpyridine antiplatelet prodrug that requires CYP2C19 for its bioactivation: 44.9% in the first step and 20.6% in the second step of activation. The wild-type *1 is the functional allele of CYP2C19-mediated metabolism; the homozygous wild-type *1*1 has sufficient drug metabolic capacity, and such patients are NMs. Patients with poor drug metabolism usually have one or two defective alleles, *2 and *3 being the most common and dominant mutated genes causing reduced enzyme activity. It is *2 mutations that are most common in South Asia (32.5%) and East Asia (31%), followed by Africa (18%), non-Finnish Europe (15%) and Latin America (10%). *3 mutations are rare in Europe and Africa (0.025% and 0.037%, respectively), less common in South Asia (0.4%), but more common in East Asia (6.3%) (6,8). In this study, 152 patients with CHD routinely treated with clopidogrel or ticagrelor as antiplatelet therapy were enrolled for CYP2C19 gene detection. Among them, 1 patient carried the gene mutation with ultra-fast activity, that is, *17; 42.76% patients carried 1 LOF allele, 15.13% patients carried 2 LOF alleles, among which *2 and *3 mutations accounted for more than 99% of all mutations, which was similar to the results of CYP2C19 gene polymorphism in Asian population reported in relevant study (6).
Related studies showed that the incidence of clopidogrel resistance in different populations is 4–44% (14,15). Among the 79 CHD patients participating in this study who took clopidogrel regularly and did not carry the mutation of *17 gene, 41 patients experienced relapse or exacerbation of disease, with an incidence of 51.9%. Among them, the incidence of poor prognosis was 32.4% in the NM group, 71.4% in the IM group, and 64.3% in the PM group. There was statistical difference in prognosis among the three groups. After adjusting for age, sex, BMI, hypertension, diabetes, smoking and family histories and other factors, logistic regression analysis found that patients carrying a CYP2C19 LOF allele had a significantly increased risk of typical angina symptoms, stent thrombosis or exacerbation of coronary artery stenosis. The risk in carrying one CYP2C19 LOF allele was 5.208-fold higher than that of non-carriers, and the risk in carrying two CYP2C19 LOF alleles was 3.75-fold higher than that of non-carriers. There was no significant difference between carriage of two CYP2C19 LOF alleles or one CYP2C19 LOF allele. In a 2010 meta-analysis, Mega et al. found that among 9,685 CHD patients taking clopidogrel antiplatelet therapy, those carrying 1 LOF allele had a 1.55-fold increased risk of MACE compared with the homozygous wild-type *1*1. Those who had two LOF alleles had a 1.76-fold increased risk compared with those who did not. The risk of stent thrombosis also increased, 2.67-fold for the heterozygous type and 3.97-fold for the homozygous type (16). Studies have shown that the bioavailability of clopidogrel in PMs is 3-fold higher than that in NMs, and the bioavailability of clopidogrel’s active metabolites is only 30% of that in NMs. Therefore, the inhibitory effect of antiplatelet drugs in PMs is 10–30% lower than in NMs, and the results of this study were basically in line with those expectations (17-19). Previous study has shown that although there is no statistical difference in the incidence of adverse cardiovascular events between the IM and PM groups, those who carry two LOF alleles often have a higher incidence of MACE (20) and the increased risk was more significant than that of non-carriers. In this study, the results were not consistent with this, which may be related to the small number of subjects included, especially the small sample size of PMs and the large sampling error.
There have been some studies of alternative therapies for CHD patients carrying *2 and/or *3 mutations. In the PLATO study [2009] (21), a total of 18,624 patients with middle and high-risk ACS received clopidogrel and ticagrelor antithrombotic therapy respectively for 12 months, and the results showed that compared with clopidogrel, ticagrelor significantly reduced the incidence of cardiogenic death and adverse cardiovascular events in these ACS patients. Ticagrelor does not increase the risk of major bleeding (12). In the present study, 72 CHD patients who received standard antithrombotic therapy with ticagrelor were compared with 79 CHD patients who received standard antiplatelet therapy with clopidogrel. The results suggested that for all patients with CHD included in the study, taking ticagrelor did not significantly improve the prognosis compared with taking clopidogrel, which was not consistent with the finding of the PLATO study that the efficacy of ticagrelor was significantly better than that of clopidogrel (21), possibly because the present study was not a randomized controlled trial. In this study, patients with CHD were diagnosed and given antiplatelet therapy with ticagrelor or clopidogrel according to the judgment of clinicians outside the experimental group. In the ticagrelor group compared with the clopidogrel group, there were more male patients, smokers, and more cases of ACS. PCI was performed in 93.1% of patients in the ticagrelor group compared with 51.9% in the clopidogrel group. Patients in the ticagrelor group were sicker and expected to have a poorer prognosis than those in the clopidogrel group, which may have offset the superior efficacy of ticagrelor. However, when the patients with CHD were limited to those undergoing PCI, the risk of an adverse prognosis was significantly reduced with ticagrelor compared with clopidogrel, and ticagrelor had the same effect as clopidogrel in patients with CHD without PCI. Therefore, for patients with CHD treated with PCI, regular administration of ticagrelor has a better inhibitory effect on platelet aggregation.
We further compared the prognosis for the CYP2C19 genotypes. There was a significant difference in the clinical efficacy of replacing the antiplatelet therapy with ticagrelor for patients in the IM group. For CHD patients in the NM group and PM group, there was no statistical difference in prognosis between regular administration of ticagrelor and regular administration of clopidogrel; that is, for CHD patients carrying one CYP2C19 mutation, regular administration of ticagrelor after discharge can achieve better clinical efficacy. For CHD patients without a CYP2C19 mutation or with two CYP2C19 mutations, regular clopidogrel administration did not increase the incidence of an adverse prognosis compared with ticagrelor. In this study, there were 23 people in the PM group, which was a small sample size and the error was large, which may affect the experimental results. However, previous study by Cavallari et al. (22) also pointed out that in CHD patients carrying a single LOF allele, the difference in the incidence of adverse cardiovascular events was more significant between the ticagrelor group and the clopidogrel group. Therefore, for patients with two LOF alleles, the clinical benefits of an alternative therapy to clopidogrel need to be further confirmed. In addition, in this study, there were significant differences between the ticagrelor and clopidogrel groups in age, sex, smoking history, and concurrent oral aspirin and PPI, which may have led to bias in the experimental results.
Conclusions
A total of 0.66% of patients carried one gene mutation with ultra-fast activity (i.e., *17), 42.76% of patients carried one LOF allele *2 or *3, and 15.13% of patients carried two LOF alleles, among which *2 and *3 mutations accounted for >99% of all mutations.
CYP2C19 polymorphism was associated with the prognosis of patients with CHD taking clopidogrel antiplatelet therapy. When patients with CHD carry *2 and/or *3 mutations, the incidence of poor prognosis after oral clopidogrel treatment significantly increased, and the risk was doubled compared with that of non-carriers.
Patients with CHD and a single LOF allele who take ticagrelor have a better prognosis than those who take clopidogrel. In CHD patients with no LOF allele or CHD patients with two LOF alleles, the clinical benefits of antiplatelet therapy with ticagrelor instead of clopidogrel were not significant.
For patients with CHD treated with PCI, the prognosis of those treated with ticagrelor as replacement therapy was better. For patients with CHD who did not undergo PCI, the clinical benefits of antiplatelet therapy with ticagrelor instead of clopidogrel were not significant.
Acknowledgments
Funding: This work was supported by the Science and Technology Projects of Wuxi City (Nos. WX18IVJN016, Z201804).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-740/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-740/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-740/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the Wuxi Second People’s Hospital of Nanjing Medical University (No. Y-50). All subjects gave written informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1789-858. [Crossref] [PubMed]
- Guo C, Cao H, Shan G, et al. Elevated lipoprotein(a) and risk of coronary heart disease according to different lipid profiles in the general Chinese community population: the CHCN-BTH study. Ann Transl Med 2021;9:26. [Crossref] [PubMed]
- Neumann FJ, Hochholzer W, Siepe M. ESC/EACTS guidelines on myocardial revascularization 2018: The most important innovations. Herz 2018;43:689-94. [Crossref] [PubMed]
- Sienkiewicz-Oleszkiewicz B, Wiela-Hojeńska A. CYP2C19 polymorphism in relation to the pharmacotherapy optimization of commonly used drugs. Pharmazie 2018;73:619-24. [PubMed]
- Jia DM, Chen ZB, Zhang MJ, et al. CYP2C19 polymorphisms and antiplatelet effects of clopidogrel in acute ischemic stroke in China. Stroke 2013;44:1717-9. [Crossref] [PubMed]
- Shuldiner AR, O'Connell JR, Bliden KP, et al. Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA 2009;302:849-57. [Crossref] [PubMed]
- Amin AM, Sheau Chin L, Azri Mohamed Noor D, et al. The Personalization of Clopidogrel Antiplatelet Therapy: The Role of Integrative Pharmacogenetics and Pharmacometabolomics. Cardiol Res Pract 2017;2017:8062796. [Crossref] [PubMed]
- Ragia G, Arvanitidis KI, Tavridou A, et al. Need for reassessment of reported CYP2C19 allele frequencies in various populations in view of CYP2C19*17 discovery: the case of Greece. Pharmacogenomics 2009;10:43-9. [Crossref] [PubMed]
- Roden DM, Shuldiner AR. Responding to the clopidogrel warning by the US food and drug administration: real life is complicated. Circulation 2010;122:445-8. [Crossref] [PubMed]
- Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007;357:2001-15. [Crossref] [PubMed]
- Price MJ, Berger PB, Teirstein PS, et al. Standard- vs high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the GRAVITAS randomized trial. JAMA 2011;305:1097-105. [Crossref] [PubMed]
- Yoon HY, Lee N, Seong JM, et al. Efficacy and safety of clopidogrel versus prasugrel and ticagrelor for coronary artery disease treatment in patients with CYP2C19 LoF alleles: a systemic review and meta-analysis. Br J Clin Pharmacol 2020;86:1489-98. [Crossref] [PubMed]
- Breet NJ, van Werkum JW, Bouman HJ, et al. Comparison of platelet function tests in predicting clinical outcome in patients undergoing coronary stent implantation. JAMA 2010;303:754-62. [Crossref] [PubMed]
- Müller I, Besta F, Schulz C, et al. Prevalence of clopidogrel non-responders among patients with stable angina pectoris scheduled for elective coronary stent placement. Thromb Haemost 2003;89:783-7. [Crossref] [PubMed]
- Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, et al. Platelet function profiles in patients with type 2 diabetes and coronary artery disease on combined aspirin and clopidogrel treatment. Diabetes 2005;54:2430-5. [Crossref] [PubMed]
- Mega JL, Simon T, Collet JP, et al. Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta-analysis. JAMA 2010;304:1821-30. [Crossref] [PubMed]
- Hulot JS, Collet JP, Cayla G, et al. CYP2C19 but not PON1 genetic variants influence clopidogrel pharmacokinetics, pharmacodynamics, and clinical efficacy in post-myocardial infarction patients. Circ Cardiovasc Interv 2011;4:422-8. [Crossref] [PubMed]
- Oh IY, Park KW, Kang SH, et al. Association of cytochrome P450 2C19*2 polymorphism with clopidogrel response variability and cardiovascular events in Koreans treated with drug-eluting stents. Heart 2012;98:139-44. [Crossref] [PubMed]
- Mega JL, Close SL, Wiviott SD, et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med 2009;360:354-62. [Crossref] [PubMed]
- Pereira NL, Rihal CS, So DYF, et al. Clopidogrel Pharmacogenetics. Circ Cardiovasc Interv 2019;12:e007811. [Crossref] [PubMed]
- Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009;361:1045-57. [Crossref] [PubMed]
- Cavallari LH, Lee CR, Beitelshees AL, et al. Multisite Investigation of Outcomes With Implementation of CYP2C19 Genotype-Guided Antiplatelet Therapy After Percutaneous Coronary Intervention. JACC Cardiovasc Interv 2018;11:181-91. [Crossref] [PubMed]
(English Language Editor: K. Brown)