
Prognostic value of left ventricular hypertrophy in postoperative outcomes in type A acute aortic dissection
Introduction
Acute aortic dissection (AD) is an urgent, life-threatening medical condition with rapid chest pain as the most common symptom at onset, that has an extremely high mortality (1,2). Acute aortic dissection is classified as acute type A AD (ATAAD) and acute type B AD based on the involvement of the ascending aorta, that differs in symptom, management, and outcome (3). Usually, ATAAD, in which the ascending aorta was involved, needs swift open surgical repair after initial diagnosis, including classic Bentall procedure, wheat procedure and frozen elephant trunk technique. Despite the improvement of clinical outcomes after surgical repair over time, the mortality of ATAAD is still high, about 1 in 5 patients died after surgery (1,2,4-7).
Hypertension is a common condition in AD, with a prevalence of 75–80% among patients with AD (8). Hypertension can be triggered by many factors, such as obesity, genetic background and salt intake (9-12). Heart is one of the major target organs in hypertension-related organs damage (13,14). Left ventricular hypertrophy (LVH), which presents in approximately two-fifth of hypertension patients, is reported to be associated with increased cardiovascular morbidity and mortality, including sudden cardiac death, heart failure, arrhythmias, etc. (14-16). Also, research indicates that LVH is a risk factor of enlarged aorta and dissection (17).
A previous study demonstrates LVH as a biomarker to predict increased mortality in type B AD patients (18). However, the association between LVH and ATAAD remains unknown. Herein, we investigated the prognostic value of LVH in AD patients after surgical repair, and developed nomogram models to predict postoperative outcomes in ATAAD patients. We present the following article in accordance with the TRIPOD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-193/rc).
Methods
Study population and data collection
From 1 January 2018 to 31 November 2021, all adult patients (≥18 years) diagnosed with ATAAD in Renmin Hospital of Wuhan University were included. Imaging data (computed tomography angiography and transthoracic/transesophageal echocardiogram) was checked for confirmation. The predefined exclusion criteria were as follows: (I) simple intramural hematoma; (II) traumatic/iatrogenic AD, AD with pregnancy, or patients who had previous cardiac surgery; (III) patients with congenital aortic abnormalities; (IV) patients without complete medical records available.
Demographic and clinical data were extracted individually from original medical records, including gender, age, weight, height, symptoms, medical background (diabetes mellitus, hypertension, coronary artery disease), smoking, alcohol consumption, laboratory biomarkers, electrocardiogram, ultrasound imaging, operation data, and in-hospital outcome. Hypertension was defined as follows, (I) patients with a history of previously diagnosed hypertension, regardless of blood pressure (BP) status. (II) patients with increased BP on admission (systolic BP >140 mmHg or diastolic BP >90 mmHg), or patients who were taking antihypertensive agents with normal BP level on admission.
Left ventricular mass index (LVMI) was calculated based on echocardiogram data, as reported previously (19). LVH was defined as LVMI ≥115 g·m-2 for males, or LVMI ≥95 g·m−2 for females. The malperfusion was presented with Penn Classification as reported (20). The patients were divided into two groups, LVH and non-LVH (nLVH) group, based on their LVMI.
Study endpoints and operation procedure
The primary endpoints were postoperative complications within 30 days as follows: operative mortality, strokes, paraplegia, continuous renal replacement therapy (CRRT), and cardiac events. Cardiac events were defined as low cardiac output syndrome or ventricular arrhythmias. To evaluate the in-hospital outcomes, a parameter named composite major outcomes (CMO) was utilized for patients with at least one primary endpoint event. The secondary endpoints were re-exploration for postoperative bleeding, tracheotomy, and new-onset atrial fibrillation after surgery.
The operation plan was decided by experienced surgeons. Moderate hypothermic circulatory arrest was applied for patients required arch replacement. Cold antegrade custodial-histidine-trypthophan-ketoglutarate solution (Custodial-HTK) was applied for myocardial preservation.
Statistical analysis
Statistical analysis was performed utilizing the R 4.0.5 (R Foundation for Statistical Computing, Vienna, Austria). A two-tailed P value of <0.05 was considered statistically significant.
Normally distributed continuous variables were expressed as mean ± standard deviation (SD) and compared with student’s t-test. Skewed continuous variables were expressed as the median and interquartile range (IQR) and compared with Mann-Whitney U-test. Categorical variables are described as frequencies with percentages, and analyzed by Fisher’s exact test. Shapiro-Wilk-test was used to evaluate the normality of continuous data. Logistic regression analysis was performed to evaluate the correlation and select predictors for the nomogram model. The bootstrap method was applied for internal validation. Calibration curve and decision curve analysis were applied to assess model performance. Propensity score matching was applied for confounding control.
Nomogram models were developed based on multivariable logistic regression. Variables with a P value <0.05 were selected for model development. LVH and LVMI were used separately for model development. Calibration curve and decision curve analysis were used to assess model performance.
Patient and public involvement statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the ethics commission of Renmin Hospital of Wuhan University (WDRM2020-K230). Informed consent was not required due to its retrospective nature.
Results
Demographic and clinical data
One hundred and ninety-three patients were included in the final analysis (Figure S1). Demographic and clinical data were summarized in Table 1. The two groups did not differ in age. However, there were more females in patients with LVH (32.7% vs. 17.4%, P=0.03). Patients without LVH had a higher median BMI of 25.0 kg·m−2. However, the proportion of overweight/obesity was similar among the two groups. Sudden anterior chest pain was a common symptom in both groups. No significant difference in the prevalence of hypertension (94.5% in LVH group vs. 89.1% in nLVH group, P=0.29). Patients with LVH had higher presenting diastolic BP (82.2±17.2 vs. 76.0±19.2 mmHg, P=0.04) and higher presenting pulse pressure (79.8±17.5 vs. 67.9±20.1 mmHg, P<0.01), but similar systolic BP (138.3±24.1 vs. 141.0±29.6 mmHg, P=0.51). Renal dysfunction was more common in the LVH group (25.5% in the LVH group vs. 12.3% in the nLVH group, P=0.03). No other apparent differences were found among the 2 groups in terms of hyperlipidemia, coronary artery disease, diabetes mellitus, alcohol consumption, smoking, liver lesions, or hypoxemia at admission.
Table 1
| Characteristics | Overall (N=193) | Left ventricular hypertrophy | P value | |
|---|---|---|---|---|
| Presence (N=55) | Absence (N=138) | |||
| Age (y) | 52.9±10.8 | 53.2±10.5 | 52.8±10.9 | 0.817 |
| Gender | ||||
| Male | 151 (78.2) | 37 (67.3) | 114 (82.6) | 0.032* |
| Female | 42 (21.8) | 18 (32.7) | 24 (17.4) | |
| LVMI (g·m−2) | 99.4 (87.2–111.9) | 123.7 (115.7–142.8) | 91.1 (82.8–102.4) | <0.001* |
| Body mass index (kg·m−2) | 24.8 (22.7–27.2) | 23.7 (21.9–26.0) | 25.0 (23.0–27.7) | 0.016* |
| Overweight | 70 (36.3) | 15 (27.3) | 55 (39.9) | 0.086 |
| Obesity | 42 (21.8) | 10 (18.2) | 32 (23.2) | |
| Blood type | ||||
| A | 60 (31.1) | 12 (21.8) | 48 (34.8) | 0.091 |
| B | 42 (21.8) | 18 (32.7) | 24 (17.4) | |
| O | 83 (43.0) | 23 (41.8) | 60 (43.5) | |
| AB | 8 (4.1) | 2 (3.6) | 6 (4.3) | |
| Presenting symptoms | ||||
| Chest pain (anterior) | 162 (83.9) | 43 (78.2) | 119 (86.2) | 0.194 |
| Back pain | 113 (58.5) | 33 (60.0) | 80 (58.0) | 0.872 |
| Syncope | 12 (6.2) | 3 (5.5) | 9 (6.5) | 1.000 |
| Medical background | ||||
| Hyperlipidemia | 71 (36.8) | 21 (38.2) | 50 (36.2) | 0.869 |
| CAD | 17 (8.8) | 6 (10.9) | 11 (8.0) | 0.576 |
| Diabetes mellitus | 11 (5.7) | 3 (5.5) | 8 (5.8) | 1.000 |
| Hypertension | 175 (90.7) | 52 (94.5) | 123 (89.1) | 0.287 |
| Presenting blood pressure | ||||
| Systolic BP (mmHg) | 140.2±28.2 | 138.3±24.1 | 141.0±29.6 | 0.514 |
| Diastolic BP (mmHg) | 77.8±18.8 | 82.2±17.2 | 76.0±19.2 | 0.038* |
| PP (mmHg) | 71.3±20.1 | 79.8±17.5 | 67.9±20.1 | <0.001* |
| Drinking history | 43 (22.3) | 9 (16.4) | 34 (24.6) | 0.253 |
| Smoking history | 71 (36.8) | 18 (32.7) | 53 (38.4) | 0.511 |
| Ultrasound-detected liver lesions | 47 (24.4) | 11 (20.0) | 36 (26.1) | 0.459 |
| Hypoxemia† | 68 (35.2) | 18 (32.7) | 50 (36.2) | 0.739 |
| Renal function | ||||
| Cr >140 mmol/L | 31 (16.1) | 14 (25.5) | 17 (12.3) | 0.031* |
Data are expressed as mean ± standard deviation or medians and interquartile ranges or numbers (percentages). *, P value <0.05; †, hypoxemia was defined as an artery oxygen partial pressure <60 mmHg on admission. LVMI, left ventricular mass index; CAD, coronary artery disease; BP, blood pressure; PP, pulse pressure; Cr, creatinine.
Perioperative data and laboratory examination
Laboratory examination results were presented in Table 2. Patients with LVH had slightly decreased hemoglobin and alanine aminotransferase concentrations. There were no significant differences among the two groups in terms of white blood cell counts, neutrophil counts, platelet counts, total bilirubin urea, creatine, uric acid, blood glucose, fibrinogen, or d-dimer concentration.
Table 2
| Biomarkers | Overall (N=193) | Left ventricular hypertrophy | P value | |
|---|---|---|---|---|
| Presence (N=55) | Absence (N=138) | |||
| WBC (109/L) | 12.32 (10.30–14.29) | 11.71 (8.74–15.12) | 12.51 (10.48–14.23) | 0.396 |
| Neu (109/L) | 10.26 (8.29–12.49) | 9.76 (7.50–12.62) | 10.46 (8.65–12.47) | 0.423 |
| Hb (g/L) | 130.1±18.8 | 125.8±21.6 | 132.0±17.5 | 0.040* |
| Plt (109/L) | 162.0 (134.0–191.0) | 150.5 (135.0–191.0) | 163.0 (134.0–191.0) | 0.340 |
| ALT (U/L) | 23.0 (15.0–34.5) | 19.5 (13.0–25.0) | 24.0 (17.0–36.0) | 0.006* |
| TBil (μmol/L) | 16.03 (11.62–22.62) | 15.80 (10.75–22.19) | 16.80 (11.86–22.75) | 0.287 |
| Urea (mmol/L) | 6.85 (5.70–8.48) | 6.91 (5.97–9.86) | 6.77 (5.57–8.20) | 0.070 |
| Cr (μmol/L) | 84.0 (66.5–118.5) | 89.0 (65.0–140.0) | 83.0 (67.0–118.0) | 0.456 |
| UA (μmol/L) | 399.0 (320.5–489.0) | 409.0 (339.0–490.0) | 398.0 (313.0–488.0) | 0.270 |
| Glucose (mmol/L) | 7.14 (6.18–8.40) | 7.36 (6.55–8.60) | 7.01 (6.05–8.40) | 0.226 |
| FIB (g/L) | 2.16 (1.68–3.14) | 2.13 (1.62–2.78) | 2.18 (0.75–3.32) | 0.555 |
| D-dimer (mg/L) | 6.44 (3.38–13.92) | 7.96 (4.42–15.01) | 5.73 (3.32–12.80) | 0.080 |
*, P value <0.05. WBC, white blood cell counts; Neu, neutrophil; Hb, hemoglobin; Plt, platelets; ALT, alanine aminotransferase; TBil, total bilirubin; Cr, creatinine; UA, uric acid; FIB, fibrinogen.
Table 3 presented the perioperative and postoperative information data of the two groups. Patients with LVH were more likely to experience cardiac tamponade and decreased left ventricular ejection function. Ultra-sound detected aortic valve insufficiency was common in both two groups, that 58.2% of the LVH group and 50.7% of the nLVH group had aortic insufficiency. About 43.6% of patients with LVH underwent surgical repair within the first 24 hours of admission, while 37.7% of patients without LVH underwent emergency surgery. The overall median (IQR) of cardiopulmonary bypass (CPB) duration, aortic cross-clamping duration, and circulatory arrest duration were 267.0 (241.0–297.0), 140.0 (124.0–165.0), and 31.0 (20.0–38.0) min, respectively. In terms of surgical procedures, there was no apparent difference among the two groups.
Table 3
| Characteristics | Left ventricular hypertrophy | P value | |
|---|---|---|---|
| Presence (N=55) | Absence (N=138) | ||
| Penn classification | |||
| Penn Aa | 26 (47.3) | 70 (50.7) | 0.874 |
| Penn Ab | 26 (47.3) | 59 (42.8) | |
| Penn Ac/Ab&c | 3 (5.5) | 9 (6.5) | |
| Myocardial infarction | 4 (7.3) | 11 (8.0) | 1.000 |
| Maximum AAoD (mm) | 41.9±7.6 | 40.9±8.0 | 0.448 |
| Echocardiogram | |||
| Decreased LVEF† | 8 (14.5) | 2 (1.4) | 0.001* |
| Pericardial effusion | |||
| Absence | 28 (50.9) | 63 (45.7) | 0.030* |
| Presence | 20 (36.4) | 70 (50.7) | |
| Cardiac tamponade | 7 (12.7) | 5 (3.6) ‡ | |
| Aortic insufficiency | |||
| Mild | 16 (29.1) | 37 (26.8) | 0.162 |
| Middle | 10 (18.2) | 29 (21.0) | |
| Severe | 6 (10.9) | 4 (2.9) | |
| Surgical repair | |||
| Within 24 h | 24 (43.6) | 52 (37.7) | 0.514 |
| After 24 h | 31 (56.4) | 86 (62.3) | |
| Cannulation strategy | |||
| Femoral artery | 28 (50.9) | 75 (54.3) | 0.889 |
| Axillary artery | 1 (1.8) | 2 (1.4) | |
| Femoral artery & axillary artery | 26 (47.3) | 61 (44.2) | |
| Operation durations | |||
| CPB durations (min) | 269.0 (238.5–308.0) | 265.0 (243.0–297.0) | 0.710 |
| ACx durations (min) | 139.0 (123.0–161.0) | 142.5 (125.0–165.0) | 0.526 |
| CA durations (min) | 33.0 (20.0–37.5) | 31.0 (20.0–38.0) | 0.736 |
| CABG | 3 (5.5) | 10 (7.2) | 0.761 |
| Proximal reconstruction | |||
| Modified Bentall | 9 (16.4) | 20 (14.5) | 0.933 |
| Aortic valve replacement/repair | 6 (10.9) | 17 (12.3) | |
| Valve conservative surgery | 40 (72.7) | 101 (73.2) | |
| Arch replacement | 41 (74.5) | 117 (84.8) | 0.102 |
| Distal aortic operation | |||
| Frozen elephant trunk | 30 (54.5) | 67 (48.6) | 0.683 |
| Hybrid | 24 (43.6) | 65 (47.1) | |
| Automatic heart resuscitation | 12 (21.8) | 38 (27.5) | 0.470 |
| In-hospital outcome | |||
| Composite major outcomes | 17 (30.9) | 21 (15.2) | 0.017* |
| Operative mortality | 10 (18.2) | 10 (7.2) | 0.035* |
| Stroke | 4 (7.3) | 2 (1.4) | 0.056 |
| Paraplegia | 2 (3.6) | 3 (2.2) | 0.624 |
| CRRT | 9 (16.4) | 12 (8.7) | 0.131 |
| Cardiac events | 7 (12.7) | 6 (4.3) | 0.053 |
| Re-exploration | 1 (1.8) | 4 (2.9) | 1.000 |
| Tracheotomy | 5 (9.1) | 6 (4.3) | 0.299 |
| Atrial fibrillation | 6 (10.9) | 4 (2.9) | 0.033* |
Data are expressed as mean ± standard deviation or medians and interquartile ranges or numbers (percentages). *, P value <0.05. †, decreased LVEF was defined as a LVEF <50%; ‡, compared with LVH group. Post hoc test was adjusted with Bonferroni method. AAoD, ascending aortic diameter; LVEF, left ventricular ejection fraction; CPB, cardiopulmonary bypass; ACx, aortic cross-clamping; CA, circulatory arrest; CABG, coronary artery bypass graft; CRRT, continuous renal replacement therapy.
As showed in Table 3, CMO occurred in 17 (30.9%) of 55 LVH patients and 21 (15.2%) of 138 nLVH patients. LVH group had higher operative mortality of 18.2%, while the nLVH group had operative mortality of 7.2% (P=0.04). LVH patients had a higher prevalence of postoperative stroke (4 in 55 patients) and cardiac events (7 in 55 patients). In the nLVH group, the prevalence of stroke and cardiac events were 1.4% (2 in 138) and 4.3% (6 in 138), respectively. However, they narrowly missed the significant point. New-onset atrial fibrillation after surgery was present in 6 (10.9%) patients with LVH, while in the nLVH group only 4 (2.9%) patients had new-onset atrial fibrillation (10.9% vs. 2.9%, P=0.03). Two groups had no significant differences in paraplegia, CRRT, re-exploration, or tracheotomy after surgical repair.
Risk factors for CMO and cardiac events
To investigate risk factors for postoperative CMO, a univariate logistic regression was performed. Perioperative data, including baseline characteristics and operative information, was included in univariate logistic regression as summarized in Table 4. Results indicated that, LVH (OR: 2.5, 95% CI: 1.2–5.2, P=0.02), LVMI (per 10 g·m−2) (OR: 1.2, 95% CI: 1.0–1.3, P<0.01), ischemia (Penn Classification Ac, or Ab&c) (OR: 15.4, 95% CI: 4.0–59.9, P<0.01), hyperlipidemia (OR: 3.4, 95% CI: 1.6–7.1, P<0.01), renal dysfunction (OR: 3.3, 95% CI: 1.4–7.6, P<0.01) and emergency surgery (OR: 3.4, 95% CI: 1.6–7.1, P<0.01) were risk factors for postoperative CMO in ATAAD patients. Moreover, the increased durations of operation, including CPB duration (per 10 minutes) (OR: 1.1, 95% CI: 1.0–1.2, P<0.01), aortic cross-clamping duration (per 10 minutes) (OR: 1.1, 95% CI: 1.0–1.2, P=0.05) and circulatory arrest duration (per 5 minutes) (OR: 1.0, 95% CI: 1.0–1.4, P≤0.01), were associated with CMO.
Table 4
| Characteristics | CMO | Cardiac events | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | ||
| Left ventricular hypertrophy | 2.492 | 1.193–5.207 | 0.015* | 3.208 | 1.027–10.02 | 0.045* | |
| LVMI | |||||||
| Linear, per 10g·m−2 | 1.169 | 1.051–1.301 | 0.004* | 1.194 | 1.061–1.343 | 0.003* | |
| Age (y) | |||||||
| Linear, per 10 y | 0.865 | 0.630–1.186 | 0.367 | 1.252 | 0.733–2.140 | 0.410 | |
| ≥60 y | 0.588 | 0.241–1.436 | 0.244 | 1.875 | 0.584–6.024 | 0.291 | |
| Male gender | 2.070 | 0.753–5.685 | 0.158 | 1.571 | 0.335–7.382 | 0.567 | |
| BMI (kg·m−2) | |||||||
| Linear | 1.018 | 0.928–1.116 | 0.701 | 1.035 | 0.899–1.193 | 0.630 | |
| Overweight | 0.779 | 0.343–1.768 | 0.550 | 0.311 | 0.062–1.549 | 0.154 | |
| Obesity | 1.027 | 0.413–2.552 | 0.955 | 1.113 | 0.307–4.039 | 0.871 | |
| Penn classification | |||||||
| Penn Aa | – | – | Reference | – | – | Reference | |
| Penn Ab | 2.224 | 0.990–4.996 | 0.053 | 1.382 | 0.406–4.703 | 0.604 | |
| Penn Ac/Ab&c | 15.45 | 3.988–59.89 | <0.001* | 3.640 | 0.623–21.26 | 0.151 | |
| Medical background | |||||||
| Hyperlipidemia | 3.418 | 1.640–7.122 | 0.001* | 2.971 | 0.933–9.465 | 0.065 | |
| CAD | 2.454 | 0.845–7.127 | 0.099 | 2.000 | 0.405–9.873 | 0.395 | |
| Diabetes mellitus | 0.392 | 0.049–3.159 | 0.379 | – | – | 0.999 | |
| Drinking history | 0.914 | 0.384–2.174 | 0.839 | 1.050 | 0.276–3.999 | 0.943 | |
| Smoking history | 1.981 | 0.966–4.061 | 0.062 | 2.115 | 0.681–6.561 | 0.195 | |
| Decreased LVEF† | 1.812 | 0.446–7.362 | 0.406 | 3.909 | 0.740–20.66 | 0.108 | |
| Cardiac tamponade | 3.203 | 0.957–10.72 | 0.059 | 3.091 | 0.602–15.87 | 0.176 | |
| Ultrasound-detected liver lesions | 1.139 | 0.506–2.563 | 0.753 | 1.416 | 0.415–4.828 | 0.578 | |
| Elevated total bilirubin | 1.061 | 0.484–2.325 | 0.882 | 1.156 | 0.340–3.922 | 0.817 | |
| Hypoxemia | 0.700 | 0.323–1.518 | 0.367 | 0.806 | 0.239–2.720 | 0.728 | |
| Renal dysfunction | 3.304 | 1.432–7.619 | 0.005* | 0.417 | 0.052–3.326 | 0.409 | |
| Myocardial infarction | 0.272 | 0.035–2.137 | 0.216 | – | – | 0.999 | |
| Surgical timing | |||||||
| After 24 h | – | – | Reference | – | – | Reference | |
| Within 24 h | 3.396 | 1.622–7.107 | 0.001* | 1.877 | 0.606–5.816 | 0.275 | |
| Operation duration | |||||||
| CPB duration (per 10 min) | 1.133 | 1.057–1.214 | <0.001* | 1.131 | 1.036–1.233 | 0.006* | |
| ACx duration (per 10 min) | 1.098 | 1.000–1.206 | 0.049* | 1.047 | 0.907–1.209 | 0.528 | |
| CA duration (per 5 min) | 1.025 | 1.055–1.376 | 0.006* | 1.111 | 0.917–1.348 | 0.283 | |
| CABG | 1.908 | 0.555-6.565 | 0.305 | 2.793 | 0.550–14.19 | 0.215 | |
| Proximal reconstruction | |||||||
| Valve conservative root surgery | – | – | Reference | – | – | Reference | |
| Modified Bentall procedure | 1.101 | 0.409–2.969 | 0.848 | 2.347 | 0.670–8.215 | 0.182 | |
| Aortic valve replacement/repair | 1.173 | 0.400–3.440 | 0.772 | – | – | 0.998 | |
| Arch replacement | 1.228 | 0.470–3.209 | 0.676 | 2.795 | 0.351–22.23 | 0.331 | |
*, P value <0.05; †, decreased LVEF was defined as a LVEF <50%. CMO, composite major outcomes; LVMI, left ventricular mass index; BMI, body mass index; CAD, coronary artery disease; LVEF, left ventricular ejection fraction; CPB, cardiopulmonary bypass; ACx, aortic cross-clamping; CA, circulatory arrest; CABG, coronary artery bypass graft.
Another univariate analysis (cardiac events as endpoint) was performed and presented in Table 4. Consistent with previous results, LVH (OR: 3.2, 95% CI: 1.0–10.0, P=0.04), LVMI (per 10 g·m−2) (OR: 1.2, 95% CI: 1.1–1.3, P<0.01) and CPB duration (per 10 minutes) (OR: 1.1, 95% CI: 1.0–1.2, P<0.01) were predictors for postoperative cardiac events. However, hyperlipidemia narrowly missed the significant point (OR: 3.0, 95% CI: 0.9–9.5, P=0.06).
Multivariate logistic regression model for CMO
Multivariate logistic regression was performed to identify independent predictors for CMO. Indicators with a P value of less than 0.05 in univariate analysis were included in the multivariate model. Aortic cross-clamping duration and circulatory arrest duration were excluded from the model, as shown in Table S1. Variables included in the final multivariate analysis for CMO were LVH/LVMI, Penn Classification, hyperlipidemia, smoking, renal dysfunction, coronary artery disease, emergency surgery, and CPB duration. Table 5 presented result of the multivariate analysis.
Table 5
| Characteristics | β | S.E. | Wald | OR | 95% CI | P value |
|---|---|---|---|---|---|---|
| LVH | ||||||
| Left ventricular hypertrophy | 0.942 | 0.448 | 4.409 | 2.564 | 1.065–6.175 | 0.036* |
| Penn Ab | 0.558 | 0.488 | 1.306 | 1.748 | 0.671–4.552 | 0.253 |
| Penn Ac/Ab&c | 2.601 | 0.798 | 10.62 | 13.48 | 2.819–64.42 | 0.001* |
| Hyperlipidemia | 1.085 | 0.441 | 6.066 | 2.960 | 1.248–7.020 | 0.014* |
| Renal dysfunction | 0.768 | 0.559 | 1.890 | 2.156 | 0.721–6.448 | 0.169 |
| Emergency surgical repair† | 1.045 | 0.441 | 5.624 | 2.845 | 1.199–6.750 | 0.018* |
| CPB durations (per 10 min) | 0.115 | 0.040 | 8.329 | 1.122 | 1.038–1.213 | 0.004* |
| Intercept | -6.638 | 1.274 | 27.16 | – | – | – |
| LVMI | ||||||
| LVMI (per 10 g·m−2) | 0.149 | 0.063 | 5.686 | 1.161 | 1.027–1.312 | 0.017* |
| Penn Ab | 0.574 | 0.491 | 1.366 | 1.776 | 0.678–4.653 | 0.242 |
| Penn Ac/Ab&c | 2.381 | 0.826 | 8.302 | 10.81 | 2.141–54.59 | 0.004* |
| Hyperlipidemia | 1.084 | 0.442 | 6.013 | 2.958 | 1.243–7.036 | 0.014* |
| Renal dysfunction | 0.797 | 0.552 | 2.082 | 2.219 | 0.752–6.554 | 0.149 |
| Emergency surgical repair† | 1.137 | 0.448 | 6.423 | 3.116 | 1.294–7.504 | 0.011* |
| CPB durations (per 10 min) | 0.119 | 0.040 | 8.807 | 1.126 | 1.041–1.219 | 0.003* |
| Intercept | −8.087 | 1.488 | 29.55 | – | – | – |
†, emergency surgical repair was defined as surgery within first 24 h of admission; *, P value <0.05. CMO, composite major outcomes; S.E., standard error; OR, odds ratio; CI, confidence interval; LVH, left ventricular hypertrophy; CPB, cardiopulmonary bypass; LVMI, left ventricular mass index.
The results indicated that, LVH (OR: 2.6, 95% CI: 1.1–6.2, P=0.04), ischemia (Penn Classification Ac, or Ab&c) (OR: 13.5, 95% CI: 2.8–64.4, P<0.01), hyperlipidemia (OR: 3.0, 95% CI: 1.2–7.0, P=0.01), emergency surgery (OR: 2.8, 95% CI: 1.2–6.8, P=0.02) and increased CPB duration (per 10 minutes) (OR: 1.1, 95% CI: 1.0–1.2, P<0.01) were independent risk factors for postoperative CMO in ATAAD patients. Consistent with previous results, increased LVMI was independent risk factor for CMO when LVH was replaced with LVMI, with an OR of 1.2 (95% CI: 1.0–1.3, P=0.02) for every 10 g·m-2 increase in LVMI.
Clinical features and in-hospital outcomes after propensity score matching
Propensity score matching was applied to reduce potential baseline confounding. Cardiac tamponade, hyperlipidemia, Penn classification, emergency surgery and renal dysfunction were included as covariates in the model Based on logistic regression results showed in Table 4 and Table 5. Cases were matched in a 1:2 ratio to cases without LVH based on the propensity score with a standard caliper width of 0.2. Jitter plot and line plot for matching were presented in Figure S2.
Clinical features and in-hospital outcomes after matching were summarized in Table 6. After matching, ATAAD patients with LVH had higher rates of postoperative CMO (16/52 vs. 13/94, P=0.02). Despite the relatively higher rates of decreased LVEF in LVH patients, no association between decreased LVEF and postoperative CMO was found by logistic regression analysis (OR: 2.6, 95% CI: 0.6–11.5, P=0.21).
Table 6
| Characteristics | Left ventricular hypertrophy | P value | |
|---|---|---|---|
| Presence (N=52) | Absence (N=94) | ||
| Age (years) | 53.0±10.5 | 52.7±11.0 | 0.899 |
| Gender | |||
| Male | 36 | 77 | 0.099 |
| Female | 16 | 17 | |
| LVMI (g·m−2) | 125.2 (115.7–145.2) | 90.8 (82.8–102.4) | <0.001* |
| Body mass index (kg·m−2) | 23.7 (22.0–26.0) | 25.0 (23.0–26.6) | 0.150 |
| Medical background | |||
| Hyperlipidemia | 19 | 37 | 0.859 |
| Diabetes mellitus | 3 | 8 | 0.747 |
| Hypertension | 49 | 83 | 0.380 |
| Ultrasound-detected liver lesions | 11 | 18 | 0.830 |
| Renal function | |||
| Cr >140 mmol/L | 13 | 17 | 0.393 |
| Penn classification | |||
| Penn Aa | 26 | 44 | 0.802 |
| Penn Ab | 23 | 46 | |
| Penn Ac/Ab&c | 3 | 4 | |
| Myocardial infarction | 3 | 6 | 0.700 |
| Echocardiogram | |||
| Decreased LVEF† | 7 | 1 | 0.003* |
| Cardiac tamponade | 4 | 4 | 0.456 |
| Surgical repair | |||
| Within 24 h | 23 | 38 | 0.727 |
| After 24 h | 29 | 56 | |
| Cannulation strategy | |||
| Femoral artery | 27 | 51 | 0.936 |
| Axillary artery | 1 | 1 | |
| Femoral artery & axillary artery | 24 | 42 | |
| Operation durations | |||
| CPB durations (min) | 268.5 (236.0–308.0) | 267.5 (245.0–296.0) | 0.933 |
| ACx durations (min) | 138.0 (121.5–161.0) | 142.5 (126.0–166.0) | 0.313 |
| CA durations (min) | 33.0 (20.0–37.5) | 31.0 (23.0–38.0) | 0.871 |
| Automatic heart resuscitation | 10 | 24 | 0.421 |
| In-hospital outcome | |||
| Composite major outcomes | 16 | 13 | 0.018* |
| Operative mortality | 8 | 7 | 0.158 |
| Stroke | 4 | 2 | 0.187 |
| Paraplegia | 2 | 2 | 0.616 |
| CRRT | 8 | 7 | 0.158 |
| Cardiac events | 6 | 4 | 0.167 |
| Re-exploration | 1 | 3 | 1.000 |
| Tracheotomy | 5 | 3 | 0.133 |
| Atrial fibrillation | 6 | 3 | 0.069 |
*, P value <0.05. †, decreased LVEF was defined as a LVEF <50%. LVMI, left ventricular mass index; Cr, creatinine; LVEF, left ventricular ejection fraction; CPB, cardiopulmonary bypass; ACx, aortic cross-clamping; CA, circulatory arrest; CRRT, continuous renal replacement therapy.
Univariable logistic regression analyses were applied to evaluate the prognostic value of LVH, as showed in Table 7. Two main variables, LVH and LVMI, were analyzed respectively. The results indicated that LVH was the risk factor for postoperative CMO (OR: 2.8, 95% CI: 1.2–6.4, P=0.02), while increasing LVMI was associated with higher risks of postoperative CMO (OR: 1.2, 95% CI: 1.0–1.3, P<0.01) and cardiac events (OR: 1.2, 95% CI: 1.0–1.3, P<0.01). In addition, increasing LVMI was associated with increased risk of postoperative CRRT (OR: 1.2, 95% CI: 1.0–1.3, P<0.01), tracheotomy (OR: 1.2, 95% CI: 1.0–1.3, P=0.02) and atrial fibrillation (OR: 1.2, 95% CI: 1.0–1.3, P=0.01).
Table 7
| Characteristics | β | S.E. | Wald | OR | 95% CI | P value |
|---|---|---|---|---|---|---|
| LVH | ||||||
| CMO | 1.019 | 0.424 | 5.778 | 2.769 | 1.207–6.354 | 0.016* |
| Operative mortality | 0.815 | 0.550 | 2.200 | 2.260 | 0.770–6.636 | 0.138 |
| Stroke | 1.344 | 0.884 | 2.310 | 3.833 | 0.678–21.68 | 0.129 |
| Paraplegia | 0.610 | 1.015 | 0.361 | 1.840 | 0.252–13.46 | 0.548 |
| CRRT | 0.815 | 0.550 | 2.200 | 2.260 | 0.770–6.636 | 0.138 |
| Cardiac events | 1.077 | 0.670 | 2.579 | 2.935 | 0.789–10.92 | 0.108 |
| Re-exploration | -0.520 | 1.168 | 0.198 | 0.595 | 0.060–5.867 | 0.656 |
| Tracheotomy | 1.172 | 0.752 | 2.427 | 3.227 | 0.739–14.09 | 0.119 |
| Atrial fibrillation | 1.375 | 0.730 | 3.551 | 3.957 | 0.946–16.54 | 0.060 |
| LVMI (per 10 g·m−2) | ||||||
| CMO | 0.174 | 0.060 | 8.490 | 1.190 | 1.059–1.337 | 0.004* |
| Operative mortality | 0.088 | 0.058 | 2.286 | 1.092 | 0.974–1.223 | 0.131 |
| Stroke | 0.096 | 0.076 | 1.603 | 1.101 | 0.949–1.278 | 0.205 |
| Paraplegia | −0.039 | 0.166 | 0.056 | 0.962 | 0.695–1.331 | 0.813 |
| CRRT | 0.153 | 0.059 | 6.821 | 1.165 | 1.039–1.307 | 0.009* |
| Cardiac events | 0.180 | 0.064 | 7.986 | 1.197 | 1.057–1.356 | 0.005* |
| Re-exploration | 0.017 | 0.130 | 0.017 | 1.017 | 0.789–1.311 | 0.898 |
| Tracheotomy | 0.145 | 0.064 | 5.109 | 1.156 | 1.019–1.310 | 0.024* |
| Atrial fibrillation | 0.154 | 0.063 | 5.994 | 1.166 | 1.031–1.318 | 0.014* |
*, P<0.05. S.E., standard error; OR, odds ratio; CI, confidence interval; LVH, left ventricular hypertrophy; CMO, composite major outcomes; CRRT, continuous renal replacement therapy; LVMI, left ventricular mass index.
Prognostic nomogram models for postoperative outcomes in ATAAD patients
Based on data from 193 enrolled patients, nomograms of postoperative CMO and cardiac events in ATAAD patients were developed and established, as shown in Figure 1 and Figure 2. Two models for postoperative CMO, model LVH and model LVMI, were developed (details were present in Table S2). Nomograms can be interpreted by adding up the points assigned to each variable, as indicated at the top of the point scale. The total point projected on the bottom scale represents the probability of postoperative CMO or cardiac events. Collinearity analyses of model LVH and model LVMI were performed, as showed in Table S3. Collinearity was not found in both models. Figure 3 showed the results of the calibration curve and decision curve analysis. Model LVH and model LVMI for postoperative CMO contained different indicators used in the nomogram. The area under curve was 0.825 (95% CI: 0.749–0.900) for Model LVH and 0.841 (95% CI: 0.776–0.905) for Model LVMI. The calibration curve and decision curve analysis indicated good clinical utility and consistency in both models in predicting postoperative CMO.


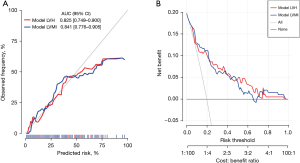
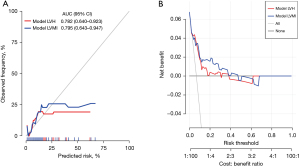
In addition, two models for postoperative cardiac events, model LVH and model LVMI, were developed and presented in Figure 2 (details were present in Table S4). Collinearity analyses of model LVH and model LVMI for cardiac events were performed, as showed in Table S5. Figure 4 showed the results of the calibration curve and decision curve analysis. The area under curve was 0.782 (95% CI: 0.640–0.923) for Model LVH and 0.795 (95% CI: 0.643–0.947) for Model LVMI. The calibration curve and decision curve analysis indicated good clinical utility and consistency in both models for postoperative cardiac events.
Discussion
Our main results were: (I) LVH was more prevalent in female patients with ATAAD. (II) Decreased left ventricular ejection fraction and cardiac tamponade were more prevalent in patients with LVH. (III) Increasing LVMI was associated with a higher risk of postoperative CMO and cardiac events. (IV) Nomogram models based on LVH/LVMI were developed for predicting postoperative CMO and cardiac events in ATAAD patients.
As a disastrous medical condition, acute aortic dissection has a high mortality rate, despite the 30-day mortality rate having decreased to 12.6% from 18.1% in recent two decades (5). Hypertension is diagnosed in approximately 80% of aortic dissection patients, which promotes aortic degeneration and weakens the aortic wall (21). Previous study has highlighted that established hypertension is associated with target organ damage, in particular, the heart, kidney, brain, etc. (22). Ventricular hypertrophy is regarded as a result of uncontrolled hypertension. Increased BP leads to left ventricular remodeling, including concentric or eccentric LVH, which results in an increased risk of adverse cardiovascular diseases (15).
Our results confirmed that LVH, which was diagnosed with an increased LVMI, was the independent risk factor for both postoperative CMO and cardiac events (showed in Table 5 and Table S2). The major concerns about LVH are adverse cardiovascular events (including sudden death, ischemic heart disease, heart failure, arrhythmias, and stroke), and impaired left ventricular diastolic/systolic function that associated with geometric changes (15,23,24). Our results indicated that LVH was related to higher risk of CMO (30.9% vs. 15.2%, P=0.02) and new-onset postoperative atrial fibrillation (10.9% vs. 2.9%, P=0.03), especially in operative mortality (18.2% vs. 7.2%, P=0.04) before matching. These results differ from previous reports by Rocha et al. (25). Rocha et al. reported that left ventricular concentricity, instead of hypertrophy, was related to a higher risk of mortality (25). However, one-fourth of involved type A aortic dissection patients were subacute/chronic. The previous study has demonstrated the significant difference in early and late outcomes among acute and subacute/chronic aortic dissection (26). The different compositions of subjects involved might contribute to the different results.
Since LVH was a binary variable with predefined diagnostic criteria, we then assessed the prognostic value of LVMI as a continuous variable, as showed in Table 7. After propensity score matching, the increasing LVMI was associated with worse outcomes, including postoperative CMO, CRRT, cardiac events, tracheotomy and atrial fibrillation. Our results suggested a better predictive value of LVMI as a continuous variable compared with binary defined LVH. Previously study demonstrated LVMI as a strong independent predictor of perioperative mortality after adult cardiac surgery, including coronary artery bypass grafting and transcatheter aortic valve replacement (27-29). Increased LVMI indicated poor controlled hypertension or unaware hypertension, which was associated with other hypertensive mediated organ damage, including renal damage and vascular dysfunction (30). In fact, decreased left ventricular ejection fraction and renal dysfunction were more prevalent in patients with LVH, as showed in Table 1 and Table 3. The hypertensive mediated organ damage, such as ventricular hypertrophy and renal dysfunction, might lead to poor prognosis for patients underwent cardiovascular surgery performed with CPB (31).
The results also indicated that the risk of CMO and cardiac events rapidly increased with prolonged CPB duration. Despite contemporary cardioprotective strategies having been well developed, ischemia-reperfusion injury and systemic inflammation that occurs during cardiopulmonary bypass may cause inevitable damage to the body (32). However, several studies reported that hypertrophic hearts are more vulnerable to ischemic–reperfusion injury, resulting in a larger infract area, higher peak cardiac troponin concentration and decreased LVEF (33-36). In addition, coronary microvascular dysfunction, which might present in some LVH patients, could also have an adverse effect on cardiomyocytes (37). Wever et al. reported that cardiac grafts with LVH from older donors contributed to a 6-fold increase in the risk of mortality after heart transplantation (38). Therefore, patients with LVH may be more susceptible to CPB-related injury due to their present cardiac abnormalities.
In conclusion, we conducted a retrospectively study with a relatively large sample to evaluate the impact of LVH in ATAAD patients who received surgical repair. We found that LVH was more prevalent in female patients. In addition, we confirmed the prognostic value of LVH/LVMI in predicting postoperative CMO and cardiac events for ATAAD patients. We also developed nomogram models for predicting postoperative CMO and cardiac events in ATAAD patients based on LVH or LVMI, that may help clinicians estimate prognosis in the early period after surgery. Future studies are required to investigate LVH’s effects on long-term prognosis in ATAAD patients.
This study has several limitations. First, our conclusion may not be generalizable to other populations and regions due to its single-central retrospective nature. Second, the study was based on data from acute type A aortic dissection patients who underwent surgical repairs. Therefore, results may be different in other aortic dissection patients. Third, genetic evidence is required for the diagnosis of hypertrophic cardiomyopathy. Therefore, subgroup analysis was not applied for hypertrophic cardiomyopathy. Patients with hypertrophic cardiomyopathy may differ in outcomes. In addition, LVH contributes to an increased risk of heart failure, which might result in poorer prognosis. Lastly, our model lacked external validation, therefore it should be regarded as a preliminary tool.
Acknowledgments
We would like to thank Dr. Jianglong Han for his assistance in statistical analyses and writing. We would like to thank Dr. Xianming Zhu for his help in polishing our paper.
Funding: This study was partially supported by the National Natural Science Foundation of China (Grant No. 82070481).
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-193/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-193/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-193/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-193/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the ethics commission of Renmin Hospital of Wuhan University (WDRM2020-K230). Informed consent was not required due to its retrospective nature.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hagan PG, Nienaber CA, Isselbacher EM, et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA 2000;283:897-903. [Crossref] [PubMed]
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the diagnosis and management of patients with thoracic aortic disease. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology,American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons,and Society for Vascular Medicine. J Am Coll Cardiol 2010;55:e27-e129. [Crossref] [PubMed]
- Nienaber CA, Clough RE. Management of acute aortic dissection. Lancet 2015;385:800-11. [Crossref] [PubMed]
- Andersen ND, Ganapathi AM, Hanna JM, et al. Outcomes of acute type a dissection repair before and after implementation of a multidisciplinary thoracic aortic surgery program. J Am Coll Cardiol 2014;63:1796-803. [Crossref] [PubMed]
- Zhu Y, Lingala B, Baiocchi M, et al. Type A Aortic Dissection-Experience Over 5 Decades: JACC Historical Breakthroughs in Perspective. J Am Coll Cardiol 2020;76:1703-13. [Crossref] [PubMed]
- Malvindi PG, Modi A, Miskolczi S, et al. Acute type A aortic dissection repair in elderly patients. Eur J Cardiothorac Surg 2015;48:664-70; discussion 671. [Crossref] [PubMed]
- Pape LA, Awais M, Woznicki EM, et al. Presentation, Diagnosis, and Outcomes of Acute Aortic Dissection: 17-Year Trends From the International Registry of Acute Aortic Dissection. J Am Coll Cardiol 2015;66:350-8. [Crossref] [PubMed]
- Bossone E, Eagle KA. Epidemiology and management of aortic disease: aortic aneurysms and acute aortic syndromes. Nat Rev Cardiol 2021;18:331-48. [Crossref] [PubMed]
- Seravalle G, Grassi G. Obesity and hypertension. Pharmacol Res 2017;122:1-7. [Crossref] [PubMed]
- Arnett DK, Claas SA. Omics of Blood Pressure and Hypertension. Circ Res 2018;122:1409-19. [Crossref] [PubMed]
- Manosroi W, Williams GH. Genetics of Human Primary Hypertension: Focus on Hormonal Mechanisms. Endocr Rev 2019;40:825-56. [Crossref] [PubMed]
- He FJ, Tan M, Ma Y, et al. Salt Reduction to Prevent Hypertension and Cardiovascular Disease: JACC State-of-the-Art Review. J Am Coll Cardiol 2020;75:632-47. [Crossref] [PubMed]
- Eirin A, Lerman A, Lerman LO. Mitochondrial injury and dysfunction in hypertension-induced cardiac damage. Eur Heart J 2014;35:3258-66. [Crossref] [PubMed]
- Shlomai G, Grassi G, Grossman E, et al. Assessment of target organ damage in the evaluation and follow-up of hypertensive patients: where do we stand? J Clin Hypertens (Greenwich) 2013;15:742-7. [Crossref] [PubMed]
- Yildiz M, Oktay AA, Stewart MH, et al. Left ventricular hypertrophy and hypertension. Prog Cardiovasc Dis 2020;63:10-21. [Crossref] [PubMed]
- Wang SX, Xue H, Zou YB, et al. Prevalence and risk factors for left ventricular hypertrophy and left ventricular geometric abnormality in the patients with hypertension among Han Chinese. Chin Med J (Engl) 2012;125:21-6. [Crossref] [PubMed]
- Iarussi D, Caruso A, Galderisi M, et al. Association of left ventricular hypertrophy and aortic dilation in patients with acute thoracic aortic dissection. Angiology 2001;52:447-55. [Crossref] [PubMed]
- Taylor AP, Freeman RV, Bartek MA, et al. Left ventricular hypertrophy is a possible biomarker for early mortality after type B aortic dissection. J Vasc Surg 2019;69:1710-8. [Crossref] [PubMed]
- Marwick TH, Gillebert TC, Aurigemma G, et al. Recommendations on the use of echocardiography in adult hypertension: a report from the European Association of Cardiovascular Imaging (EACVI) and the American Society of Echocardiography (ASE)†. Eur Heart J Cardiovasc Imaging 2015;16:577-605. [PubMed]
- Augoustides JG, Geirsson A, Szeto WY, et al. Observational study of mortality risk stratification by ischemic presentation in patients with acute type A aortic dissection: the Penn classification. Nat Clin Pract Cardiovasc Med 2009;6:140-6. [Crossref] [PubMed]
- Nienaber CA, Clough RE, Sakalihasan N, et al. Aortic dissection. Nat Rev Dis Primers 2016;2:16053. [Crossref] [PubMed]
- Messerli FH, Williams B, Ritz E. Essential hypertension. Lancet 2007;370:591-603. [Crossref] [PubMed]
- Oktay AA, Lavie CJ, Milani RV, et al. Current Perspectives on Left Ventricular Geometry in Systemic Hypertension. Prog Cardiovasc Dis 2016;59:235-46. [Crossref] [PubMed]
- Haider AW, Larson MG, Benjamin EJ, et al. Increased left ventricular mass and hypertrophy are associated with increased risk for sudden death. J Am Coll Cardiol 1998;32:1454-9. [Crossref] [PubMed]
- Rocha WEM, Oliveira MFRA, Soares JD, et al. Left Ventricular Concentric Geometric Patterns Are Associated With Worse Prognosis Among Patients With Type-A Aortic Dissection. J Am Heart Assoc 2021;10:e018273. [Crossref] [PubMed]
- Wu J, Xie E, Qiu J, et al. Subacute/chronic type A aortic dissection: a retrospective cohort study. Eur J Cardiothorac Surg 2020;57:388-96. [PubMed]
- Weiner MM, Reich DL, Lin HM, et al. Influence of increased left ventricular myocardial mass on early and late mortality after cardiac surgery. Br J Anaesth 2013;110:41-6. [Crossref] [PubMed]
- Zhu P, Dai Y, Qiu J, et al. Prognostic implications of left ventricular geometry in coronary artery bypass grafting patients. Quant Imaging Med Surg 2020;10:2274-84. [Crossref] [PubMed]
- Rozenbaum Z, Finkelstein A, Zhitomirsky S, et al. Impact of preprocedural left ventricle hypertrophy and geometrical patterns on mortality following TAVR. Am Heart J 2020;220:184-91. [Crossref] [PubMed]
- Korhonen PE, Kautiainen H, Järvenpää S, et al. Target organ damage and cardiovascular risk factors among subjects with previously undiagnosed hypertension. Eur J Prev Cardiol 2014;21:980-8. [Crossref] [PubMed]
- Apostolakis EE, Baikoussis NG, Parissis H, et al. Left ventricular diastolic dysfunction of the cardiac surgery patient; a point of view for the cardiac surgeon and cardio-anesthesiologist. J Cardiothorac Surg 2009;4:67. [Crossref] [PubMed]
- De Hert S, Moerman A. Myocardial injury and protection related to cardiopulmonary bypass. Best Pract Res Clin Anaesthesiol 2015;29:137-49. [Crossref] [PubMed]
- Stiermaier T, Pöss J, Eitel C, et al. Impact of left ventricular hypertrophy on myocardial injury in patients with ST-segment elevation myocardial infarction. Clin Res Cardiol 2018;107:1013-20. [Crossref] [PubMed]
- Fernández-Jiménez R, Silva J, Martínez-Martínez S, et al. Impact of left ventricular hypertrophy on troponin release during acute myocardial infarction: new insights from a comprehensive translational study. J Am Heart Assoc 2015;4:e001218. [Crossref] [PubMed]
- Mølgaard S, Faricelli B, Salomonsson M, et al. Increased myocardial vulnerability to ischemia-reperfusion injury in the presence of left ventricular hypertrophy. J Hypertens 2016;34:513-23; discussion 523. [Crossref] [PubMed]
- Nepper-Christensen L, Lønborg J, Ahtarovski KA, et al. Left Ventricular Hypertrophy Is Associated With Increased Infarct Size and Decreased Myocardial Salvage in Patients With ST-Segment Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention. J Am Heart Assoc 2017;6:004823. [Crossref] [PubMed]
- Camici PG, Tschöpe C, Di Carli MF, et al. Coronary microvascular dysfunction in hypertrophy and heart failure. Cardiovasc Res 2020;116:806-16. [Crossref] [PubMed]
- Wever Pinzon O, Stoddard G, Drakos SG, et al. Impact of donor left ventricular hypertrophy on survival after heart transplant. Am J Transplant 2011;11:2755-61. [Crossref] [PubMed]


