Lung transplantation at Duke
Lung transplantation remains the only potentially life-saving therapy for many individuals with end-stage lung disease. Utilization has continued to rise over the past 20 years. Most recently, the ISHLT registry reports that over 4,000 adult and pediatric lung transplants were performed internationally in 2013 (1).
Established in 1992, the lung transplant program at Duke University Medical Center remains one of the largest volume lung transplant centers in the world. Since its inception, our program has performed more than 1,600 lung transplants. We have previously reported our experience in the first 15 years of the program (2). This report describes more recent practice and management strategies, as well as a reflection upon the impact of the first ten years of the lung allocation score (LAS) in the U.S.
Since the implementation of the LAS in May of 2005, the lung transplant volume experience at Duke has grown and included 1,059 transplant procedures. This includes multi-organ transplants such as heart-lung, lung-liver, lung-kidney, heart-lung-liver, and lung-bone marrow transplants. Table 1 provides demographic data for lung only transplants performed at Duke University Medical Center following the implementation of the LAS.
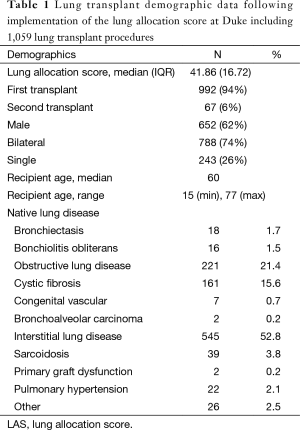
Full table
Transplant candidacy
The ISHLT recently updated its guidelines for selection criteria for lung transplant (1). In an attempt to balance the scarcity of donors and maximize societal benefit of lung transplantation, the indications for lung transplant have been updated to denote greater attention paid to the potential life years gained. It is now recommended that lung transplant only be considered in patients with >50% risk of death from lung disease within two years without transplant, >80% chance of 90-day survival after transplant and >80% expected 5-year survival with transplant from general medical perspective, provided adequate graft function.
The broadness of this document reflects our practice of considering the candidacy of every patient with end-stage lung disease. While numerous relative contraindications to transplantation may be present, a holistic risk assessment of each patient’s medical comorbidities, functional status, psychosocial milieu, and potential life expectancy with transplant opens up the possibility of lung transplantation to many who otherwise may not have previously been offered this therapy.
This strategy has led to our experience with multi-organ transplant combinations in those with severe multi-organ dysfunction. We will also offer combined lung transplant and cardiac surgery, including coronary artery bypass grafting (CABG), valve repair and complex vascular reconstructions to carefully selected patients. Our center has successfully bridged many critically ill patients to transplant using extracorporeal membrane oxygenation (ECMO). We have also performed successful staged lung—hematopoietic stem cell transplants in individuals with severe immunodeficiency syndromes with a goal of both allograft tolerance and cure of underlying immunodeficiency. Some exceptional circumstances, such as high degree of HLA sensitization, may require living lobar donation for lung transplantation as well.
Transplant evaluation
Our standard evaluation includes a multidisciplinary approach defined by pulmonary, cardiothoracic surgery, transplant psychology, social work, physical therapy and financial consultations. A nutritionist screens all candidates and performs a nutritional assessment in all patients with cystic fibrosis as well as those felt to be at increased risk for malnutrition. A clinical pharmacist meets with each patient for medication review and education prior to listing.
Based on data indicating an association between pre-transplant physical fitness and improved post-transplant survival, we require all patients who are able to participate in pre-transplant physical therapy (3,4). Our rigorous program maximizes respiratory muscle strength as well as total body conditioning in preparation for surgery. Physical requirements and exercise plan prior to transplantation are outlined in Table 2. We require candidates to walk at least 1,000 feet in 6 minutes (without limitation on oxygen usage) as well as 1/2 miles in 20 minutes on a track using as much oxygen as is necessary to maintain oxygen saturations >88%. While level surface walking is the most important component of the physical therapy program, it also includes stationary bike and strengthening, stretching and diaphragmatic breathing exercises. Included in the physical therapy program are educational classes intended to prepare both the candidate and his or her caregivers for routine post-transplant care as well as anticipatory guidance for common complications they might expect. These classes include teaching directed at self-monitoring of vital signs and home spirometry, transplant medications, diabetes management, managing a feeding tube and coping skills training.

Full table
Required pulmonary testing includes full pulmonary function testing, an arterial blood gas on room air, PA and lateral chest X-ray, a 6-minute walk test, non-contrast chest CT scan, quantitative ventilation and perfusion scan, and fluoroscopy of the diaphragms. Abnormal results will prompt additional testing. For instance, if abnormalities in swallowing function are identified on barium swallow, we perform functional endoscopic evaluation of swallowing (FEES) testing. Impaired diaphragm function may prompt maximal inspiratory and expiratory pressure measurements (MIP/MEP) with pulmonary function testing.
Cardiac evaluation includes an electrocardiogram, right heart catheterization and echocardiogram with bubble study on all patients. Those over the age of 40 undergo left heart catheterization or CT coronary angiography. If significant cardiac disease is found, intervention and a follow up stress test may be required. For good risk patients (e.g., less than 65 years old, high functional status, etc.) concomitant revascularization via CABG and lung transplant can be considered if necessary. For higher risk patients, pre-transplant percutaneous coronary revascularization via stenting is preferred. Drug eluting stents are avoided during the evaluation period because of the need to be off dual antiplatelet platelet therapy around the time of transplant. If it is anticipated a patient will require intra-aortic balloon pump support at the time of transplant, we will obtain a reconstructed CT angiogram of the abdomen and pelvis for vascular access planning. Patients with underlying sarcoidosis undergo cardiac MRI test to look for evidence of sarcoid infiltration of the heart. If significant involvement is seen, patients are considered for heart/lung transplant.
Our gastrointestinal evaluation includes a barium swallow, 24-hour pH probe testing, and esophageal manometry. Solid gastric emptying testing is performed when there is concern for gastroparesis. Imaging of the liver is also required to screen for cirrhosis. This is done with ultrasound in patients under the age of 55 and a CT scan in those over the age of 55. Patients with suspicious imaging, laboratory results, or clinical history may need liver fibroscan and/or biopsy with portal venous pressure gradient monitoring for additional investigations.
Lab testing includes routine hematologic, chemistry and coagulation studies as well as viral serologies for cytomegalovirus (CMV), herpes simplex virus (HSV), Epstein-Barr virus (EBV), and varicella zoster virus (VZV). We also screen for the indolent chronic infections such as syphilis, hepatitis B, C and HIV. Positive screening tests prompt polymerase chain reaction (PCR) testing and would require treatment prior to proceeding with transplant. Routine, age-appropriate cancer screening as recommended by the US Preventive Services Task Force (USPSTF) is required on all patients. We also perform serologic screening for malignancy using tumor markers, including prostate specific antigen (PSA), carcinoembryonic antigen (CEA), beta human chorionic gonadohormone (β-HCG) and follicle stimulating hormone (FSH). Positive results prompt more invasive testing. The identification of low-grade, indolent malignancies within two years of transplant listing requires specialty consultation, but is not necessarily considered an absolute contraindication to transplant at our center. These include Gleason stage 6 or less prostate cancer as well as localized non-melanoma skin cancers. Additionally, we will consider transplant in patients with stage 1 non-small cell lung cancer when the transplant would be curative treatment for disease.
Data suggest that patients with idiopathic pulmonary fibrosis related to telomerase mutations are at increased risk of bone marrow and renal failure after lung transplantation (5-7). Because of this, we have recently begun evaluating telomere length in patients with familial or early onset pulmonary fibrosis, premature greying of the hair and leukopenia or thrombocytopenia. If telomere length is confirmed to be in the bottom decile, we will trial the patient on immunosuppressives prior to transplant listing to ensure they are tolerant of therapies.
Management of HLA antibodies
All candidates are screened for the presence of HLA antibodies using flow cytometry. A positive result will prompt specificity testing using Luminex single antigen bead testing. Our lab uses a mean fluorescence intensity (MFI) cutoff of 1,000 to be considered a positive result. All circulating HLA antibodies are considered unacceptable antigens and are avoided by means of a virtual crossmatch in all patients with calculated panel reactive antibody (cPRA) of less than 25%. For patients with a cPRA of greater than 25%, the HLA lab will generate a chart with all antibodies and their intensity over time for all patients prior to listing. The transplant physicians then, in consultation with the HLA lab, determine which antibodies are most likely to result in a positive crossmatch. Generally, this includes all antibodies present at 1:16 dilution and those with MFI >4,000. These HLA antibodies are all considered unacceptable antigens and avoided by means of a virtual crossmatch. Antibodies with less intensity or antigens with less cell surface expression that are thought to be possibly non-specific or not clinically significant are included on the waitlist and considered unacceptable on virtual crossmatch. However, if a donor is available and a prospective crossmatch can be performed and is negative, that organ may be accepted for that recipient. All patients with a cPRA ≥25% are treated with intravenous immunoglobulin (IVIG) intraoperatively at 2 grams/kilogram dose.
On some occasions, we have accepted a donor for a highly sensitized recipient who is clinically deteriorating when there is a positive virtual crossmatch for an antigen that is of questionable intensity, and a prospective crossmatch is not possible. Plasmapheresis is performed intraoperatively and rabbit antithymocyte globulin (rATG, 3 g/kg dose) is used for induction instead of basiliximab. Patients then receive rituximab 1 gram IV on post-operative day 1. Further plasmapheresis and antibody-directed therapy may be continued depending on the results of the retrospective crossmatch. Another option in this scenario would be to perform ex-vivo perfusion of the lungs in order to delay transplant until a negative crossmatch is confirmed.
Donor selection and management
Aggressive potential donor evaluation and management, paired with prudent selection of donors, has at our center led to minimal wait list mortality without adversely impacting short- or long-term outcomes following transplantation. In particular, appropriate donor management is critical to the optimization of potential allografts.
International guidelines from the ISHLT inform rough criteria with which to evaluate a potential donor. Our group has demonstrated that the donor pool may be safely increased through the careful selection of donors outside the concept of an “ideal” donor as described by early international guidelines. We evaluate donors over the age of 55, as well as organs that may require periods of cold ischemia greater than 6 hours, as the more conservative measures excluding those donors do not result in improved outcomes in the available published evidence (8).
Frequently, donor management during the period of evaluation may not reflect the optimum strategy for lung preservation. In particular, low volume and low pressure ventilatory settings may lead to donor lung atelectasis that manifests as inadequate gas exchange and abnormal chest roentogram. Low PaO2/FiO2 ratios (less than 300) may frequently be due to reversible conditions such as atelectasis, pulmonary edema, or mucous plugging. With appropriate recruitment of the donor lungs and pulmonary secretion clearance, significant improvement in gas exchange is achievable and excellent post-transplant outcomes may be attained with organs initially felt to be unsuitable for transplantation. In addition to recruitment maneuvers to improve oxygenation, the donor’s hemodynamics and physiology should be optimized. Hemodynamic stability should be achieved with minimal use of inotropic support. When needed, vasopressin arginine may support blood pressure and permit diuresis to optimize donor fluid balance and acid-base status. The use of a pulmonary artery catheter is frequently advised to permit goal-directed therapies for the attainment of appropriate loading conditions and optimum volume status. Published data from randomized trials demonstrate that a judicious use of diuretics, conservative fluid management, and protective ventilator protocols for donors leads to improved lung allograft utility, without adversely affecting other organ function (9).
Although donor cultures from bronchoalveolar lavage are important for appropriate tailoring of post-transplant antibiotics, rarely should donor microbiologic results prohibit or dissuade use of the allograft for transplantation. Culture-directed antibiotic therapy successfully prevents fatal complications in the event of donor to recipient transmission of infectious organisms (10). However, potential donors colonized with Genomovar 3 Burkholderia cenocepacia or other highly virulent, multi-drug resistant organisms may need to be excluded from donation on account of extremely complex resistance patterns (11).
The candidate donor should be size-matched to the anticipated recipient. In our practice, horizontal and vertical measurements based on plain chest radiographs are used to assist with appropriate matching. The predicted total lung capacity of recipients and donors can also be calculated using standard formulation. Extreme size mismatch, either too large or too small, confers a survival disadvantage in published series (12,13). In the event of a large donor matched to a smaller recipient, lung reduction can be performed at the time of transplantation. Our preferred method of pneumoreduction is anatomic resection of the right middle lobe and/or lingulectomy. Very rarely cadaveric lobar transplantation can be performed; however, this appears to increase the perioperative risks of transplantation to some degree (14).
Ex vivo lung perfusion (EVLP) is deployed clinically in selected donors as a means to further interrogate allograft function prior to committing to transplant. Although it may possibly improve the quality of an otherwise marginal candidate allograft, current EVLP technologies permits further assessment of the graft prior to proceeding with transplantation. Candidate grafts in which the suitability for transplantation is uncertain may be serially assessed on the EVLP device during a period of optimum ventilator management in order to ascertain if the graft is appropriate for transplantation. We use clinically the XVIVO Perfusion System (XPS™) as was used in the NOVEL clinical trial. This is currently the only device for EVLP approved for use in the United States by the FDA. In our experience, nearly half of the allografts evaluated on the XPS system were subsequently used for transplantation. We have transplanted 20 recipients thus far using this device, with short and long terms outcomes no different from our standard lung donor cohort. The final results of the trial have yet to be published and the sponsor is actively accruing additional patients for an extension of the trial.
Surgical approach
As introduced previously, every attempt is made to match the candidate recipient with the optimum procedure. Comprehensive evaluation identifies the appropriateness of the available therapies. Single-lung, bilateral-lung, bilateral lobar, heart-lung, and lung with concomitant cardiac surgery are all available therapies based on pre-transplant evaluation.
For the majority of patients, bilateral orthotopic lung transplantation (BOLT) is the preferred procedure. Patients receiving bilateral allografts enjoy improved long-term survival and a lower rate of chronic allograft dysfunction (15). Septic lung disease such as cystic fibrosis mandates BOLT, as does severe pulmonary hypertension. In the case of interstitial lung disease, we do stratify patients based on age, functional status, and other comorbidities. We pursue BOLT for lower risk patients and single orthotopic lung transplantation (SOLT) for patients thought to be at a high perioperative risk. We generally consider higher risk patients to be those over age 65, with coronary disease, marginal renal function, or increased frailty. Data suggest that in older patients with idiopathic pulmonary fibrosis (IPF), the long-term benefits of BOLT may not be fully realized due to increased perioperative risk (16). In very select circumstances we have considered staging a bilateral procedure by performing two single lung transplants at discrete time points. We have done this electively in 12 recipients. Results suggest similar perioperative outcomes except for diminished rates of renal dysfunction in the staged BOLT approach. Long-term benefit of the staged approached is still under investigation.
For single lung transplantation, an anterolateral thoracotomy incision in the 4th or 5th intercostal space permits excellent exposure for the transplant procedure. This can also be done via a posterolateral approach if the surgeon prefers. For double lung transplantation, we prefer a clamshell incision by way of bilateral anterolateral thoracotomies in the 4th intercostal space, in conjunction with a transverse sternotomy. The clamshell incision yields generous exposure and can facilitate rapid deployment of cardiopulmonary bypass or ECMO if needed. In patients with a planned concomitant cardiac procedure that mandates cardiopulmonary bypass (CPB), a median sternotomy may be optimal if pleural adhesions are thought to be minimal. Our preference is to avoid CPB if possible as it has been associated with increased rates of primary graft dysfunction (PGD) and transfusion requirements. Mechanical support intraoperatively should be tailored to the needs of the present scenario; however, ECMO remains our preferred support method if full bypass is not required. Intra-aortic balloon pump (IABP) is a useful adjunct for patients with depressed left ventricular (LV) function and those with coronary artery disease.
Though our evaluation and listing process aims to identify patients sick enough to benefit from transplantation but otherwise healthy enough to tolerate the procedure, a small subset of our patient population progresses to respiratory failure pre-transplant. In highly selected patients, we provide ECMO support as a bridge to transplantation. In this setting, our most common practice is to support these patients with veno-venous (VV) ECMO through a percutaneously inserted Avalon catheter into the right internal jugular vein, then initiate pre-transplant active rehabilitation as a means to recover the debilitated patient prior to transplant. Nutritional support is via a gastrojejunostomy tube and sedation and ventilator support are weaned as low as possible or off as tolerated once ECMO is initiated. Active rehabilitation while on ECMO includes passive resistance exercises, as well as ambulation. Figure 1 depicts a patient ambulating while supported by VV-ECMO. Our early experience included patients supported in this manner with VV-ECMO as a bridge to transplantation, with 100% survival to one year. Patients able to ambulate and participate in physical therapy while supported by VV-ECMO pre-transplant demonstrated significantly shorter times to extubation, shorter ICU stays, and shorter index hospitalizations. Economic analysis suggests that these benefits associated with ambulatory ECMO lead to decreased total cost of index hospitalization associated with lung transplantation (17-19). Veno-arterial (VA) ECMO may be necessary in patients with severe PH and RV failure who require mechanical support prior to bridging. Utilizing an axillary arterial and right IJ cannulation strategy, our strategy of active rehab while on ECMO can still be attained. The transplant procedure may be conducted while on ECMO support, or transitioned to cardiopulmonary bypass if required.

Duke has made additional contributions to the field of lung transplant in pioneering novel procedures to treat complex vascular abnormalities that might otherwise pose a contraindication to transplantation. We described the use of simultaneous lung and RVOT allograft as a means to treat aneurysmal disease of the pulmonary artery at the time of lung transplant (20). Transplantation of the RVOT avoids the need for concomitant heart transplant or the need for a complex repair with prosthetic material to treat a pulmonary artery aneurysm. This approach has been utilized in both single and bilateral lung transplant procedures, as well as in the setting of reoperation after a remote correction of tetralogy of Fallot led to pseudoaneurysm of the RVOT. In each setting, the use of RVOT allograft can minimize morbidity and permit transplantation in patients who might otherwise be turned down for the procedure. If the RVOT allograft is not available, then at times a homograft has been utilized with good success.
Primary graft dysfunction (PGD)
PGD after lung transplantation remains a significant source of early morbidity and mortality. Patients surviving PGD are also at risk for long-term alloimmune consequences and decreased overall survival, suggesting a link between PGD and subsequent development of BOS. Prompt diagnostic workup is mandatory to evaluate for alternative causes of respiratory failure, to include vascular torsion, infection, cardiogenic edema, or hyperacute rejection. During the transplant procedure, several steps are taken at our center to minimize the extent of reperfusion injury experienced by the allograft. In addition to the use of extracellular preservation solutions, we administer intravenous methylprednisolone (500 mg) and mannitol (25 mg) prior to reperfusion of both allografts. Importantly, reperfusion is performed in a controlled fashion over a period of 10-15 minutes. Similarly, ventilation and lung recruitment should be held until the newly implanted lung has rewarmed. Inhaled nitric oxide (iNO) is used to decrease pulmonary vascular resistance during the operation. If additional pulmonary vasodilation is thought to be necessary, the patient can be weaned from iNO to inhaled epoprostenol (Veletri) after initial stabilization in the intensive care unit and prior to extubation.
Those patients exhibiting PGD despite preventive measures are considered for ECMO support. Those with peak inspiratory pressures approaching 30 cm H2O and requiring FiO2 greater than 0.60 after excluding other causes for failure are considered candidates for post-transplant ECMO. VV ECMO provides short-term support while lung recovery is anticipated. Since 2001, approximately 5% of lung transplant recipients at our center have required VV-ECMO support for primary graft dysfunction following transplant. Support can be initiated at the bedside by way of a single dual-lumen cannula in the right internal jugular (RIJ) vein. In consultation with our anesthesia teams, our preference is to place central lines in the left internal jugular vein pre-transplant in order to more easily facilitate initiation of ECMO by way of the RIJ if needed post-transplant. Once ECMO support is established, patients are transitioned to lung-protective ventilatory settings with low pressures and FiO2 of 0.21. Of those patients requiring VV-ECMO post-transplant at our center, over 95% are successfully weaned from support as their graft performance improves. Patients are typically weaned from ECMO within 24–72 hours as evidence of pulmonary recovery is observed. Though survival rates of those experiencing PGD continue to improve with advances in ECMO technology, PGD continues to decrease overall survival rates and leads to a decrease in overall graft function once free from ECMO support (21-23).
Immunosuppression
Our standard immunosuppression regimen consists of basiliximab for induction and tacrolimus, prednisone and mycophenolate mofetil for maintenance immunosuppression. Basiliximab 20 mg is administered intraoperatively and again on postoperative day 4. Intraoperatively we also administer 500 mg IV methylprednisolone at the time of each allograft reperfusion and mycophenolate mofetil 1,000 mg intravenously once. The recipient starts tacrolimus prior to the transplant, at the time the donor lungs are deemed acceptable and the decision to proceed with transplant is made, with a single dose of 1 mg tacrolimus sublingual (0.5 mg for patients >age 65 or on a triazole antifungal).
Tacrolimus troughs are measured starting post-operative day 2. We typically target tacrolimus trough levels 12–15 mcg/L in the first year, with lower target troughs in patients over the age of 65 or with significant renal dysfunction. Target troughs are generally decreased over time depending on rejection episodes and renal function. Patients are given methylprednisolone 125 mg IV q12h ×4 doses and then maintained on prednisone 20 mg daily for the first three months. Prednisone is typically tapered in 5 mg increments every three months until a basal dose of 5 mg daily is reached. Mycophenolate is continued at 1,000 mg twice daily, with discontinuation or dose reductions in the setting of leukopenia or severe infectious complications.
All patients with a cPRA ≥25% are treated with intravenous immunoglobulin (IVIG) intraoperatively as stated previously. IVIG is continued weekly for six weeks after the transplant, then monthly for three months and then every three months for the first year after transplant. If the HLA antibody screen is negative on two samples, IVIG is discontinued.
Infection prophylaxis
Standard intraoperative antibiotic prophylaxis includes cefepime for gram negative coverage, vancomycin for gram positive coverage and fluconazole for candida prophylaxis. The cefepime is typically discontinued after 7–10 days once all intraoperative cultures are finalized as negative. Vancomycin is generally continued for the duration of chest tubes being in place. We have recently begun extending fluconazole duration for 90 days after the transplant to decrease risk of invasive candidiasis. We also use inhaled liposomal amphotericin for additional fungal prophylaxis to target airway mold colonization. This starts POD 1, and continues daily ×4 days prior to going to weekly for the duration of the transplant hospitalization. Patients with known pretransplant colonization with antimicrobial pathogens, such as those with cystic fibrosis, are evaluated by transplant infectious disease for development of a customized perioperative antibiotic regimen. We typically continue pathogen-directed antimicrobials for a minimum of 14 days post transplant.
We use sulfamethoxazole/trimethoprim 80/360 mg daily as our first line agent for pneumocystis jeroveci prophylaxis starting seven days after the transplant and continuing indefinitely. Inhaled pentamidine, dapsone and atovaquone are second line agents used in those with intolerance to sulfamethoxazole/trimethoprim. Patients take nystatin swish and swallow four times daily for the first six months post transplant for oral candida prophylaxis.
Our viral prophylaxis protocol is dependent on donor and recipient CMV status. Recipients who are at risk for CMV going into transplant (either recipient CMV IgG positive or donor CMV IgM/IgG positive) are treated initially with ganciclovir 5 mg/kg IV q24h and transitioned to valganciclovir 900 mg PO daily. Recipients with prior exposure to CMV going into transplant are continued on CMV prophylaxis for 12 months following transplant. Those who are high risk for CMV disease due to donor CMV IgG positivity without pre-transplant recipient exposure, i.e., CMV recipient IgG negative, are continued on prophylaxis indefinitely as tolerated. Gancilcovir and valganciclovir dosing is adjusted based on renal function. In patients who are both donor and recipient CMV IgG negative, acyclovir prophylaxis is given IV initially and then at a dose of 400 mg PO bid for the first six months after transplant.
Post-transplant monitoring
After discharge from the transplant hospitalization, patients return to lung transplant physical therapy for reconditioning and strength training. All are required to complete a minimum of 23 sessions. They are followed in the transplant pulmonary clinic on a weekly basis during this time. We monitor radiographic imaging, spirometry, blood gases, immunosuppressive drug levels and routine labs.
In the first year after the transplant we perform regularly scheduled surveillance bronchoscopies with bronchoalveolar lavage and transbronchial lung biopsy at 2–4 weeks, 3, 6, 9 and 12 months after the transplant. Bronchoscopies are also performed as clinically indicated (new respiratory symptoms, radiographic abnormalities, drop in lung function). We monitor for CMV with serum PCR testing and the development of HLA antibodies at the time of routine surveillance bronchoscopies. If acute rejection is found, two follow-up bronchoscopies are performed at 4-6-week intervals after treatment to ensure the rejection has been effectively managed. Thereafter, we generally perform an annual bronchoscopy long term as a screening for indolent rejection and infection.
The bronchoalveolar lavage return is sent for cell differential, bacterial, fungal, mycobacterial culture and an extended respiratory viral PCR. The viral PCR analyzes for the presence of influenza, RSV, adenovirus, parainfluenza, human metapneumovirus and rhinovirus. Patients with a neutrophilic-predominant cell differential on bronchoalveolar lavage are considered for treatment with azithromycin 250 mg PO three times weekly.
Management of rejection
Our standard, first line treatment of acute cellular rejection is with corticosteroids. We use methylprednisolone 10 mg/kg IV daily (rounded to the nearest 250 mg) ×3 days followed by a taper of prednisone starting at 60 mg daily and decreasing by 5 mg daily until the patient reaches his or her baseline dose. The protocol for the treatment of antibody-mediated rejection is outlined in Table 3.
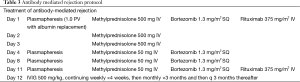
Full table
For patients with severe or refractory rejection, we treat with anti-thymocyte globulin. We typically use rabbit-derived anti-thymoglobulin at a dose of 1.5 mg/kg ×3 doses as first choice, but also use equine-derived formulations on occasion. In the setting of refractory rejection, we also evaluate for possible drivers of the rejection, such as CMV infection, inadequate calcineurin inhibitor levels, aspiration injury, medication nonadherence, community-acquired respiratory viruses and development of HLA antibodies. We consider adjusting basal immunosuppression. This may mean changing route of tacrolimus administration from PO to SL, changing from tacrolimus to cyclosporine, or an alternative to mycophenolate such as azathioprine or sirolimus.
Patients who experience either acute cellular rejection after thymoglobulin or in those with evidence of chronic lung allograft dysfunction (CLAD) are considered for alemtuzumab. This is given as a one-time, 30 mg dose. We routinely initiate extended antifungal and antiviral prophylaxis after alemtuzumab to decrease the risk of opportunistic infections. Preferred antifungal prophylaxis is posaconazole delayed release. Viral prophylaxis is dependent on the CMV status of the donor and recipient. Prophylaxis is continued until the CD4 count is greater than 100.
Because of the evidence indicating worse outcomes in patients with donor specific anti-HLA antibodies, we routinely monitor our patients for the development of anti-HLA antibodies (24,25). Patients who are highly sensitized prior to transplant or who develop new onset HLA antibodies after transplant are managed with intravenous immunoglobin (IVIG). Those who develop donor specific HLA antibodies, but do not have evidence of graft dysfunction are treated with rituximab 375 mg/m2 IV weekly ×4 doses. This is followed by IVIG monthly for three months and then every three months for a year or until resolution of the donor specific antibodies. Rituximab is a chimeric monoclonal antibody directed against CD20 expressing cells which results in depletion of B cells. When there is a concern for antibody mediated rejection, typically based on the presence of donor specific HLA antibodies, pathologic findings and graft dysfunction, we initiate our DSA—pheresis protocol. This multimodal strategy includes plasmapheresis, high dose steroids, rituximab, bortezomib which is a proteasome inhibitor which results in plasma cell apoptosis, and IVIG (Table 4).

Full table
Management of gastroesophageal reflux disease (GERD)
Our program takes an aggressive approach to management of gastroesophageal reflux disease. As stated above, all patients are evaluated for reflux prior to transplant. Those with significant GERD prior to transplant (acid contact times >10% total or demeester score >20) are arranged to undergo early fundoplication after transplant, with a goal of having the procedure within the first 90 days of the transplant, depending on clinical stability and fitness for surgery. Those without significant GERD prior to transplant have repeated testing done after they are discharged from the transplant hospitalization. Studies from our center, as well as others, have shown that early fundoplication confers advantage both with respect to overall survival and in freedom from bronchiolitis obliterans (26,27).
Long-term management
While freedom from chronic allograft dysfunction decreases over time, the rates of malignancy and renal dysfunction after lung transplant increase and are not inconsequential (28,29). Therefore, long term management of lung transplant recipients requires continued close monitoring of allograft function balanced against risks of immunosuppression. We generally evaluate patients every 3-4 months in lung transplant clinic for the duration of their lifespan. In addition to assessing allograft function, we screen for complications such as hypertension, hyperlipidemia, diabetes mellitus, cardiovascular disease, chronic kidney disease, hematologic disorders and malignancy routinely. We then collaborate closely with primary care providers and other subspecialists to manage these conditions and optimize outcomes for our patients.
Conclusions
Lung transplant remains an important and growing treatment option for patients with many kinds of end-stage lung disease. Since the establishment of a lung transplant program in 1992, Duke has strived for excellence in the management of patients with thoracic disease. A continuous commitment to the delivery of high-quality care has enabled Duke to meet increasing demand for this life-saving therapy. Pioneering technologies, techniques, and management strategies have enabled the program to offer this therapy to those who might previously have been denied eligibility for transplant, to rescue those who suffer graft dysfunction postoperatively, and to use early interventions to minimize post-transplant complications. In this update, we have reviewed the evidence that guides these changes in practice. Patients continue to become sicker and more complex in their comorbidities. Steady improvement in survival metrics reflects an increasing ability to safely treat these patients. More radical improvements in the field remain just ahead as we learn to take advantage of new technologies such as EVLP and novel immunosuppression. Discoveries such as these will increase the limited donor pool, allow for organ manipulation leading to improved long-term outcomes, and selectively protect the organ from immunologic injury.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Yusen RD, Edwards LB, Kucheryavaya AY, et al. The registry of the International Society for Heart and Lung Transplantation: thirty-first adult lung and heart-lung transplant report--2014; focus theme: retransplantation. J Heart Lung Transplant 2014;33:1009-24. [Crossref] [PubMed]
- Hartwig MG, Snyder LD, Finlen-Copeland A, et al. Lung transplantation at Duke University. Clin Transpl 2009.197-210. [PubMed]
- Castleberry AW, Englum BR, Snyder LD, et al. The utility of preoperative six-minute-walk distance in lung transplantation. Am J Respir Crit Care Med 2015;192:843-52. [Crossref] [PubMed]
- Martinu T, Babyak MA, O'Connell CF, et al. Baseline 6-min walk distance predicts survival in lung transplant candidates. Am J Transplant 2008;8:1498-505. [Crossref] [PubMed]
- Tokman S, Singer JP, Devine MS, et al. Clinical outcomes of lung transplant recipients with telomerase mutations. J Heart Lung Transplant 2015;34:1318-24. [Crossref] [PubMed]
- Borie R, Kannengiesser C, Hirschi S, et al. Severe hematologic complications after lung transplantation in patients with telomerase complex mutations. J Heart Lung Transplant 2015;34:538-46. [Crossref] [PubMed]
- Silhan LL, Shah PD, Chambers DC, et al. Lung transplantation in telomerase mutation carriers with pulmonary fibrosis. Eur Respir J 2014;44:178-87. [Crossref] [PubMed]
- Grimm JC, Valero V 3rd, Kilic A, et al. Association Between Prolonged Graft Ischemia and Primary Graft Failure or Survival Following Lung Transplantation. JAMA Surg 2015;150:547-53. [Crossref] [PubMed]
- Mascia L, Pasero D, Slutsky AS, et al. Effect of a lung protective strategy for organ donors on eligibility and availability of lungs for transplantation: a randomized controlled trial. JAMA 2010;304:2620-7. [Crossref] [PubMed]
- Ruiz I, Gavaldà J, Monforte V, et al. Donor-to-host transmission of bacterial and fungal infections in lung transplantation. Am J Transplant 2006;6:178-82. [Crossref] [PubMed]
- Alexander BD, Petzold EW, Reller LB, et al. Survival after lung transplantation of cystic fibrosis patients infected with Burkholderia cepacia complex. Am J Transplant 2008;8:1025-30. [Crossref] [PubMed]
- Eberlein M, Reed RM, Bolukbas S, et al. Lung size mismatch and primary graft dysfunction after bilateral lung transplantation. J Heart Lung Transplant 2015;34:233-40. [Crossref] [PubMed]
- Eberlein M, Reed RM, Bolukbas S, et al. Lung size mismatch and survival after single and bilateral lung transplantation. Ann Thorac Surg 2013;96:457-63. [Crossref] [PubMed]
- Keating DT, Marasco SF, Negri J, et al. Long-term outcomes of cadaveric lobar lung transplantation: helping to maximize resources. J Heart Lung Transplant 2010;29:439-44. [Crossref] [PubMed]
- Hadjiliadis D, Chaparro C, Gutierrez C, et al. Impact of lung transplant operation on bronchiolitis obliterans syndrome in patients with chronic obstructive pulmonary disease. Am J Transplant 2006;6:183-9. [Crossref] [PubMed]
- Thabut G, Christie JD, Ravaud P, et al. Survival after bilateral versus single-lung transplantation for idiopathic pulmonary fibrosis. Ann Intern Med 2009;151:767-74. [Crossref] [PubMed]
- Rehder KJ, Turner DA, Hartwig MG, et al. Active rehabilitation during extracorporeal membrane oxygenation as a bridge to lung transplantation. Respir Care 2013;58:1291-8. [Crossref] [PubMed]
- Bain JC, Turner DA, Rehder KJ, et al. Economic Outcomes of Extracorporeal Membrane Oxygenation With and Without Ambulation as a Bridge to Lung Transplantation. Respir Care 2016;61:1-7. [Crossref] [PubMed]
- Gulack BC, Hirji SA, Hartwig MG. Bridge to lung transplantation and rescue post-transplant: the expanding role of extracorporeal membrane oxygenation. J Thorac Dis 2014;6:1070-9. [PubMed]
- Zanotti G, Hartwig MG, Davis RD. A simplified technique for pulmonary artery aneurysm repair in a lung transplant recipient with right ventricular outflow tract obstruction. J Thorac Cardiovasc Surg 2013;145:295-6. [Crossref] [PubMed]
- Hartwig MG, Appel JZ 3rd, Cantu E 3rd, et al. Improved results treating lung allograft failure with venovenous extracorporeal membrane oxygenation. Ann Thorac Surg 2005;80:1872-9; discussion 1879-80.
- Hartwig MG, Walczak R, Lin SS, et al. Improved survival but marginal allograft function in patients treated with extracorporeal membrane oxygenation after lung transplantation. Ann Thorac Surg 2012;93:366-71. [Crossref] [PubMed]
- Castleberry AW, Hartwig MG, Whitson BA. Extracorporeal membrane oxygenation post lung transplantation. Curr Opin Organ Transplant 2013;18:524-30. [Crossref] [PubMed]
- Snyder LD, Wang Z, Chen DF, et al. Implications for human leukocyte antigen antibodies after lung transplantation: a 10-year experience in 441 patients. Chest 2013;144:226-33. [Crossref] [PubMed]
- Morrell MR, Pilewski JM, Gries CJ, et al. De novo donor-specific HLA antibodies are associated with early and high-grade bronchiolitis obliterans syndrome and death after lung transplantation. J Heart Lung Transplant 2014;33:1288-94. [Crossref] [PubMed]
- Cantu E 3rd, Appel JZ 3rd, Hartwig MG, et al. J. Maxwell Chamberlain Memorial Paper. Early fundoplication prevents chronic allograft dysfunction in patients with gastroesophageal reflux disease. Ann Thorac Surg 2004;78:1142-51; discussion 1142-51. [Crossref] [PubMed]
- Hartwig MG, Anderson DJ, Onaitis MW, et al. Fundoplication after lung transplantation prevents the allograft dysfunction associated with reflux. Ann Thorac Surg 2011;92:462-8; discussion; 468-9.
- Osho AA, Castleberry AW, Snyder LD, et al. Assessment of different threshold preoperative glomerular filtration rates as markers of outcomes in lung transplantation. Ann Thorac Surg 2014;98:283-9; discussion 289-90. [Crossref] [PubMed]
- Osho AA, Castleberry AW, Snyder LD, et al. Determining eligibility for lung transplantation: A nationwide assessment of the cutoff glomerular filtration rate. J Heart Lung Transplant 2015;34:571-9. [Crossref] [PubMed]


