Tracheal replacement
Immediate repair of long-segmental defects
Prosthetic tracheal repair
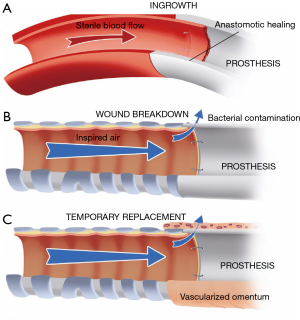
In recent years, most synthetic materials used for tracheal replacement have been tested in experimental animal research. From these studies, it became clear that definitive prosthetic replacement of the airway wall is not possible (1). To date, nearly all surgical prostheses that have been successful were observed in potentially sterile mesenchymal tissues. No example of successful prosthetic repair can be cited in the respiratory or gastrointestinal tract. The internal site of the airway tract belongs to the outside world, and bacterial contamination at the interface between the airway and prosthesis prevent its in-growth (Figure 1). The complications of wound breakdown at the anastomoses can be temporarily delayed by wrapping the prosthesis in vascularized tissue, mostly transposed omentum.
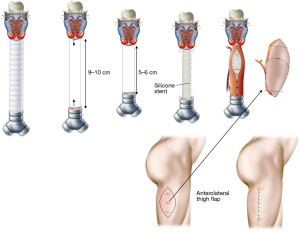
Palliative treatment of long-segmental defects
Long-segmental tracheal defects, which result after removal of malignant tumors are extremely rare. The only possibility for immediate reconstruction of these defects is to reduce the length of the defect by inserting a silicone stent, which is sutured to the upper and lower margins of the defect. A free fasciocutaneous skin tube (lateral thigh flap, radial forearm flap) can be used to wrap the silicone stent as a temporary closure (Figure 2) (2).
Tracheal allotransplantation
Introduction
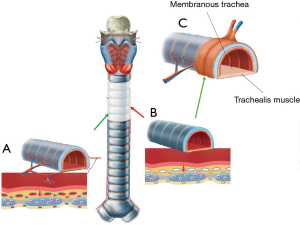
The trachea is one of the few organs that are exceptionally difficult to transplant because of the technical difficulty to restore the blood supply to the graft. The blood supply of the 12 cm-long trachea depends in its entirety on small blood vessels branching out into numerous even smaller vessels, each of them subsequently penetrating the trachea in between the cartilage rings to provide blood supply to segments of the mucosal lining. If a part of the trachea is removed from the airway, all blood supply is interrupted. The removed part of the trachea cannot survive, even if it were to be placed back into the airway straightaway (Figure 3). Our group has a 20 year-long research record in the field of tracheal revascularization and holds a leading position in the development of tracheal transplantation by means of vascularized segmental units.
A tracheal transplant may be necessary to repair surgical defects of the laryngotracheal airway tract that are unsuitable for segmental resection and autologous tissue repair. With the exception of some anecdotal, poorly documented cases performed without blood supply restoration (3) or immunosuppressive medication (4), no clinical tracheal allotransplants have been transplanted orthotopically as an isolated composite tissue graft. In tracheal allotransplantation, it is important to deal with both immunosuppression and indirect revascularization in a heterotopic position. The first documented preserved viability of a heterotopically revascularized allotransplant was published by Klepetko et al. in 2004 (5). The graft was revascularized in the omentum of a patient who underwent lung transplantation from the same donor. Ultimately, the trachea transplant was not used, but its viability was documented for at least 60 days.
The first documented revascularized tracheal allotransplant to be reported was published in 2010 (6).
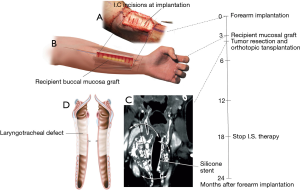
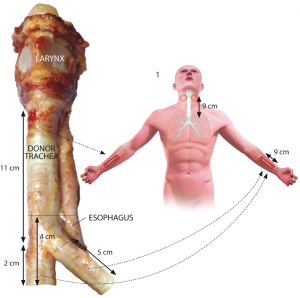
Our approach to tracheal heterotopic revascularization, orthotopic transplantation, and withdrawal of immunosuppressive medication is based on a series of six cases (Figure 4) (7). For tracheal allotransplantation, we consider a “good match” to mean that the donor is of the same blood group as the patient.

Surgical technique
Revascularization of the trachea is the first step towards successful tracheal transplantation. The typical arterial and venous blood supply, consisting of several small tracheo-esophageal branches, does not enable direct tracheal transplantation. Currently, the only reliable way to achieve tracheal revascularization is to wrap the isolated trachea with a well-vascularized soft tissue flap perfused by a vascular pedicle, which then allows for transfer of the revascularized trachea to an airway defect. The forearm fascia flap pedicled on the radial artery and vein has proven to be reliable for tracheal revascularization (7). It is important to have complete immobility between the trachea and the surrounding recipient’s vascular bed to obtain a fast revascularization of the blood vessels of the tracheal adventitia (Figure 5).
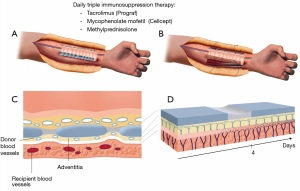
Revascularization has to be achieved by the outgrowth of capillary buds from the fascia flap (recipient blood vessels) uniting with those on the adventitia (donor blood vessels) of the tracheal segment. Inosculation is the establishment of direct vascular anastomoses between the vascularized soft tissue flap and the adventitia of the trachea.
Compared to a free skin graft, there are two additional barriers to revascularization for a tracheal allograft. The cartilage rings and intercartilaginous ligaments may interfere with the revascularization of the mucosal lining of the cartilaginous trachea. Cartilaginous tissue does not allow for the ingrowth of blood vessels. Revascularization of the mucosal layer of an avascular tracheal segment occurs through the intercartilaginous ligaments (Figure 6). Full revascularization and mucosal regeneration of the cartilaginous trachea can be achieved within 2−4 months of the trachea being implanted in the forearm. Incision of the intercartilaginous ligaments will foster the revascularization process by bringing the recipient blood vessels closer to the submucosal capillaries.
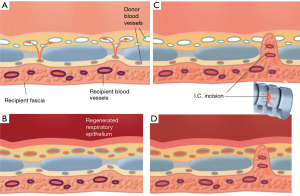
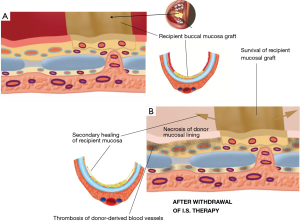
A tracheal allotransplant is a composite tissue transplant that may be used to restore the airway, with the goal of improving quality of life. The benefits garnered by tracheal allotransplantation have to be balanced against the morbidity of long-term immunosuppression therapy. Immunosuppressive medication should be withdrawn before immunosuppressant-related complications occur. The cartilage tissue seems to escape immunologic rejection owing to the absence of blood vessels, and because the chondrocytes are protected within a matrix (6,8,9). In our initial patient series of tracheal transplantations, it became clear that the intercartilaginous ligaments formed an obstruction for the ingrowth of native blood vessels (Figure 7). The placement of intercartilaginous incisions at the time of forearm implantation was an important adaptation. The incisions of the intercartilaginous ligaments facilitated revascularization, enabling the ingrowth of recipient vessels into the submucosal space of the transplant. When incisions through the intercartilaginous ligaments were made at regular intervals, full revascularization and mucosal regeneration of the cartilaginous allotransplant could be obtained in a shorter time period. Moreover, regularly spaced intercartilaginous incisions provide avenues for angiogenic recipient vessels to breach the ligamentous barrier and thus grow into the submucosal space of the transplant tissue after withdrawal of immunosuppressants.
Clinical examples
Of the six patients treated so far, five patients were treated for a long-segment stenosis and one patient was transplanted to resolve a long-segment laryngotracheal involvement by a chondrosarcoma. Our approach to a long-segement stenosis is shown in Figure 8.
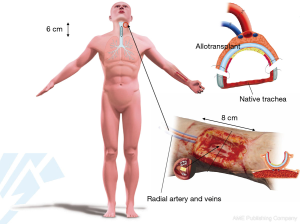
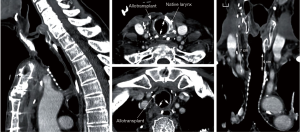
Tracheal allotransplantation was used in the treatment of a patient with an extended laryngotracheal chondrosarcoma. The patient involved was a 63-year-old man. The tumor developed over a period of more than 10 years. His airway could be preserved by the placement of a silicone stent. Due to the stagnation of secretions, he required periodical bronchoscopic cleaning of the stent. Since the last time, he had developed several acute episodes of stent blockages, which made definitive treatment necessary. Four months after implantation of a suitable allograft in the left forearm, the tumor was resected through an anterior cervical incision with a sternotomy extension (Figure 9). The potential for tumor progression while under immunosuppression for a low-grade malignancy was considered to be low and was confirmed by CT scan at the time of orthotopic transplantation, which demonstrated a nearly unchanged tumor bulk. Immunosuppressive medication was gradually phased out between 15 and 18 months after orthotopic transplantation. The transplant’s morphology remained intact after withdrawal of immunosuppressive therapy. It seems that the mucosal repopulation of the transplant after cessation of immunosuppressants can occur with minimal loss of airway lumen (Figure 10).
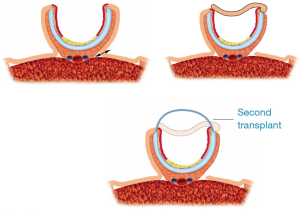
A circumferential airway repair may be necessary after resection of malignant tumors. Tracheal allotransplantation at the time of tumor resection will be possible only for low-grade malignancies and not for other malignant tumors, because of the risk of tumor progression in the 3-month period of pretransplant immunosuppression. A circumferential defect left by tumor resection can be reconstructed temporarily with a stent wrapped in vascularized tissue. This type of reconstruction must be considered temporary due to inevitable stent-related complications. Tracheal allotransplantation may be considered in those patients with a temporary repair who remain tumor-free.
The best protocol for circumferential allotransplantation may lie in a bilateral transplantation of the cartilaginous trachea (Figures 11,12).
Tracheal regeneration
Regeneration versus secondary healing
The relative contribution of tissue regeneration versus scarring in the healing of the airway mucosal lining depends on the extent of injury inflicted. A superficial epithelial wound can heal by way of regeneration of the surface epithelium (Figure 13) (10). Indeed tissues with a high proliferative capacity, such as airway tract epithelia, renew themselves continuously and, after injury, can regenerate above the basal membrane as long as the stem cells in these tissues have not been destroyed.
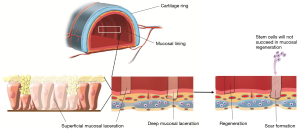
If a tissue injury is severe and involves damage of both epithelial cells and the submucosal layer, healing cannot be accomplished by regeneration alone. Under these conditions, the main healing process is repair by deposition of collagen, causing the formation of a scar. Future therapies should aim to promote regeneration and reduce scar tissue formation when dealing with full-thickness mucosal tracheal defects. Exploration of the potential use of stem cells for true regenerative healing is ongoing. The present challenge for regenerative medicine is to overcome the barriers to regeneration of the mucosal and epithelial lining in full-thickness epithelial defects. However, regeneration of full-thickness mucosal defects is not yet possible.
The unrealistic prospect of tracheal regeneration
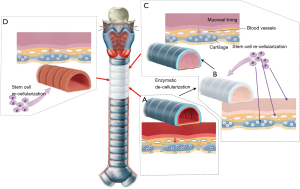
Since 2008 the trachea has been termed the first human organ that can be man-made with stem cells (11). Meanwhile an engineered trachea has been implanted in several patients. This achievement has received a lot of attention in medical journals as well as in the press. Indeed, the engineered windpipe was seen to be the first step towards other forms of organ regeneration. Classic organ transplantations with their typical side effects due to anti-rejection medication could then be replaced by growing organs from the body’s own cells. However, the optimism surrounding organ regeneration has proved to be completely unfounded. In fact, the engineered trachea is an example of blatant scientific deception.
The engineered trachea was represented as a regenerated trachea after applying bone marrow cells to a de-cellularized (12) or synthetic scaffold (Figure 14) (13). There is no scientific foundation whatsoever to assume why stem cells would support airway tissue regeneration in this setting. In addition, even if a trachea-like organ would be generated, it would irrefutably fail after implantation if adequate blood supply had not been restored. As expected, the implantation of de-cellularized and synthetic scaffolds resulted in extremely high morbidity and mortality rates (14). At this point in time, this form of airway regeneration should be regarded as hypothetical and scientifically unfounded (15,16).
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Grillo HC. Tracheal replacement: a critical review. Ann Thorac Surg 2002;73:1995-2004. [PubMed]
- Beldholm BR, Wilson MK, Gallagher RM, et al. Reconstruction of the trachea with a tubed radial forearm free flap. J Thorac Cardiovasc Surg 2003;126:545-50. [PubMed]
- Levashov YuN, Yablonsky PK, Cherny SM, et al. One-stage allotransplantation of thoracic segment of the trachea in a patient with idiopathic fibrosing mediastinitis and marked tracheal stenosis. Eur J Cardiothorac Surg 1993;7:383-6. [PubMed]
- Rose KG, Sesterhenn K, Wustrow F. Tracheal allotransplantation in man. Lancet 1979;1:433. [PubMed]
- Klepetko W, Marta GM, Wisser W, et al. Heterotopic tracheal transplantation with omentum wrapping in the abdominal position preserves functional and structural integrity of a human tracheal allograft. J Thorac Cardiovasc Surg 2004;127:862-7. [PubMed]
- Delaere P, Vranckx J, Verleden G, et al. Tracheal allotransplantation after withdrawal of immunosuppressive therapy. N Engl J Med 2010;362:138-45. [PubMed]
- Delaere PR, Vranckx JJ, Meulemans J, et al. Learning curve in tracheal allotransplantation. Am J Transplant 2012;12:2538-45. [PubMed]
- Sykes M. Immune evasion by chimeric trachea. N Engl J Med 2010;362:172-4. [PubMed]
- Delaere PR, Vranckx JJ, Den Hondt M, et al. Tracheal allograft after withdrawal of immunosuppressive therapy. N Engl J Med 2014;370:1568-70. [PubMed]
- Puchelle E, Zahm JM. Repair process of the airway epithelium. In: Lenfant C, Dekker M, editors. Airway environment: from injury to repair. Series: Lung biology in health and diseases. New York: Marcel Dekker, 1996:1576-82.
- Macchiarini P, Jungebluth P, Go T, et al. Clinical transplantation of a tissue-engineered airway. Lancet 2008;372:2023-30. [PubMed]
- Elliott MJ, De Coppi P, Speggiorin S, et al. Stem-cell-based, tissue engineered tracheal replacement in a child: a 2-year follow-up study. Lancet 2012;380:994-1000. [PubMed]
- Jungebluth P, Alici E, Baiguera S, et al. Tracheobronchial transplantation with a stem-cell-seeded bioartificial nanocomposite: a proof-of-concept study. Lancet 2011;378:1997-2004. [PubMed]
- Vogel G. Trachea transplants test the limits. Science 2013;340:266-8. [PubMed]
- Cyranoski D. Investigations launched into artificial tracheas. Nature 2014;516:16-7. [PubMed]
- Delaere PR, Van Raemdonck D. The trachea: the first tissue-engineered organ? J Thorac Cardiovasc Surg 2014;147:1128-32. [PubMed]