Reresection for recurrent stenosis after primary tracheal repair
Introduction
The reported rate of tracheal restenosis after primary resection and reconstruction for benign tracheal disease is low, occurring in less than 4% of resections at our center (1). The incidence of unreported restenosis, however, may be higher. Treatment of tracheal restenosis involves careful consideration of whether the patient and the operative factors leading to initial failure may be modified. Most failed anastomoses can be temporarily stented with placement of T-tubes. Successful operative treatment of tracheal restenosis may be undertaken in selected patients in a meticulous fashion at high-volume centers.
Definition
Restenosis refers to events during and after primary tracheal reconstruction that lead to either immediate or delayed failure of the anastomosis. Late restenosis with symptoms occurring 3 or more months following operation is very uncommon and may indicate the presence of an underlying systemic disease involving the airway. The causes of early restenosis are limited and accessible to study. A recapitulation of the first operation, the consideration of all potential factors leading to failure and the opportunities to modify them in a future repair should therefore precede a second attempt at reconstruction.
Risk factors predisposing to tracheal restenosis
Any tracheal anastomosis is constructed under tension; the formation of a durable scar, good vascularization of the anastomosed tracheal ends, careful reduction of excessive tension and the absence of excessive anastomotic stress during recovery are all important for a good outcome. In 2014, a review of 94 patients who underwent tracheal resection found that 16% of patients had restenosis (2). At another center of experience, 7.1% of 450 patients had failure of primary tracheal resection and construction (1). For those collecting the experience for publication, the etiology of restenosis is often difficult to determine. Granulations developed in 20% of patients who presented with restenosis (1), but granulations are a phenomenon and not a cause. In patients without granulations (50%), failure was likely due to excessive tension and devascularization (1). Patient-specific factors such as diabetes, connective tissue disorders and poor nutrition also contributed to restenosis.
The risk for developing tracheal restenosis may be grouped into factors specific to the patient or to the operation (Tables 1,2). Patient-specific factors concern the preoperative use of steroids, for example for airway obstruction; diabetes, as it affects vascularity at the anastomosis; malnutrition, less common as resection for benign disease is usually elective; a young, immature airway; marked kyphosis, creating tension even when the resected airway is short; prior radiation; or, uncommonly, an unrecognized inflammatory or infectious disease.

Full table
Operation-specific factors involve the sum of judgments surrounding the conduct of the first operation and include the selection of unsuitable strictures, incomplete mobilization of the trachea or excessive circumferential dissection at the tracheal margins prepared for anastomosis, causing devascularization; excessive tension at the anastomosis when the stricture was long; and anastomotic inflammation resulting from suture-associated foreign body reaction with formation of granuloma (1,4,5). The latter issue is now mitigated by the use of absorbable braided or monofilament suture. In 2004, a single-institution, retrospective review of 901 patients who underwent tracheal resection found that reoperation, diabetes, long-segment (>4 cm) resection, laryngotracheal resection, age less than 17 years, need for preoperative tracheostomy and need for release maneuvers predicted anastomotic complications (3). In addition, incomplete resection at the time of initial surgery or the presence of tracheomalacia may manifest as tracheal restenosis.
In choosing patients who would benefit from reresection, a critical eye is needed to assess whether risk factors predisposing to tracheal restenosis present at the initial, failed operation can be reversed or modified before another attempt at a resection. The surgeon should note the history of initial presentation and operative details. Candidates suitable for another attempt at resection typically present with short strictures or an avoidable error of judgment during the first operation (Table 2). The perioperative care environment must be optimized, and patients likely benefit from undergoing treatment at an experienced, high-volume center.
Evaluation of tracheal restenosis
Patients with tracheal restenosis usually present within 2 weeks after their primary operation (1) with stridor and dyspnea from a narrowed airway. Anastomotic dehiscence may manifest early and dramatically with fever, pain, crepitus and abscess formation.
The history leading to the first operation and prior medications are reviewed. On examination, any airway obstruction, the location of any tracheal stoma and its proximity to the cricoid are noted. Computed tomography is used to assess any extraluminal component to a stenosis and the relation of the innominate artery and other mediastinal structures to the anastomosis.
Bronchoscopy and laryngoscopy are performed to determine the extent of residual normal trachea and the condition of the stricture, the integrity of vocal cords and the presence or absence of tracheomalacia. Performed as a separate procedure prior to operative intervention, endoscopy helps guide the decision to proceed with operative intervention. Key observations include the length of stenosis, the length of both proximal and distal normal trachea, presence of tracheal mucosa inflammation, and signs of vocal cord impairment. No absolute length of residual trachea indicates the success of another operation; conversely, a short distance of normal trachea may predict the futility of repeat resection. A paretic vocal cord in the medial position indicates damage to one recurrent laryngeal nerve, and the risk reoperation poses to the second nerve must be carefully considered. Avoiding reoperation altogether may be prudent in some patients with overt laryngeal dysfunction lest the remaining recurrent laryngeal nerve suffer damage as well.
Pre-operative management of tracheal restenosis
The surgeon’s first priority after failed resection should be to optimize the alternative airway prior to any reresection, so that an ill-fitting tracheostomy or a lack of voice do not create any false urgency for surgeon or patient to “fix the problem” with reresection. Delaying reoperative intervention for a minimum of 4 to 6 months until inflammation subsides is critical to the success of reresection; at our institution, the average time interval between the initial operation to reresection was 8 months (1). Reoperation should also be delayed if infection persists.
Depending on the severity of airway stenosis, in our experience 52% of patients were managed with observation or dilatation before reoperation (1). If the patient remains symptomatic, a T-tube or tracheostomy should be considered. T-tube insertion is our preferred method to optimize the airway as it minimizes inflammation, preserves speech and is easy to manage. The main reason not to use a T-tube is a short distance between the tracheal stoma and the vocal cords, for example after prior laryngotracheal resection, leading to granulations from contact between vocal cords and the upper limb of the tube. A stoma for the T-tube is placed through the prior anastomosis, through the most damaged or stenotic portion of the trachea or at a previously marked, anatomically suitable location in the trachea. If the airway was just dilated during the same procedure, ventilation is maintained through the rigid bronchoscope that is removed once the tube is in place. Some patients with tracheal restenosis may discover that the T-tube is the optimal long-term option. In a series of 140 patients who underwent T-tube placement at our institution, 20% of patients could not tolerate initial T-tube insertion because of obstruction of the upper limb or aspiration (6). However, only 3.6% of patients required tube removal for obstructive problems more than 2 months after placement and long-term intubation of greater than 5 years was achieved in close to 10% of patients (6). About 1 to 2 weeks prior to planned reoperation, the T-tube should be removed and replaced with a tracheostomy to allow the subglottic larynx to recover from irritation caused by the proximal end of the T-tube (6).
The selection of patients for reoperation may be difficult. A “Diagnostic and Therapeutic Main Airway Protocol” was developed in Barcelona, Spain to aid in staging and therapeutic options (7). Stenotic lesions were classified according to stage of development (fibrosis, inflammation/granuloma, malacia, tracheoesophageal fistula), caliber (diameter of stenosis), and length of stenosis (7). This group recommended laser therapy for smaller lesions and resection for larger lesions. In the absence of a preserved cartilaginous airway, we caution that laser therapy of a circumferential cicatricial process affords at best temporary restoration of a functional lumen.
Reoperation for tracheal restenosis
After carefully selecting and preparing a patient for a second resection, the critical determinant of successful reconstruction is a meticulous surgical technique. As for the first operation, the lateral tracheal blood supply must be preserved, extensive anterior tracheal mobilization is provided while protecting the lateral blood supply and excessive anastomotic tension avoided. These steps are particularly important in the reoperative field where the presence of dense scar tissue and limited amount of trachea available for reconstruction decrease the surgeon’s margin for error.
The most common approach is an anterior cervical collar incision. About 25% of patients require additional partial upper sternotomy (1). A trans-thoracic approach is selected in the uncommon case of a stricture with carinal proximity. The previous cervical scar and any tracheal stoma are excised.
The sternocleidomastoid muscle is identified. Dissection is performed in the midline and carried down to the surface of the trachea. The dissection should be close to the trachea and midline to avoid impairing the recurrent laryngeal nerve or lacerating the esophagus; circumferential exposure of the trachea is only necessary at the level of the failed anastomosis. The trachea is exposed from cricoid to carina in this fashion. The innominate artery may be incorporated in scar tissue on the anterior tracheal wall; if exposed, the artery should be buttressed with a flap of sternothyroid strap muscle to help prevent formation of tracheoinnominate fistula. At the border between stricture and lower trachea, circumferential dissection is carried immediately on the tracheal wall; any distance taken from the wall risks injury to the recurrent laryngeal nerves. This part of the dissection must not be hurried.
To address the stenotic trachea, first the trachea distal to the stenosis is opened by an anterior incision. The endotracheal tube is withdrawn and lateral traction sutures are placed in the distal healthy trachea. The distal trachea is intubated with a sterile endotracheal tube and cross-field ventilation is initiated. The stenotic segment is excised; the average resected specimen is 3.5 cm (1); since some or most of this distance represents scar, its removal cannot be equated with loss of tracheal length. Judgment is required to balance complete resection of abnormal trachea with tolerable anastomotic tension. In benign strictures, abnormal trachea may be accepted provided cartilage is stable and luminal diameter acceptable. Strictures after resection for malignancy should accept the margin status found at the time of the first resection.
Our preference is to reconstruct the anastomosis with interrupted 4-0 vicryl sutures; polydioxanone sutures may also be used. Once placed, the sutures are tied with the patient’s head in the flexed position. If tension at the anastomosis is excessive, release maneuvers should be performed. About 25% of patients undergoing reresection at our institution required release maneuvers, compared to 6% of patients who underwent primary resection (1).
The most common maneuver to gain additional tracheal length is the Montgomery suprahyoid release (8,9). Useful for cervical reconstruction, the suprahyoid release results in downward displacement of larynx and cervical trachea by 1–2 cm after severing muscular attachments and lateral segments of the central hyoid. This release maneuver often results in temporary postoperative dysphagia. To gain additional length for reconstruction of the lower trachea, intrathoracic tracheal mobilization is performed with division of the pulmonary ligament, release of the pulmonary veins by partial or complete circumcision of the attached pericardium and mobilization of the mainstem bronchi; depending on the type of reconstruction, between 2 and 6 cm of additional tracheal length are generated (10). After wound closure, a heavy suture is placed between chin and presternal skin to prevent extension of the neck during early recovery.
After the operation, the patient is monitored in the intensive care unit. Postoperative bronchoscopy is performed within 7 to 14 days of the operation to evaluate the anastomosis.
Outcomes after reoperation for tracheal restenosis
Donahue and coauthors evaluated outcomes after reoperation for tracheal restenosis and analyzed 75 patients who underwent reoperation from 1965 to 1992 (1). A good or satisfactory outcome was achieved in 91.9% of patients: 78.6% of patients had no physical limitation in activity and good voice and 13.3% of patients had dyspnea on exertion only and adequate voice. Four patients (5.3%) had failed reoperations and were managed with permanent tracheostomy or T-tube. Two patients died (2.7%) from anastomotic dehiscence and mediastinitis. In a series by Jović and coauthors that analyzed 22 patients with recurrent tracheal stenosis who underwent reresection from 2002 to 2010 (11), 95.3% of patients had satisfactory airway lumen with undisturbed breathing. We do not know from these studies the total number of patients evaluated and the proportion of those rejected for reresection.
Another series examined 12 patients who presented with restenosis after tracheal resection from 2000 to 2009 (12). Three patients achieved good outcomes with reresection (two patients underwent dilatation and endobronchial treatments first). The remaining nine patients achieved good results with a combination of dilatations, endobronchial stents, or placement of T-tubes. The circumstances of restenosis therefore seemed to favor non-resectional management.
While the incidence of complications after primary tracheal resection is at least 15%, the risks of reresection are greater. Among the 75 patients who underwent reoperation for tracheal restenosis, Donahue and coauthors found that 39% of patients suffered postoperative complications, anastomotic granulations being most common (15%), followed by retained secretions (5%), wound infection (5%), dysphagia (4%), anastomotic dehiscence (3%), deep venous thrombosis (1%), and pneumopericardium (1%) (1). Complications occurred more frequently in patients who underwent laryngeal release procedures, indicating the deleterious role of tension. Similarly, Jović and coauthors found 31.8% complications among 22 reoperations (11). These included vocal cord immobility, laryngeal edema, granulation, wound infection, wound dehiscence, restenosis, cardiac arrest, and death.
Case examples
The following three cases evaluated and managed by members of the Division of Thoracic Surgery at Massachusetts General Hospital, Boston MA present the range of strictures and outcomes.
Patient 1
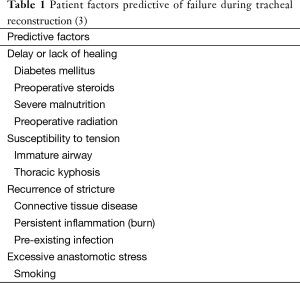
This is a 54-year-old man with high tracheostomy for facial injuries. Tracheal stenosis following decannulation recurred after multiple dilations and he underwent a tracheal resection in an outside hospital that failed immediately. The airway was maintained with biweekly dilatations. The first resection failed presumably because a subglottic stricture was not resected. Computed tomography before reresection showed a 1 cm-long high-grade stenosis with a subglottic component (Figure 1). During reresection, a scarred anterior cricoid was removed while the posterior subglottic mucosa was intact. Bronchoscopy 1 week after reconstruction demonstrated a widely patent anastomosis. Reresection succeeded because the second operation removed the subglottic part of the stricture.
Patient 2
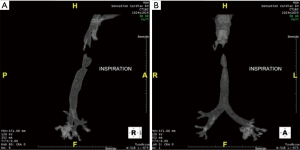
This 22-year-old man with Opitz syndrome (manifested by midline defects) underwent repair of a laryngotracheoesophageal cleft as a neonate and a second repair of a subglottic stenosis with cartilage graft at 4 years of age. He presented with noisy breathing and chronic stridor. Computed tomography (Figure 2) and bronchoscopy before reresection showed high tracheal bar creating a 1 cm-long stenotic luminal slit 2 mm wide and 1.5 cm deep. He underwent a tracheal reresection through the cricoid cartilage and reconstruction. Bronchoscopy 1 week later demonstrated a widely patent anastomosis. Reresection succeeded because a tracheal bar remaining after cartilage graft reconstruction was removed.
Patient 3
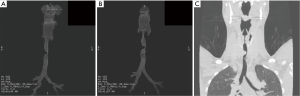
This 30-year-old woman with adenoid cystic carcinoma underwent tracheal resection (6.5 cm) and reconstruction. Four weeks later, she presented with wheezing and stridor. Dilatation failed to improve her stridor. Computed tomography (Figure 3) and bronchoscopy before reresection demonstrated a 6-mm stricture at the level of the anastomosis that was 2 cm long. She underwent tracheal reresection through the previous upper sternotomy and reconstruction. Bronchoscopy 1 week later demonstrated a widely patent anastomosis. Reresection succeeded because the residual stricture was short and reconstruction did not result in excessive tension.
Conclusions
Reoperation for tracheal restenosis after failed primary reconstruction is worthwhile in patients selected for favorable characteristics when performed in an optimal care environment. Neither the true incidence of restenosis nor the precise proportion of patients selected for reresection is known; the former we suspect to be higher than reported, and the latter lower than desirable. One 2012 case report detailing how a patient successfully underwent a third resection and anastomosis of the trachea emphasizes that surgical technique, patient selection and preoperative preparation all play important roles in the success of tracheal reoperation (13). To optimize these factors, surgeons should make liberal use of temporizing measures such as T-tubes or tracheostomy and consider referral to a high-volume center (Table 3).
Full table
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Donahue DM, Grillo HC, Wain JC, et al. Reoperative tracheal resection and reconstruction for unsuccessful repair of postintubation stenosis. J Thorac Cardiovasc Surg 1997;114:934-8; discussion 938-9. [PubMed]
- Bibas BJ, Terra RM, Oliveira Junior AL, et al. Predictors for postoperative complications after tracheal resection. Ann Thorac Surg 2014;98:277-82. [PubMed]
- Wright CD, Grillo HC, Wain JC, et al. Anastomotic complications after tracheal resection: prognostic factors and management. J Thorac Cardiovasc Surg 2004;128:731-9. [PubMed]
- Couraud L, Jougon JB, Velly JF. Surgical treatment of nontumoral stenoses of the upper airway. Ann Thorac Surg 1995;60:250-9; discussion 259-60. [PubMed]
- Grillo HC, Donahue DM, Mathisen DJ, et al. Postintubation tracheal stenosis. Treatment and results. J Thorac Cardiovasc Surg 1995;109:486-92; discussion 492-3. [PubMed]
- Gaissert HA, Grillo HC, Mathisen DJ, et al. Temporary and permanent restoration of airway continuity with the tracheal T-tube. J Thorac Cardiovasc Surg 1994;107:600-6. [PubMed]
- Amorós JM, Ramos R, Villalonga R, et al. Tracheal and cricotracheal resection for laryngotracheal stenosis: experience in 54 consecutive cases. Eur J Cardiothorac Surg 2006;29:35-9. [PubMed]
- Donahue DM. Reoperative tracheal surgery. Chest Surg Clin N Am 2003;13:375-83. [PubMed]
- Montgomery WW. Suprahyoid release for tracheal anastomosis. Arch Otolaryngol 1974;99:255-60. [PubMed]
- Grillo HC, Dignan EF, Miura T. Extensive resection and reconstruction of mediastinal trachea without prosthesis or graft: an anatomical study in man. J Thorac Cardiovasc Surg 1964;48:741-9. [PubMed]
- Jović RM, Dragičević D, Komazec Z, et al. Laryngotracheal stenosis and restenosis. What has the influence on the final outcome? Eur Arch Otorhinolaryngol 2012;269:1805-11. [PubMed]
- Erelel M, Kaya S, Toker A. Anastomotic stenotic complications after tracheal resections. J Bronchology Interv Pulmonol 2010;17:142-5. [PubMed]
- Saghebi SR, Abbasidezfouli A, Sheikhy K, et al. A successful third resection-anastomosis in a tracheal restenosis. Interact Cardiovasc Thorac Surg 2012;15:174-5. [PubMed]