Video-assisted thoracoscopic surgery lobectomy for lung cancer versus thoracotomy: a less decrease in sVEGFR2 level after surgery
Introduction
Vascular endothelial growth factor (VEGF), the most potent angiogenic factor noted to date, is vital to both wound healing and tumor growth by directly inducing vascular endothelial cell proliferation, migration, tube formation and angiogenesis (1). The plasma level of VEGF rises and reaches the maximal level at two weeks after major surgical injury, and then gradually decreases until four weeks after operation (2). The VEGF family consists of five related homodimeric glycoproteins in mammals, including VEGF-A, B, C, D, E and placenta growth factor (PIGF)-1 and 2 (3). The biological effects of VEGF are mediated by VEGF receptors (VEGFRs), a major type of cell surface receptor, leading to their subsequent hetero- or homo-dimerization and activation through transphosphorylation (4-6).
VEGFR1 and VEGFR2 are the two major VEGF receptors and exhibit high structure similarities. But both recepotors have substantial difference such as VEGF-binding properties, signal transduction and angiogenesis (7,8). sVEGFR1 and sVEGFR2 are the soluble forms of each receptor through alternative splicing of VEGFR1 and VEGFR2 mRNA. Due to lack of the intracellular kinase domains, they can’t induce signaling transduction. But the presence of extracelluar VEGF-binding domains in sVEGRF1 and sVEGRF2 ensure that they can sequester plasma VEGF and decrease the level of free VEGF, thus decreasing pro-angiogenic effect on vascular endothelial cell. sVEGFR1 and sVEGFR2 are considered as negative regulators in the process of angiogenesis by decreasing the availability of VEGF to the endothelial cells (9-11).
Surgical resection remains the standard therapy for lung cancer, however, a high percentage of patients develop recurrences in recent years (12). As mentioned above, the levels of VEGF are progressively increased after surgery for at least two weeks in lung cancer patients. The persistent increase of VEGF level may stimulate the growth of residual tumor in cancer patients and the formation of new blood vessels in solid lung cancer. Therefore, the balance of VEGF and its soluble form of receptors, sVEGFR1 and sVEGFR2, is critical for regulating vessel formation.
Presently, minimally invasive video-assisted thoracoscopic surgery (VATS) and OT are the two major types of thoracic surgery, and pulmonary resection remains the mainstay of treatment for lung cancer patients. Although considerable controversy about the advantages of VATS compared with conventional approach still remains (13), VATS has been used more and more in daily practice for the treatment of lung cancers especially NSCLC in the last decade (14,15).
Little is known about the influences of VATS lobectomy and open by thoracotomy for early stage non-small cell lung cancer (NSCLC) on postoperative circulating sVEGFR1 and sVEGFR2 levels. The purpose of this study was to determine and compare the impact of VATS and OT on the plasma levels of sVEGFR1 and sVEGFR2 during the first week after surgery in lung cancer patients.
Materials and methods
Study population
In this study, 48 patients with stage I NSCLC, who were found solitary pulmonary mass by chest X-ray or CT scan at the First People’s hospital of Yunnan Province between January 2013 and June 2014, were recruited. The pathologic classification of lung cancer was confirmed by histological examination. Patients who had received preoperative radiotherapy and chemotherapy were excluded. Written informed consents were given and approval for this study was obtained from the research ethics committee of Kunming University of Science and Technology.
Patients were operated by either VATS (n=26) or OT (n=22). The VATS lobectomy procedure was performed by a standardized three-port anterior approach described by us (16-19). The OT procedure was performed by either the direct anterior approach or the posterolateral approach. Contraindications to the VATS approach include the followings: T3 or T4 lung cancer, tumor larger than 6 cm, centrally placed tumors in the hilum and adherent to vessels, tumors visible in the bronchus. There was no significant difference in baseline variables including age, gender, cancer stage and histology between VATS group and OT group.
Blood sampling, processing and the determination of sVEGFR1 and sVEGFR2 levels
Blood samples were obtained preoperatively (PreOP) within 4 h of surgery and on postoperative days (POD) 1, 3 and 7 for all patients. Plasma was used to evaluate the sVEGFR1 and sVEGFR2 concentration in blood, and EDTA was used as an anticoagulant. Within 30 minutes after the collection, the plasma was isolated via centrifugation at 500 g and stored frozen at −20 °C to avoid loss of bioactive of sVEGFR1 and sVEGFR2. The concentrations of sVEGFR1 and sVEGFR2 were determined by commercially available enzyme-linked immunosorbent assay (ELISA) kits (eBioscience, San Diego, CA, USA). All procedures were performed according to the manufacturer’s instructions.
Statistical analyses
SPSS 19.0 statistics package was used for statistical analysis. The experimental data were reported with means and standard deviations, and compared by independent samples t-tests. The non-parametric Mann-Whitney U-test was used to analyze clinical variables. All categorical data such as sex and tumor stage were expressed as frequencies and compared by Fisher’s exact test. A two-sided P value of less than 0.05 was considered significant.
Results
Clinical finding
In this study, a total of 48 NSCLC patients met the study criteria and were recruited. Twenty six of them were in the VATS group and 22 were in the OT group. There were no significant demographic differences between these two groups (Table 1). The duration of surgery for patients of both VATS and OT groups were not significantly different in this study, although there was a weak tendency towards a slightly longer procedure for VATS. The number and location of lymph nodes dissected were comparable between the two groups. No blood transfusion was required in the surgical procedures for any patients, and there was no perioperative mortality or severe complications after surgery.
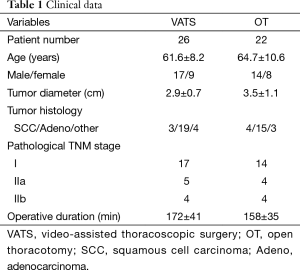
Full table
Effect of different treatments on sVEGFR1 level
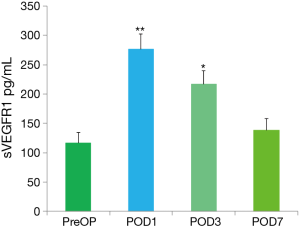
No significant difference was observed in the levels of sVEGFR1 between VATS and OT groups at any time point measured, so the data were reported as the mean value of sVEGFR1 from all patients. The mean levels of sVEGFR1 detected in all patients at each time point are presented in Figure 1. The mean preoperative sVEGFR1 value or baseline for all patients was 119.5±15.6 pg/mL. The mean sVEGFR1 levels on POD1 (274.6±23.7 pg/mL, P<0.01), on POD3 (222.5±20.9 pg/mL, P<0.05) were significantly higher than the preoperative result (Figure 1). No significant difference was observed between the POD7 time point and the baseline.
Effect of different treatments on sVEGFR2 level
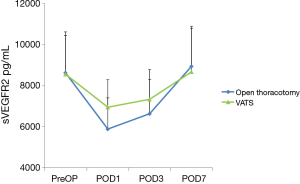
The mean sVEGFR2 levels detected in both VATS group and OT group at each time point are presented in Figure 2. When compared with preoperative sVEGFR2 basal level (VATS, 8,556.3±1,627.5 pg/mL; OT, 8,621.1±2,060.7 pg/mL), significant decreases were noted in both groups on POD1 (VATS, 6,753.5±1,535.9 pg/mL, P<0.05; OT, 6,640.1±1,452.3 pg/mL, P<0.01), on POD3 (VATS, 7,336.4±1,672.5 pg/mL, P<0.05; OT, 6,640.1±1,452.3 pg/mL, P<0.05). Furthermore, there was a significant difference in the level of sVEGFR2 between the two groups on POD1 (P<0.05), sVEGFR2 level in VATS group decreased less than in OT group. While no significant difference was observed on POD3. On POD7, sVEGFR2 had a tendency to return to normal level in both groups.
Discussion
Lung cancer remains the leading cause of cancer death in the world. VATS has completely revolutionized modern thoracic surgery. In the past 20 years, VATS lobectomy technique transform from an experimental procedure to the standard of care for patients with early stage NSCLC (20). Potential benefits of VATS for lung cancer resections compared to OT include smaller incision, less pain, less blood loss, reduced postoperative complications, shortened length of stay and hospital cost reduction (21-23). The potential advantages following VATS major lung resection have given rise to numerous speculations on the possible mechanisms, including weakened acute inflammatory responses (24), better preserved immune functions resulting in improved tumor immune-surveillance (25,26), alterations to tumor microenvironment and its effect on tumor angiogenesis.
Angiogenesis is essential for tumor growth and metastasis, and VEGF is presently the most potent inducers of angiogenesis. The VEGF release after surgery may have undesirable effects on residual tumor cells, and could promote tumor growth and metastasis formation (4). Previous studies have shown there was significantly higher level of circulating VEGF at POD3 in the Open group in comparison with the VATS group (1). VEGF exerts its function by binding to VEGFR on endothelial cells. The VEGF binding sites on the soluble and EC-bound VEGFR are identical. So it is assumed that once VEGF is bound to sVEGFR, it cannot bind to EC-bound VEGFR, sVEGFR are thought to sequester plasma VEGF and counteract the functions of VEGF.
In recent years, many researches are attempting to investigate the relationship between sVEGFRs and cancer, and the role of sVEGFRs in the formation of metastasis. The results of some studies have shown significantly higher levels of sVEGFR1 and sVEGFR2 in the plasma of women with breast cancer than in the control group (27,28), higher concentrations of sVEGFR1 in malignant pleural effusion than in benign pleural effusion (29). Additionally, other studies indicated that the high levels of sVEGFR1 or the high ration of sVEGFR1:VEGF within tumor tissue and in plasma correlated with high stage of cancer or worst prognosis, although the potential mechanisms remain unknown (30).
In this study, we found an early increase in circulating sVEGFR1 level, whereas a great decrease in sVEGFR2 level after surgery in both groups. Furthermore, a less sVEGFR2 decrease was observed in VATS group when compared with Open group. This study also found that the plasma sVEGFR2 level was greater than the corresponding sVEGFR1 level at all time points, and this phenomenon has been observed in patients following major colorectal surgery (9,10). Thus, it is demonstrated that the effect of the decrease in sVEGFR2 level predominates over the effect of the increase in sVEGFR1 level during the early postoperative days with respect to the total soluble receptor concentration. Moreover, VATS had little impact on the level of sVEGFR2 in comparison with OT, sVEGFR2 level was maintained at a constant concentration to sequester and discourage angiogenesis in the perioperative period following VATS. The less impact of VATS on the level of anti-angiogenic factor sVEGFR2 may be one of the potential mechanisms supporting advantages of VATS lobectomy.
However, it is notable that numerous cytokines and cofactors including neuropilin-1, neuropilin-2 and heparin differentially modulate the affinity of VEGF for VEGFR1 and VEGFR2 or sVEGFR1 and sVEGFR2 (9). Without measuring the levels of these molecules during the first postoperative days, so it is difficult to evaluate the exact effects of sVEGFRs on free plasma VEGF. In addition, it is reported that there is higher level of sVEGFR2 on the endothelial cell membrane in comparison with sVEGFR1 (10), we did not investigate the impact of different types of surgery on endothelial cell surface expression of VEGFR1 and VEGFR2, so it remains unknown whether the relative portion of VEGFRs in the endothelial cell membrane changes responding to surgical procedures. Selection bias is another important factor to affect the accuracy of research results, because it is impossible to ethically make a prospective and randomized comparison between VATS and open lobectomy. Perioperative investigations concerning these aspects will lead to better characterization of the effect of surgical procedures on early postoperative plasma protein composition with respect to angiogenesis and tumor growth.
Conclusions
In conclusion, major lung resection for early stage NSCLC is associated with changes in postoperative anti-angiogenic factors sVEGFR1 and sVEGFR2 levels. Dramatic increase of sVEGFR1 levels and great decrease of sVEGFR2 levels were observed in both VATS group and OT group. More important, there was a less decrease of sVEGFR2 level in VATS group when compared with OT group. Minimally invasive VATS approach resulted in relatively stronger antiangiogenic response in the early postoperative period in comparison with the OT approach. The effect of postoperative changes in anti-angiogenic factors on tumor angiogenesis after lung cancer surgery warrants further research.
Acknowledgements
Funding: This work was supported by Fund of Application Basis Research of Yunnan Province (2010ZC216), Health Science and Technology Plan Projects of Yunnan Province (2014NS227) and National Natural Science Foundation of China (U1302225).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Eswarappa SM, Fox PL. Antiangiogenic VEGF-Ax: A New Participant in Tumor Angiogenesis. Cancer Res 2015;75:2765-9. [Crossref] [PubMed]
- Belizon A, Balik E, Feingold DL, et al. Major abdominal surgery increases plasma levels of vascular endothelial growth factor: open more so than minimally invasive methods. Ann Surg 2006;244:792-8. [Crossref] [PubMed]
- Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol 2005;23:1011-27. [Crossref] [PubMed]
- Lee SH, Jeong D, Han YS, et al. Pivotal role of vascular endothelial growth factor pathway in tumor angiogenesis. Ann Surg Treat Res 2015;89:1-8. [Crossref] [PubMed]
- Subramanian J, Morgensztern D, Govindan R. Vascular endothelial growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. Clin Lung Cancer 2010;11:311-9. [Crossref] [PubMed]
- Lee CB, Socinski MA. Vascular endothelial growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer: a review of recent clinical trials. Rev Recent Clin Trials 2007;2:117-20. [Crossref] [PubMed]
- Brekken RA, Thorpe PE. VEGF-VEGF receptor complexes as markers of tumor vascular endothelium. J Control Release 2001;74:173-81. [Crossref] [PubMed]
- Cao Y. Positive and negative modulation of angiogenesis by VEGFR1 ligands. Sci Signal 2009;2:re1. [Crossref] [PubMed]
- Shantha Kumara HM, Cabot JC, Hoffman A, et al. Minimally invasive colon resection for malignant colonic conditions is associated with a transient early increase in plasma sVEGFR1 and a decrease in sVEGFR2 levels after surgery. Surg Endosc 2010;24:283-9. [Crossref] [PubMed]
- Shantha Kumara HM, Cabot JC, Hoffman A, et al. Minimally invasive colon resection is associated with a transient increase in plasma sVEGFR1 levels and a decrease in sVEGFR2 levels during the early postoperative period. Surg Endosc 2009;23:694-9. [Crossref] [PubMed]
- Ng CS, Wan S, Wong RH, et al. Angiogenic response to major lung resection for non-small cell lung cancer with video-assisted thoracic surgical and open access. ScientificWorldJournal 2012;2012:636754.
- Martini N, Bains MS, Burt ME, et al. Incidence of local recurrence and second primary tumors in resected stage I lung cancer. J Thorac Cardiovasc Surg 1995;109:120-9. [Crossref] [PubMed]
- Gopaldas RR, Bakaeen FG, Dao TK, et al. Video-assisted thoracoscopic versus open thoracotomy lobectomy in a cohort of 13,619 patients. Ann Thorac Surg 2010;89:1563-70. [Crossref] [PubMed]
- McKenna RJ Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg 2006;81:421-5; discussion 425-6. [Crossref] [PubMed]
- Swanson SJ, Herndon JE 2nd, D'Amico TA, et al. Video-assisted thoracic surgery lobectomy: report of CALGB 39802--a prospective, multi-institution feasibility study. J Clin Oncol 2007;25:4993-7. [Crossref] [PubMed]
- Peng J, Chen XL, Mao X, et al. Video-assisted thoracoscopic right lower lobectomy for lung cancer using the Harmonic scalpel. J Thorac Dis 2013;5:864-7. [PubMed]
- Peng J, Chen XL, Mao X, et al. VATS lobectomy: The First People’s Hospital of Yunnan Province, Kunming University of Science and Technology. Asvide 2014;1:391. doi: [Crossref]
- Peng J, Chen XL, Mao X, et al. Video-assisted thoracoscopic left upper lobe apical trisegmentectomy with the Harmonic scalpel. J Thorac Dis 2014;6:1822-5. [PubMed]
- Peng J, Chen XL, Mao X, et al. VATS segmentectomy: the First People’s Hospital of Yunnan Province, Kunming University of Science and Technology. Asvide 2014;1:363. doi: [Crossref]
- Yan TD. Video-assisted thoracoscopic lobectomy-from an experimental therapy to the standard of care. J Thorac Dis 2013;5 Suppl 3:S175-6. [PubMed]
- Lacin T, Swanson S. Current costs of video-assisted thoracic surgery (VATS) lobectomy. J Thorac Dis 2013;5 Suppl 3:S190-3. [PubMed]
- Swanson SJ, Meyers BF, Gunnarsson CL, et al. Video-assisted thoracoscopic lobectomy is less costly and morbid than open lobectomy: a retrospective multiinstitutional database analysis. Ann Thorac Surg 2012;93:1027-32. [Crossref] [PubMed]
- Nicastri DG, Wisnivesky JP, Litle VR, et al. Thoracoscopic lobectomy: report on safety, discharge independence, pain, and chemotherapy tolerance. J Thorac Cardiovasc Surg 2008;135:642-7. [Crossref] [PubMed]
- Yim AP, Wan S, Lee TW, et al. VATS lobectomy reduces cytokine responses compared with conventional surgery. Ann Thorac Surg 2000;70:243-7. [Crossref] [PubMed]
- Ng CS, Lee TW, Wan S, et al. Thoracotomy is associated with significantly more profound suppression in lymphocytes and natural killer cells than video-assisted thoracic surgery following major lung resections for cancer. J Invest Surg 2005;18:81-8. [Crossref] [PubMed]
- Ng CS, Whelan RL, Lacy AM, et al. Is minimal access surgery for cancer associated with immunologic benefits? World J Surg 2005;29:975-81. [Crossref] [PubMed]
- Toi M, Bando H, Ogawa T, et al. Significance of vascular endothelial growth factor (VEGF)/soluble VEGF receptor-1 relationship in breast cancer. Int J Cancer 2002;98:14-8. [Crossref] [PubMed]
- Thielemann A, Baszczuk A, Kopczyński Z, et al. Clinical usefulness of assessing VEGF and soluble receptors sVEGFR-1 and sVEGFR-2 in women with breast cancer. Ann Agric Environ Med 2013;20:293-7. [PubMed]
- Cao C, Sun SF, Lv D, et al. Utility of VEGF and sVEGFR-1 in bronchoalveolar lavage fluid for differential diagnosis of primary lung cancer. Asian Pac J Cancer Prev 2013;14:2443-6. [Crossref] [PubMed]
- Yamaguchi T, Bando H, Mori T, et al. Overexpression of soluble vascular endothelial growth factor receptor 1 in colorectal cancer: Association with progression and prognosis. Cancer Sci 2007;98:405-10. [Crossref] [PubMed]


