Nonintubated uniportal thoracoscopic surgery for resection of lung lesions
Introduction
Video-assisted thoracoscopic surgery (VATS) without tracheal intubation has been widely discussed in the recent decade. It has been proven feasible and safe for a variety of thoracic procedures, including pulmonary resection (1-3), lung-volume reduction (4), empyema (5), and excision of mediastinal tumors (6). The potential benefits include faster recovery, fewer perioperative complications, and shorter hospital stays. The patients are also free from intubated general anesthesia-related adverse effects, such as pressure-induced lung injury, delayed muscle blockade, and prolonged opioid effects.
The uniportal approach has grown in popularity in recent years due to its benefits of less postoperative pain and paresthesia and better patient satisfaction (7,8). This minimally invasive approach has been employed in pulmonary wedge resections for more than a decade (9), and its application has been extended to major lung resections including lobectomy and pneumonectomy in recent years (10,11).
Considering these benefits, the choice of nonintubated VATS in combination with a single incision approach decreases the invasiveness of the procedures and allows quicker postoperative recovery. Sporadic case reports have shown that nonintubated uniportal VATS may be performed for wedge and anatomical resections (12-14). After accumulating experience with nonintubated VATS for various procedures since 2009 (2,3,15,16) and intubated single-port VATS wedge resections, we began to use nonintubated uniportal VATS to manage peripheral lung nodule(s) smaller than 2 cm in January 2014, and the preliminary results are promising (17). In the current study, we further report our experience with 116 consecutive patients undergoing nonintubated uniportal VATS for lung lesions of variable size using various resection techniques to evaluate the feasibility and safety of this innovative VATS method.
Patients and methods
Study design and patients
This study was reviewed and approved by the National Taiwan University Hospital Research Ethics Committee (approval No.: 201509038RINA). From January 2014 to June 2015, the medical records of 116 patients who underwent nonintubated uniportal VATS for diagnosis or treatment of lung lesions at our hospital were reviewed. These patients were selected to receive nonintubated VATS according to the consensus of the thoracic surgery team including surgeons and anesthesiologists. Patients with American Society of Anesthesiologists (ASA) Physical Status Classification score of 4 or more, anticipated difficult airway management, evidence of pleural adhesions on chest computed tomography (CT), or spinal or thoracic deformity were contraindicated for nonintubated VATS. The surgeons made the decision to use a single incision for access after considering the tumor size and location on the preoperative CT images.
Preoperative CT-guided dye localization
For lesions not in direct contact with the visceral pleura or presenting as ground-glass nodules on low-dose CT imaging, we preferred to use preoperative CT-guided dye localization to facilitate tumor identification during VATS. After CT identification, the radiologists introduced a 22-G needle percutaneously into the target lesion. The puncture site was chosen for the shortest path to the lesion. After confirming the position of the needle tip by CT, 0.1 to 0.2 mL of Patent Blue Vital dye (Guerbet, Aulnay-sous-Bois, France) was injected into the lesion along the needle tract and at the subpleural parenchyma. After the localization procedure, the patient was transferred to the operating room as soon as possible.
Anesthetic setting, induction, and maintenance
The management of general anesthesia without intubation in our hospital has been described previously (2,3,15,17-19). In brief, the patients were premedicated with an intravenous infusion of 50 to 100 µg of fentanyl. We routinely monitored electrocardiography, arterial blood pressure, and pulse oximetry during the operation. A BIS Quatro bispectral index sensor (Aspect Medical System, Norwood, MA, USA) was applied to the forehead of the patient to monitor their level of consciousness. The respiratory rate and end-tidal carbon dioxide (ETCO2) were continuously monitored by insertion of a detector into one nostril (2). The patients were then sedated with intravenous propofol using a target-controlled infusion method to achieve a bispectral index value between 40 and 60 for adequate sedation. Incremental intravenous injections of fentanyl 25 to 50 µg were given as needed. During the operation, the patients were placed in the lateral decubitus position; they breathed oxygen spontaneously through ventilation mask.
Regional nerve block
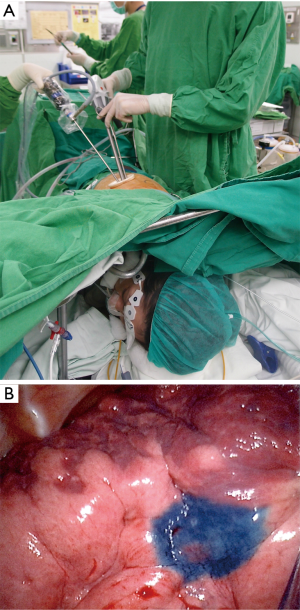
After applying local anesthesia with 2% lidocaine in fifth or sixth intercostal space away from the lesion, a 3-cm incision was made. The wound was covered by a wound protector/retractor (Alexis Wound Protector, Applied Medical, Rancho Santa Margarita, CA, USA) to maintain easy access to and protect the working space (Figure 1A). An iatrogenic pneumothorax was created after dissecting into the chest cavity, and the lung collapsed gradually while the patient remained spontaneously breathing. Under the guidance of a 5-mm thoracoscope, analgesia with intrathoracic intercostal nerve blocks was established by infiltrating 0.5% bupivacaine (1.5 mL in each intercostal space) from the third to the eighth intercostal nerve under the parietal pleura, 2 cm lateral to the sympathetic chain, using a 25-G top-winged infusion needle (20). A vagal nerve block was produced by infiltration of 3 mL of 0.5% bupivacaine at the level of the lower trachea for right-sided procedures and the level of the aortopulmonary window for left-sided procedures to inhibit the cough reflex during lung manipulation (2).
Nonintubated uniportal VATS for wedge or anatomical resections
The pulmonary lesion was identified by palpation with a ring forceps or fingers, or by visualization of the CT-tattooed dye on the lung surface (Figure 1B). After identification of the lesion, two silk anchoring sutures were set proximal and distal to the lesion. The threads of the anchoring sutures were pulled out through the chest wall above the lesion and were used for traction (21). The stapler was inserted at the base of the lifted-up lung lesion, sometimes with the assistance of another grasper to ensure an adequate safety margin (Figure 2). For anatomical resections such as segmentectomy and lobectomy, the pulmonary vessels and bronchus were elevated using the vessel loops or silk, respectively. The artery, vein, bronchus, and fissure were divided using an angulated endostapler. The resected lung parenchyma was removed in an organ retrieval bag. The silk sutures were also used for pleural traction to facilitate mediastinal lymph node dissection. For indeterminate nodules, the specimen was sent for intraoperative pathological analysis (frozen section). When the result indicated primary lung cancer, additional resection was done to ensure an adequate safety margin, and lymphadenectomy was performed for complete tumor staging.

At the end of the operation, the collapsed lung was re-expanded using mask ventilation to check for air leaks. A 28-French chest tube was inserted into the posterior aspect of the lung. The infusion of propofol was stopped. When the patients were fully awake, they were encouraged to breathe deeply and cough to expand the collapsed lung.
Conversion to multiport VATS or tracheal intubation
The attending surgeon made the decision to convert to two-port or three-port VATS if the lesion could not be identified, the margin required further resection, or the anatomical dissection was difficult. For anesthesia, the indications for conversion to tracheal-intubated, single-lung ventilation included profound respiratory movement, massive pleural adhesions, persistent hypoxemia (oxygen saturation on pulse oximetry of <80%), unstable hemodynamic status, or uncontrolled bleeding requiring an emergency thoracotomy (2,15,18,23,24). The attending surgeon and the anesthesiologist made the decision for conversion to thoracotomy jointly. The details of the conversion procedure have been described previously (20).
Postoperative analgesics and care
After the operation, the patients were able to resume water and food intake within 2 hours. Oral nonsteroidal analgesics, acetaminophen (325 mg), and tramadol (37.5 mg) were prescribed soon after the recovery of oral intake. Patient-controlled analgesia with intravenous morphine (1 mg/mL) was provided if the patient reported extreme pain and requested additional analgesia. The postoperative pain intensity was evaluated every day after the operation using a numeric pain intensity scale where 0 represented no pain and 10 represented intractable pain. A plain X-ray film of the chest was obtained the next morning. The chest tube was removed if no air leak was detected and the fluid drained was less than 200 mL per day. Prolonged air leakage was defined as persistent air leakage for 3 days after the operation. All postoperative complications were recorded.
Data collection and analysis
The demographic data, preoperative CT findings, anesthesia results, operative findings, and the complications were retrospectively collected during the chart review.
Results
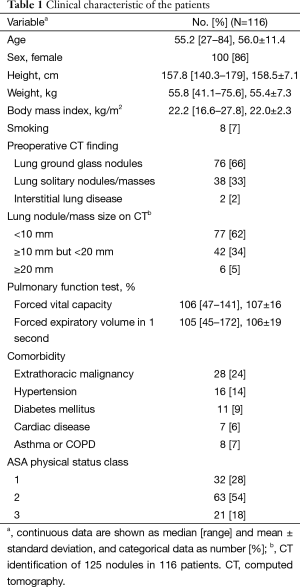
Table 1 provides the clinical and demographic data of the patients. The median patient age was 55.2 years (range, 27–84). Most (100/116, 86%) patients were women, and only eight of them smoked tobacco.
Full table
Of the 116 patients, 76 (66%) presented with ground-glass nodules on preoperative CT imaging. A total of 125 nodules or masses were identified on CT in the 116 patients. Most lesions (77/125, 62%) measured less than 10 mm in diameter.
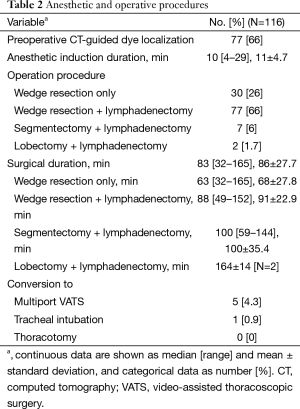
Two-thirds of the patients (77/116) underwent CT-guided dye localization before uniportal VATS to facilitate the intraoperative identification of their lesions. Wedge resection of the lung with or without lymphadenectomy was the most commonly performed procedure (107/116, 92%). Anatomical resections with lymphadenectomy were performed in nine patients; including seven segmentectomy and two lobectomy. Intraoperative frozen section pathology evaluations were done in 91/116 (78%) patients.
The median anesthesia induction time was 10 minutes. The operation time varied depending on the procedure. For a simple wedge resection without a lymphadenectomy, the median operative time was 63 minutes [32–165]. For a lobectomy with lymphadenectomy, the average operative time was 164±14 minutes (Table 2).
Full table
Overall, conversion to multiport VATS was required in five (4.3%) patients. In two patients, the lung nodules were difficult to identify via thoracoscopy with a single access incision. Therefore, two more incisions were made to facilitate palpation of the lesion. One of the two patients then underwent a lobectomy after confirmation that the lesion was malignant. In one patient, multiple incisions were made for further resection to achieve adequate surgical margins after confirmation of malignancy. In another patient, we encountered staple failure requiring another 5-mm camera port to assist with suturing. Conversion to tracheal-intubated multiport VATS was required in one patient due to significant mediastinal movement. No conversion to open thoracotomy was required (Table 2).
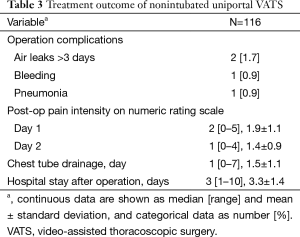
Table 3 shows the postoperative complications and surgical outcomes. Complications occurred in only four (3.4%) patients. The median numeric pain score rating on first and second days after the operation were 2 and 1 out of 10, respectively. The median postoperative hospital stay was 3 days.
Full table
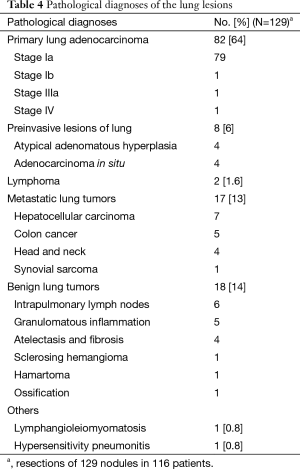
Table 4 provides the pathological diagnoses of the 129 nodules resected. The final pathology reported 82 (64%) primary lung adenocarcinomas, and most of them (79/82) were stage Ia. Benign disease was diagnosed in 18/129 (14%) lesions.
Full table
Discussion
With the rising prevalence of low-dose chest CT as a screening tool for lung cancer, increasing numbers of indeterminate lung lesions are being identified. The emerging needs for proper diagnosis and treatment of these small nodules have prompted the development of surgical approaches for precise resection, reduced invasiveness, improved cost-effectiveness, and faster recovery. The combination of VATS without intubation and with a single access incision is a novel approach, and our experience in this study shows that nonintubated uniportal VATS is technically feasible and safe for the resection of various lung lesions.
Low-dose CT has increased the discovery of small lung lesions compared to conventional X-ray imaging (25). In this study, most of the nodules identified were ground-glass nodules, and 62% (77/125) of the lesions were less than 10 mm in size. With 3-port VATS, these small lesions are identified by palpation via the nearest port. However, in the setting of single-port VATS, the incision is not necessarily close to the lesion, which makes palpation of the lesion difficult. Some surgeons have used a CT-guided percutaneous hookwire to localize pulmonary nodules (26,27). In our early experience, however, dislodgement of the hookwire and poor depth detection of the lesion were sometimes problematic. We, therefore, switched to CT-guided injection of blue dye near the lesion and at the subpleural parenchyma to mark the location of the nodule for resection. With single-incision VATS, the target area was easily located by the blue staining on the lung surface. The tattoo also identified the depth and margin of the lesion. This approach also had the advantages of minimalized manipulation of the lung to identify the lesion and avoidance of wire-related complications such as pleuritic pain (28,29). This procedure required close cooperation and communication with experienced radiologists. Furthermore, the patient should undergo VATS as soon as possible to avoid the dispersal of the dye over time.
Anesthesia management in nonintubated uniportal VATS is similar to that in nonintubated VATS. Our well-established technique using internal intercostal nerve block, vagal block, and targeted sedation have proven safety and effectiveness in various procedures including major lung resection (20). Compared to thoracic epidural anesthesia, this technique saves time, is easier to perform, and prevents potential neurological complications. In the current study, the median induction duration was only 10 minutes, which was significantly less than in our previous studies of nonintubated VATS using thoracic epidural anesthesia (2). With uniportal VATS, intercostal nerve and vagal blocks are applied with gentle traction of the lung to provide proper exposure of the target region and to avoid the cough reflex before establishing the vagal block. Theoretically, blockade of one intercostal nerve above and below the site of the port is sufficient for uniportal VATS. However, we routinely performed blocks from the third to the eighth intercostal nerves because the procedure is easy and enables to the option of conversion to multiple-incision VATS whenever indicated.
For the wedge resections, we used two-directional anchoring sutures to facilitate the application of the stapler (21). With manipulation of the traction sutures, fewer instruments were required for traction, and the operation fields for either wedge resection or lymph node dissection were better exposed, facilitating the procedure in the single-incision situation.
Nonintubated uniportal VATS for anatomical resections has been reported only sporadically. Diego reported the first uniportal VATS lobectomy under spontaneous ventilation (13). He used a laryngeal mask to control the airway and provide oxygen and sevoflurane gas via inhalation. Remifentanil was administered by continuous infusion for target-controlled sedation, and no intrathoracic vagal blockade was necessary. We reported the application of nonintubated uniportal VATS for segmentectomy. The patient breathed spontaneously through a ventilation mask and received target-controlled sedation by continuous infusion of propofol and intrathoracic intercostal and vagal nerve blockade (14). Vagus nerve blockade is necessary to prevent coughing in our setting. This may be due to less respiratory depression with propofol compared to remifentanil (30). In the current study, we demonstrated the safety and effectiveness of this technique for providing a steady operative field in various procedures, including those requiring extensive hilar dissection.
Intraoperative frozen pathology is essential for indeterminate lung lesions to determine the proper resection technique and whether or not lymphadenectomy is required. The additional procedure took approximately 30 minutes, and wound closure was delayed until the final pathologic result was obtained.
In our study, the conversion rate to multiport VATS was very low at 4.3%. The reasons for conversion included difficult localization of nodules, failure of stapling, and significant mediastinal movement. Rocco et al. reported a 3.7% conversion rate in 644 patients who underwent uniportal VATS. Among them, most patients (51.1%) underwent diagnostic procedures for pleural conditions, and only 28.9% underwent VATS for lung resection. The main reason for conversion was incomplete lung collapse (92%) (31). Liu et al. reported a similar conversion rate (3.45%, 3/87) from uniportal to multiport VATS for lobectomy and segmentectomy. These conversions were primarily due to pleura adhesion or anthracotic lymph nodes (32). Gonzalez-Rivas et al. reported converting five of 102 (4.9%) uniportal VATS lobectomies to multiport VATS or thoracotomy (10). Compared to aforementioned studies with uniportal VATS and tracheal intubation, we experienced a similar conversion rate from single-incision to multiport VATS although without tracheal intubation.
Only one patient (0.9%) in our study was converted to tracheal intubation along with incisions for additional ports due to significant mediastinal movement. The conversion rate to tracheal intubation was low in this study, which is explained by our previous experience of nonintubated VATS for various types of lung resections. With the cooperation of experienced anesthesiologists and surgeons, the respiratory pattern may be controlled to keep the diaphragm and mediastinum moving smoothly in most patients throughout the operation.
Previous studies have shown encouraging results for nonintubated uniportal VATS for excision of peripheral nodules (19). With less invasive anesthesia management and less use of opioids in our study, the patients recovered consciousness faster and were able to resume water and food intake 2 to 4 hours postoperatively. The postoperative pain intensity scores were low but did not differ from those of our previous studies of nonintubated multiport VATS (20).
The rate of operative complications was also low. In most patients, the chest tube was removed the next morning, and the hospital stay after the operation was short. These results imply that with a standardized protocol, nonintubated uniportal VATS is an alternative with least invasiveness and quick recovery for various lung procedures, including early diagnosis and management of early stage lung cancer.
We acknowledge that this study is limited by its retrospective design. Additionally, the lack of a control group that receives either intubated general anesthesia or nonintubated multiport VATS makes the comparison of advantages and disadvantages impossible. However, the low conversion rate from single to multiple-incision VATS, the low conversion rate from nonintubated to intubated general anesthesia, the fast postoperative recovery, and low complication rates are encouraging results.
Conclusions
Our experience with nonintubated uniportal VATS demonstrated that it is feasible, effective, and safe for diagnosis and treatment of various pulmonary diseases in selected patients. Larger prospective studies with appropriate control groups are needed to confirm the study results. The long-term benefits of nonintubated uniportal VATS also require further investigation.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Pompeo E, Tacconi F, Mineo D, et al. The role of awake video-assisted thoracoscopic surgery in spontaneous pneumothorax. J Thorac Cardiovasc Surg 2007;133:786-90. [PubMed]
- Chen JS, Cheng YJ, Hung MH, et al. Nonintubated thoracoscopic lobectomy for lung cancer. Ann Surg 2011;254:1038-43. [PubMed]
- Chen KC, Cheng YJ, Hung MH, et al. Nonintubated thoracoscopic lung resection: a 3-year experience with 285 cases in a single institution. J Thorac Dis 2012;4:347-51. [PubMed]
- Pompeo E, Tacconi F, Frasca L, et al. Awake thoracoscopic bullaplasty. Eur J Cardiothorac Surg 2011;39:1012-7. [PubMed]
- Tacconi F, Pompeo E, Fabbi E, et al. Awake video-assisted pleural decortication for empyema thoracis. Eur J Cardiothorac Surg 2010;37:594-601. [PubMed]
- Hung MH, Hsu HH, Cheng YJ, et al. Nonintubated thoracoscopic surgery: state of the art and future directions. J Thorac Dis 2014;6:2-9. [PubMed]
- Jutley RS, Khalil MW, Rocco G. Uniportal vs standard three-port VATS technique for spontaneous pneumothorax: comparison of post-operative pain and residual paraesthesia. Eur J Cardiothorac Surg 2005;28:43-6. [PubMed]
- Chen PR, Chen CK, Lin YS, et al. Single-incision thoracoscopic surgery for primary spontaneous pneumothorax. J Cardiothorac Surg 2011;6:58. [PubMed]
- Rocco G, Martin-Ucar A, Passera E. Uniportal VATS wedge pulmonary resections. Ann Thorac Surg 2004;77:726-8. [PubMed]
- Gonzalez-Rivas D, Paradela M, Fernandez R, et al. Uniportal video-assisted thoracoscopic lobectomy: two years of experience. Ann Thorac Surg 2013;95:426-32. [PubMed]
- Gonzalez-Rivas D, Delgado M, Fieira E, et al. Uniportal video-assisted thoracoscopic pneumonectomy. J Thorac Dis 2013;5 Suppl 3:S246-52. [PubMed]
- Rocco G, Romano V, Accardo R, et al. Awake single-access (uniportal) video-assisted thoracoscopic surgery for peripheral pulmonary nodules in a complete ambulatory setting. Ann Thorac Surg 2010;89:1625-7. [PubMed]
- Gonzalez-Rivas D, Fernandez R, de la Torre M, et al. Single-port thoracoscopic lobectomy in a nonintubated patient: the least invasive procedure for major lung resection? Interact Cardiovasc Thorac Surg 2014;19:552-5. [PubMed]
- Hung MH, Cheng YJ, Hsu HH, et al. Nonintubated uniportal thoracoscopic segmentectomy for lung cancer. J Thorac Cardiovasc Surg 2014;148:e234-5. [PubMed]
- Hung MH, Hsu HH, Chen KC, et al. Nonintubated thoracoscopic anatomical segmentectomy for lung tumors. Ann Thorac Surg 2013;96:1209-15. [PubMed]
- Tsai TM, Chen JS. Nonintubated thoracoscopic surgery for pulmonary lesions in both lungs. J Thorac Cardiovasc Surg 2012;144:e95-7. [PubMed]
- Hung MH, Cheng YJ, Chan KC, et al. Nonintubated uniportal thoracoscopic surgery for peripheral lung nodules. Ann Thorac Surg 2014;98:1998-2003. [PubMed]
- Chen KC, Cheng YJ, Hung MH, et al. Nonintubated thoracoscopic surgery using regional anesthesia and vagal block and targeted sedation. J Thorac Dis 2014;6:31-6. [PubMed]
- Hung MH, Liu YJ, Hsu HH, et al. Nonintubated video-assisted thoracoscopic surgery for management of indeterminate pulmonary nodules. Ann Transl Med 2015;3:105. [PubMed]
- Hung MH, Hsu HH, Chan KC, et al. Non-intubated thoracoscopic surgery using internal intercostal nerve block, vagal block and targeted sedation. Eur J Cardiothorac Surg 2014;46:620-5. [PubMed]
- Lee SK, Son BS, Ahn HY, et al. Single-incision thoracoscopic surgery using an anchoring suture of the lung parenchyma for two-directional traction. Ann Thorac Surg 2014;97:e89-91. [PubMed]
- Hung WT, Hsu HH, Hung MH, et al. The surgical technique of nonintubated uniportal thoracoscopic wedge resection of a CT-guided tattooed lesion. Asvide 2016;3:072. Available online: http://www.asvide.com/articles/825
- Wu CY, Chen JS, Lin YS, et al. Feasibility and safety of nonintubated thoracoscopic lobectomy for geriatric lung cancer patients. Ann Thorac Surg 2013;95:405-11. [PubMed]
- Tseng YD, Cheng YJ, Hung MH, et al. Nonintubated needlescopic video-assisted thoracic surgery for management of peripheral lung nodules. Ann Thorac Surg 2012;93:1049-54. [PubMed]
- Doria-Rose VP, Szabo E. Screening and prevention of lung cancer. In: Kernstine KH, Reckamp KL, editors. Lung cancer: a multidisciplinary approach to diagnosis and management. New York: Demos Medical Publishing, 2010:53-72.
- Ciriaco P, Negri G, Puglisi A, et al. Video-assisted thoracoscopic surgery for pulmonary nodules: rationale for preoperative computed tomography-guided hookwire localization. Eur J Cardiothorac Surg 2004;25:429-33. [PubMed]
- Ng CS, Hui JW, Wong RH. Minimizing single-port access in video-assisted wedge resection, with a hookwire. Asian Cardiovasc Thorac Ann 2013;21:114-5. [PubMed]
- Lenglinger FX, Schwarz CD, Artmann W. Localization of pulmonary nodules before thoracoscopic surgery: value of percutaneous staining with methylene blue. AJR Am J Roentgenol 1994;163:297-300. [PubMed]
- Vandoni RE, Cuttat JF, Wicky S, et al. CT-guided methylene-blue labelling before thoracoscopic resection of pulmonary nodules. Eur J Cardiothorac Surg 1998;14:265-70. [PubMed]
- Holas A, Krafft P, Marcovic M, et al. Remifentanil, propofol or both for conscious sedation during eye surgery under regional anaesthesia. Eur J Anaesthesiol 1999;16:741-8. [PubMed]
- Rocco G, Martucci N, La Manna C, et al. Ten-year experience on 644 patients undergoing single-port (uniportal) video-assisted thoracoscopic surgery. Ann Thorac Surg 2013;96:434-8. [PubMed]
- Liu CY, Lin CS, Shih CH, et al. Single-port video-assisted thoracoscopic surgery for lung cancer. J Thorac Dis 2014;6:14-21. [PubMed]


