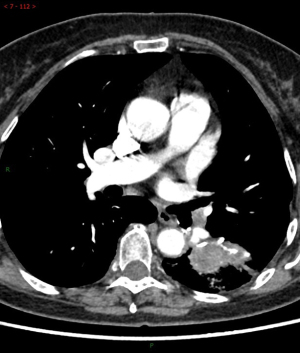
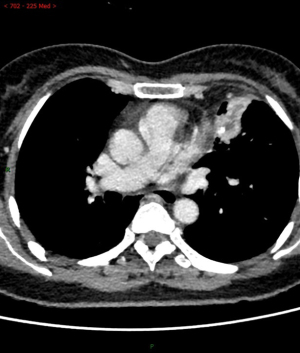
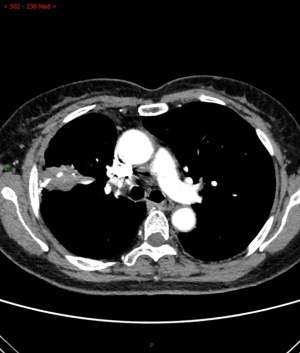
The difference of auxiliary examination parameters between margin recurrence and granuloma on enhanced computed tomography after sublobar resection
IntroductionOther Section
Lung cancer is one of the primary malignant tumors threatening people’s health (1,2), but early detection and treatment can effectively reduce mortality. After surgical treatment, the 5-year survival rate of patients with early-stage lung cancer can be over 80% (3), and the 5-year survival rate of patients with adenocarcinoma in situ and the adherent growth subtype is close to 100% (4). With the widespread use of low-dose spiral computed tomography (CT) in recent years, and the enhancement of individuals’ awareness of their health care, the early detection rate of lung cancer has increased significantly, and more lung cancer patients are being diagnosed and treated at an early stage (5). At the same time, the use of sublobar resection is gradually increasing because it preserves a greater amount of normal lung tissue and improves postoperative quality of life.
However, with the increase in the proportion of sublobar resections, the recurrence rate in the margins has also been increasing during postoperative follow-up (6,7). In some patients who experienced recurrence after sublobar resection, specific masses suspected of being recurrence tumors were located at the surgical margins, but the final postoperative pathological diagnosis proved to be granuloma, causing patients to suffer unnecessary surgery. Therefore, the differential diagnosis of margin granulomas and margin recurrence after sublobar resection is vitally important (8,9). Although some researchers have tried to confirm the advantages of examinations such as positron emission tomography (PET)-CT and diffusion-weighted imaging (DWI) in identification (10,11), false positive results still occur (12-15). As the most popular examination at present, enhanced CT imaging has the advantages of easy follow-up, simplicity, and speed and has great clinical significance in helping clinicians make a preliminary analysis of neoplasms to assist in follow-up diagnosis and treatment. We analyzed various enhanced CT image features of these neoplasms, hoping to discover meaningful indicators that could assist clinical decision-making. We present the following article in accordance with the MDAR reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-987/rc).
MethodsOther Section
Patients
This a diagnostic test examined the clinical records of 7,047 patients who underwent lung cancer surgery through the Department of Thoracic Surgery of our hospital from January 2015 to December 2021. Twenty patients who underwent sublobar resection were suspected of recurrence because of the auxiliary examination such as CT and PET-CT, during follow-up and received further care. Of these, five patients could not tolerate surgery due to the evaluation of their pulmonary function or because they refused surgery and biopsy. After multidisciplinary diagnosis and treatment (MDT), follow-up was continued. The remaining 15 patients underwent repeat surgery because of postoperative masses around the resection margin, including six cases of benign granulomas and nine cases of lung cancer recurrence.
Follow-up
For all lung cancer patients, postoperative follow-up consisted of CT examinations every 3 months during the first 6 months after surgery, every 6 months after the first half year, and every year after 3 years. If the tissue thickening around the resection margin was indeterminate during the follow-up process, the follow-up interval was shortened, and in cases where tumorous lesions could not be ruled out, the patient was admitted to hospital for further examination and evaluation. All patients’ enhanced CT examination reports underwent artificial intelligence (AI) analysis and were analyzed and reviewed by more than two radiologists. All patients admitted to hospital underwent fluorodeoxyglucose (FDG)-PET/CT examinations, and specific infection was excluded by various blood indicators. All patients received MDT to discuss the treatment plan. All patients’ data were collected and recorded with the consent of the patients or their families. Through the verification of surgical records, we ensured a sufficient distance from the primary nodule to the resection margin during surgery, and intraoperative frozen pathology indicated that the resection margin was negative.
Data
All patients included in the study were examined in our hospital and received enhanced CT and PET-CT examinations with the same mode. The maximum standardized FDG uptake value (SUVmax) of the mass on the lungs of these patients and the CT value of the mass were recorded by enhanced CT. At the same time, the patient’s medical history was collected before and after the pathological results of the patient’s first operation. Patients with a tumor diameter >2.0 cm, solid tumor, presence of vascular invasion or lymphatic infiltration, visceral pleural invasion, or tumor spread through air spaces (STAS) were defined as high risk (16). The specific surgical methods, surgical sites, and other data were also collected. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The ethical approval was waived by Ethics Committee of Renji Hospital, and individual consent for this retrospective analysis was waived.
Statistical analysis
SPSS (Version No.18.0.0) software was used for the statistical analysis. We employed t-tests and univariate analysis of variance to compare the data, where a P value <0.05 was considered statistically significant. The results were visually displayed using GraphPad Prism (Version No.9.3.1) software. The analysis was conducted to find meaningful indicators for the differentiation between tumor recurrence and granuloma.
ResultsOther Section
Patient characteristics
As shown in Table 1, 15 patients were included in this study with an average age of 64.0±10.9 years. The follow-up time to tumor recurrence and granuloma was 50.2±29.6 and 39.2±28.9 months, respectively. There were five males and ten females. The pathology of the mass around the resection margin was confirmed as lung cancer in nine patients and as granuloma in the remaining six patients. All 15 patients underwent sublobar resection in their initial surgery, with 12 cases undergoing segmental resection and three undergoing wedge resection. All nodules were ground glass opacity (GGO). Three patients with nodules greater than 2 cm in diameter were considered unable to tolerate lobectomy in their preoperative evaluation for initial surgery and underwent palliative resection.
Table 1
| Variables | Total (n=15) | Recurrence (n=9) | Granuloma (n=6) | P |
|---|---|---|---|---|
| Gender | 1.000 | |||
| Male | 5 (33.3) | 3 (33.3) | 2 (33.3) | |
| Female | 10 (66.7) | 6 (66.7) | 4 (66.7) | |
| Diameter | 0.810 | |||
| ≤2 cm | 12 (80.0) | 7 (77.8) | 5 (83.3) | |
| >2 cm | 3 (20.0) | 2 (22.2) | 1 (16.7) | |
| Surgery | 0.015 | |||
| Segment | 12 (80.0) | 9 (100.0) | 3 (50.0) | |
| Wedge | 3 (20.0) | 0 (0.0) | 3 (50.0) | |
| Position | 0.109 | |||
| Left | 4 (26.7) | 1 (11.1) | 3 (50.0) | |
| Right | 11 (73.3) | 8 (88.9) | 3 (50.0) | |
| Pathology | 1.000 | |||
| High-risk | 5 (33.3) | 3 (33.3) | 2 (33.3) | |
| Lobe | 0.693 | |||
| Up | 6 (40.0) | 4 (44.4) | 2 (33.3) | |
| Mid & lower | 9 (60.0) | 5 (55.6) | 4 (66.7) | |
| CEA+ | 5 (33.3) | 3 (33.3) | 2 (33.3) | 0.153 |
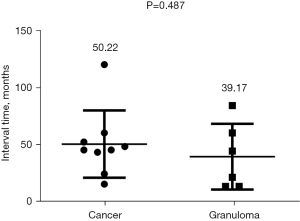
| Time to reoccurrence for cancer or granuloma (months) | 45.8±28.8 | 50.2±29.6 | 39.2±28.9 | 0.487 |
Data are expressed as n (%) or mean ± standard deviation. “+” means higher than the standard range. CEA, carcinoembryonic antigen.
FDG-PET results
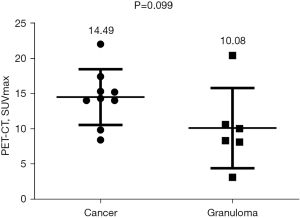
We recorded the SUVmax values of the masses in the FDG-PET examination results. As shown in Figure 1, although the average SUVmax value in the tumor group was higher than that in the granuloma group (14.49±1.320 vs. 10.08±2.328), this difference was not significant (P>0.05) due to the large variance in SUVmax values (exceeding 10) in both groups.
Enhanced CT results
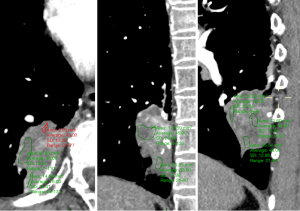
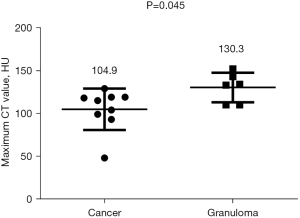
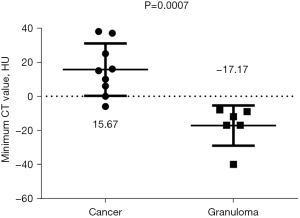
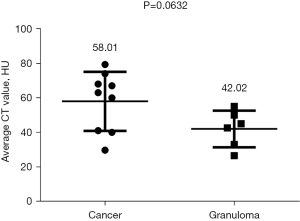
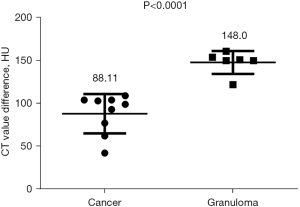
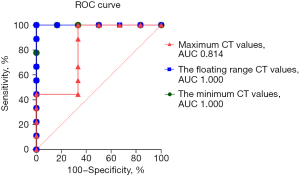
Among the various indicators of enhanced chest CT, we mainly focused on the difference in CT values of the mass around the margin in the enhanced CT arterial item. The contrast agent we used was Tylenol, 100 mL intravenously, with an injection rate of 3–4 m/s. We used the special software (Carestream Vue PACS) in the hospital imaging department to repeatedly measure the whole tissue from the sagittal, coronal, and axial positions, avoiding vascular shadows and staples. In this way, we determined the CT value range of the mass, and recorded its maximum value, minimum value, floating range, and average value (Figure 2). Figures 3-6 show that the maximum CT values of the tumor recurrence and granuloma were 104.9±8.051 and 130.3±7.017, respectively, and this difference was significant (P=0.045). There was also a highly significant difference in the minimum CT values between the tumor recurrence and granuloma groups (15.67±5.113 vs. −17.17±4.826, P=0.0007) and in the floating range CT values (148.00±5.471 vs. 88.11±7.671, P<0.0001), respectively. Figure 7 show the receiver operating characteristic (ROC) curve for maximum CT values, the minimum CT values, the floating range CT values and area under curve (AUC) of them. There was no significant difference in the overall average value of the masses. However, given the small sample size of this study and no assessment of diagnostic accuracy of CT parameters, the conclusion should be made with cautions.
Impact of primary surgery
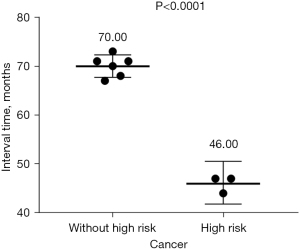
As shown in Table 1, the univariate analysis results showed no significant difference between the tumor recurrence and granuloma groups in the surgical location of the mass (left or right position, P=0.109 or different lobe, P=0.693). There was also no significant difference in the time to recurrence (P=0.487) (Figure 8). In terms of surgical methods, all patients with tumor recurrence received segmentectomy for the initial surgery, and the ratios of wedge resection and segmentectomy in patients with granulomas stood at 50% each. Among the patients with postoperative recurrence, the time to recurrence in those with high-risk factors was significantly faster than in those without high-risk factors (P<0.0001) (Figure 9).
Postoperative findings
After surgical treatment, all patients with tumor recurrence had the same pathological type as the primary tumor. One patient with granuloma was considered to have a tuberculosis infection after further pathological exam. We found some granulomatous tissue in the postoperative pathological section of a patient with tumor recurrence who was classified into the cancer group.
DiscussionOther Section
Our results showed that out of all patients with suspected recurrence at the resection margin in our center, the final pathological diagnosis of lung cancer recurrence was 60% (n=9). The methods were all segmentectomy, and all the included wedge resection patients had granulomas. This differs from the findings of Matsuoka et al. (12). In their study, the recurrence rate in wedge resections was higher. They suggested that disturbed ventilation and blood flow obstruction near the incision margin causes infection around the margin and is the main reason for the formation of granulomas. Also, with the popularization of thoracoscopic surgery, the use of cutting staplers has increased (17). In segmentectomy, the lung parenchyma cut by the stapler is usually thicker than in lobectomy and wedge resection. Therefore, ventilation and blood flow disturbances near the margins are more evident after segmentectomy, and granulomas are more likely to occur. However, the study did not describe the specific differences between the different surgical methods, which is likely to be the main reason for the difference between the two studies. At our center, we are very cautious about the choice of sublobar resection, especially wedge resection, for patients undergoing their first surgery. All patients who received wedge resection in this study had nodules less than 1 cm, and the preoperative imaging showed that the consolidation-to-tumor ratio (CTR) (solid component size of the tumor/tumor size) of these patients was less than 0.25. We also strictly ensured sufficient margins during the operation. As for segmentectomy, excluding the three patients who underwent palliative resection, the CT images and postoperative pathology of the other nine patients showed higher tumor malignancy than the three patients who underwent wedge resection. This is also in line with the view of some researchers that the malignancy of the first lung tumor is proportional to the recurrence probability after sublobar resection (12,16). Additionally, some researchers have suggested that defects in intraoperative frozen pathology techniques may be a major reason for recurrence at surgical margins. Higashiyama and colleagues (18,19) proposed a solution using intraoperative lavage cytology (ILC), and Masasyesva et al. reported a new method for detecting incisal margins using molecular assays (20). However, these new methods need further research and data to confirm their effectiveness, so it is currently difficult to verify the results of their research.
Aside from imaging all aspects of the first operation, the postoperative follow-up has also played a role. Some resection margin masses were initially suspected to be lung cancer based on imaging features but gradually reduced after follow-up and were eventually confirmed as granulomas (17). However, some benign neoplasms do not decrease over time and even increase (21). Sun et al. reported that 96% of their patients had thickening of the resection margin on the earliest postoperative follow-up CT, but subsequent follow-up CT showed that 25.3% of these areas did not change in shape or size over time, 58.7% showed degenerative changes, and only 16.0% showed progressive changes (22). We also excluded most patients with benign lesions by this method, but there were still some patients with thickening of the resection margin that could not be judged by observation alone, such as the patients included in the present study. Regarding the speed of development of the masses, patients with a higher degree of pathological malignancy had a significantly shorter time to postoperative margin recurrence than patients without risk factors. However, in the overall comparison, there was no significant difference in the development time of margin granulomas and tumor recurrence. Consistent with other cases reported around the world (23-25), our study found a large variance in the time to recurrence in both types of masses. In other reports, some patients with relatively low-grade primary lung cancer who underwent wedge resection were found to have peri-margin recurrence after 13 years (25), and there were patients with metastatic lung cancer suspected of short-term margin recurrence that eventually proved to be granuloma (26).
Since the patient’s surgical data and follow-up cannot help doctors determine the pathology of the masses, the problem has again focused on further examinations. However, many reports point out the limitations of other types of examinations. For instance, compared with CT examination, PET-CT is a better, noninvasive imaging method for detecting tumor metastasis and recurrence (27), but it also sometimes produces false positive results (17,28,29), especially in the identification of margin recurrence in lung cancer. The pathology of the neoplasms at the postoperative margins remains insufficient. Some researchers found that although tumor tissue had a higher SUVmax value than granuloma, the final results were not statistically significant (12,24). Similarly, in our study, the most important PET-CT SUVmax value could not adequately distinguish recurrent tumors from granulomas. In our study, the tuberculous granuloma case may have been the reason for the significantly higher SUVmax value than other cases. Tumor markers can also be used to determine tumor recurrence during follow-up after sublobar resection (30), but they did not play a role in differentiating the two types of masses in this study. We found the positive rate of carcinoembryonic antigen (CEA) in both types of patients was 33.3%. Normal mucosal epithelia, such as oral, colorectal, and bronchial mucosa secrete CEA, and smoking and aging also lead to elevated serum CEA (31). Some researchers have pointed out that DWIs has advantages in identifying recurrence in surgical margins and sutured granulomas after lung cancer surgery, but there are also limitations in misdiagnosis, tumor type, and the population examined (25). Even invasive biopsy cannot guarantee a complete diagnosis. Among the 15 patients in this study, one patient had both granuloma and tumor tissue in the final excised mass. Due to possible false negatives and its own risk, there are also limitations in operating risks and needle biopsy. Therefore, at present, only surgery with clear pathology can be used as the gold standard for identification.
In this study, we found that CT, as the primary postoperative follow-up examination (5), has certain advantages over other examinations, including the low risk of adverse or allergic reactions. In our country, for PET-CT and DWI, both tests are expensive and cannot be popularized in most clinical centers for the time being. Coupled with their false-positive rate and their respective contraindications, accuracy and convenience have been greatly affected. Several other researchers are also studying how to distinguish granuloma from resection margin recurrence using various features of enhanced CT. Several indicators have been proposed, such as whether the thickened tissue around the resection margin of the mass on CT images is smooth or irregular, whether there is calcification in the tissue, whether the tissue shows traction or invasion of blood vessels and bronchi, or whether there is a relatively unique growth pattern. It has also been reported that tumor recurrence tends to proliferate on the resection margin, whereas granulomas tend to grow unidirectionally from the resection margin to the medial side of the lung parenchyma (26). Mizukami et al. found that all recurrences grew radially from the incisal margin, whereas the granulomas mostly extended along the long axis of the incision margin (8). Similar conclusions were also found by Kamata et al. (17). Matsuoka and colleagues summarized the CT findings from granulomatous margins as follows: irregular nodules grew from the margins to the medial lung, and some appeared as masses with very different densities. However, the manifestations of recurrent masses in lung cancer are more complex, and some even show abscess-like changes in the lung (12). However, these findings have certain limitations. As shown in Figures 10,11, the growth patterns of tumors and granulomas on CT may be very similar. In our study, we found that in the imaging manifestations of enhanced CT, no matter which way the new organisms grew, they all had one thing in common: in the enhanced CT of the arterial phase, the difference in CT values of the granulomas compared with tumor tissues was very obvious. In our data center statistics, whether it was the maximum or minimum CT value of the mass, there was a significant statistical difference between the two kinds of masses, as shown in Figures 12,13. It can be seen that, compared with the relatively uniform tumor tissue, the enhanced CT manifestations of granuloma tissue are often markedly enhanced areas in the periphery, with necrotic tissue in the center.
Although some scholars have tried to distinguish lung cancer from granuloma using CT features (32), we have not found any other relevant literature that specifically examines the difference between recurrence of tumor at the resection margin and granuloma in CT values. It is worth noting that enhanced CT examination has been popularized in most clinical centers in China. At the same time, CT values are also data that clinicians can easily obtain and it can indeed reflect many properties of tumors, such as density, blood flow richness, etc. Some centers even can obtain these data through mobile phones and other mobile devices. Therefore, it will be of great significance to find out the different rules between tumor recurrence and granuloma through these easily obtained parameters. We surmise that the reason for the different imaging features of tumor and granuloma tissues in enhanced CT may be related to their respective pathophysiological features. There are many reasons for the formation of granulomas, such as using staplers during surgery, preoperative or postoperative infection, and foreign body reactions (33-36). It has also been reported that ventilation and blood flow disturbances near the surgical margins are the main causes of infection and granuloma formation around the surgical margins (17). Some researchers also believe that the artificial materials used in surgery, such as bioabsorbable synthetic mesh [polyglycolic acid (PGA)] and titanium nails in the stapler, can cause foreign body reactions that lead to granuloma formations (37-41). By observation of the postoperative pathological sections of these patients, we also found that in the pathological sections of patients with granuloma, vascular tissue, multinucleated giant cells, and granuloma tissue are often concentrated in the periphery of the mass, while the central part is necrotic tissue. Although necrotic tissue also exists in the pathological sections of tumor patients, its distribution is more scattered, and large aggregates of necrosis are rarely seen, which may be related to the stronger vitality and heterogeneity of tumor cells. This also explains why the overall variation of granulomatous tissue is more pronounced in contrast-enhanced CT images.
Limitations
Our study had several limitations. First, the small number of patients and the relatively low incidence of lesions limited our data screening to some extent. Furthermore, this was a single-center retrospective study with inherent selection bias in the patient population. In fact, comparing the differences between the two groups can only denote differences, not the differential diagnosis ability. Additionally, we cannot guarantee that differences in the medical images in this study were not due to individual differences between instruments and operators. Finally, because it was a retrospective study, the lack of sufficient data to refine the model may have led to some key points being overlooked. Therefore, prospective studies with well-established prespecified models are necessary.
ConclusionsOther Section
With the gradual increase in the proportion of sublobar resections, the number of postoperative margin recurrences has increased, but granulomas on surgical margins are not uncommon, and their identification is very difficult. Compared with other examinations, this study found that enhanced CT value differences can be a more convenient and accurate method to assist clinicians in further diagnosis and treatment. There are few reports on this aspect at present, so it will be necessary to collect more data and design corresponding models for additional verification to reduce unnecessary surgery for patients.
AcknowledgmentsOther Section
Funding: None.
FootnoteOther Section
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-987/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-987/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-987/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The ethical approval was waived by Ethics committee of Renji Hospital, and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
ReferencesOther Section
- Guo Y, Zeng H, Zheng R, et al. The burden of lung cancer mortality attributable to fine particles in China. Sci Total Environ 2017;579:1460-6. [Crossref] [PubMed]
- Zhi XY, Yu JM, Shi YK. Chinese guidelines on the diagnosis and treatment of primary lung cancer (2015 version). Cancer 2015;121:3165-81. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Kadota K, Villena-Vargas J, Yoshizawa A, et al. Prognostic significance of adenocarcinoma in situ, minimally invasive adenocarcinoma, and nonmucinous lepidic predominant invasive adenocarcinoma of the lung in patients with stage I disease. Am J Surg Pathol 2014;38:448-60. [Crossref] [PubMed]
- National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Yoshida J, Ishii G, Yokose T, et al. Possible delayed cut-end recurrence after limited resection for ground-glass opacity adenocarcinoma, intraoperatively diagnosed as Noguchi type B, in three patients. J Thorac Oncol 2010;5:546-50. [Crossref] [PubMed]
- Nakao M, Yoshida J, Goto K, et al. Long-term outcomes of 50 cases of limited-resection trial for pulmonary ground-glass opacity nodules. J Thorac Oncol 2012;7:1563-6. [Crossref] [PubMed]
- Mizukami Y, Takahashi Y, Adachi H. Pulmonary Staple-Stump Granuloma After Segmentectomy: Two Case Reports and Comparison with Cases of Stump Recurrence. Am J Case Rep 2019;20:1049-56. [Crossref] [PubMed]
- Chung YE, Kim EK, Kim MJ, et al. Suture granuloma mimicking recurrent thyroid carcinoma on ultrasonography. Yonsei Med J 2006;47:748-51. [Crossref] [PubMed]
- Shyn PB, Madan R, Wu C, et al. PET/CT pattern analysis for surgical staple line recurrence in patients with colorectal cancer. AJR Am J Roentgenol 2010;194:414-21. [Crossref] [PubMed]
- Fink G, Herskovitz P, Nili M, et al. Suture granuloma simulating lung neoplasm occurring after segmentectomy. Thorax 1993;48:405-6. [Crossref] [PubMed]
- Matsuoka K, Yamada T, Matsuoka T, et al. Nodule around the staple line after pulmonary resection: benign granuloma or cancer recurrence? Indian J Thorac Cardiovasc Surg 2019;35:569-74. [Crossref] [PubMed]
- Hahn SY, Ko ES, Han BK, et al. Analysis of factors influencing the degree of detectability on diffusion-weighted MRI and diffusion background signals in patients with invasive breast cancer. Medicine (Baltimore) 2016;95:e4086. [Crossref] [PubMed]
- Matsuura S, Sasaki K, Kawasaki H, et al. Silk suture granuloma with false-positive findings on PET/CT accompanied by peritoneal metastasis after colon cancer surgery. Int J Surg Case Rep 2016;28:22-5. [Crossref] [PubMed]
- Lim JW, Tang CL, Keng GH. False positive F-18 fluorodeoxyglucose combined PET/CT scans from suture granuloma and chronic inflammation: report of two cases and review of literature. Ann Acad Med Singap 2005;34:457-60. [PubMed]
- Miyoshi T, Yoshida J, Aokage K, et al. Stapling cartridge lavage cytology in limited resection for pulmonary malignant tumors: assessment of cytological status of the surgical margin. Heliyon 2019;5:e01240. [Crossref] [PubMed]
- Kamata T, Watanabe SI, Sakurai H, et al. Two cases of pulmonary granuloma difficult to differentially diagnose from resection stump recurrence of lung cancer. J Jpn Assoc Chest Surg 2015;29:700-5. [Crossref]
- Higashiyama M, Kodama K, Takami K, et al. Intraoperative lavage cytologic analysis of surgical margins as a predictor of local recurrence in pulmonary metastasectomy. Arch Surg 2002;137:469-74. [Crossref] [PubMed]
- Higashiyama M, Kodama K, Takami K, et al. Intraoperative lavage cytologic analysis of surgical margins in patients undergoing limited surgery for lung cancer. J Thorac Cardiovasc Surg 2003;125:101-7. [Crossref] [PubMed]
- Masasyesva BG, Tong BC, Brock MV, et al. Molecular margin analysis predicts local recurrence after sublobar resection of lung cancer. Int J Cancer 2005;113:1022-5. [Crossref] [PubMed]
- Koike S, Toishi M, Sakaizawa T, et al. Pulmonary granuloma or post-segmentectomy resection stump lung cancer recurrence: Differential diagnosis of two cases. The Journal of the Japanese Association for Chest Surgery 2019;33:759-64. [Crossref]
- Sun B, Kamel MK, Nasar A, et al. Staple Line Thickening After Sublobar Resection: Reaction or Recurrence? Ann Thorac Surg 2020;109:1670-6. [Crossref] [PubMed]
- Matsuoka K, Ueda M, Miyamoto Y. Mycobacterial granuloma on the staple line after pulmonary resection. Asian Cardiovasc Thorac Ann 2018;26:540-5. [Crossref] [PubMed]
- Usuda K, Iwai S, Yamagata A, et al. Differentiation between suture recurrence and suture granuloma after pulmonary resection for lung cancer by diffusion-weighted magnetic resonance imaging or FDG-PET / CT. Transl Oncol 2021;14:100992. [Crossref] [PubMed]
- Hanaoka T, Kurai M, Okada M, et al. Pulmonary adenocarcinoma possibly developed from the cut-end of small-sized adenocarcinoma in the lung periphery as recurrence 13 years after its wedge resection. Surg Case Rep 2018;4:2. [Crossref] [PubMed]
- Motono N, Okada A, Togashi K. A foreign body granuloma on the staple-line after pulmonary resection for metastatic renal cell carcinoma. Haigan 2012;52:23-36. [Crossref]
- Keidar Z, Haim N, Guralnik L, et al. PET/CT using 18F-FDG in suspected lung cancer recurrence: diagnostic value and impact on patient management. J Nucl Med 2004;45:1640-6. [PubMed]
- Kostakoglu L, Agress H Jr, Goldsmith SJ. Clinical role of FDG PET in evaluation of cancer patients. Radiographics 2003;23:315-40; quiz 533. [Crossref] [PubMed]
- Kikuchi M, Nakamoto Y, Shinohara S, et al. Suture granuloma showing false-positive finding on PET/CT after head and neck cancer surgery. Auris Nasus Larynx 2012;39:94-7. [Crossref] [PubMed]
- Mizuno K, Ohde Y, Hayashi S, et al. Clinical differentiations in stump granuloma and stump recurrence after lung resection for malignancy using a stapler. Haigan 2017;57:826-31. [Crossref]
- Okazaki M, Sano Y, Mori Y, et al. Two cases of granuloma mimicking local recurrence after pulmonary segmentectomy. J Cardiothorac Surg 2020;15:7. [Crossref] [PubMed]
- Lin X, Jiao H, Pang Z, et al. Lung Cancer and Granuloma Identification Using a Deep Learning Model to Extract 3-Dimensional Radiomics Features in CT Imaging. Clin Lung Cancer 2021;22:e756-66. [Crossref] [PubMed]
- Tomita M, Matsuzaki Y, Edagawa M, et al. Pulmonary granuloma possibly caused by staples after video-assisted thoracoscopic surgery. Ann Thorac Cardiovasc Surg 2003;9:123-5. [PubMed]
- Tanaka H, Iuchi K, Matsumura A, et al. Pulmonary tuberculosis post staple-segmentectomy for lung cancer. The Journal of the Japanese Association for Chest Surgery 2003;17:794-7. [Crossref]
- Kono Y, Endo S, Otani S, et al. Non-tuberculous mycobacterial infection along the staple-suture line after segmentectomy for small peripheral lung cancer; report of a case. Kyobu Geka 2005;58:165-8. [PubMed]
- Grismer JT, Schaefer RF, Read RC. Postsegmentectomy pseudotumor of the lung. Ann Thorac Surg 1998;65:243-5. [Crossref] [PubMed]
- Munteanu RM, Eva L, Dobrovă Ţ BI, et al. Longer survival of a patient with glioblastoma resected with 5-aminolevulinic acid (5-ALA)-guided surgery and foreign body reaction to polyglycolic acid (PGA) suture. Rom J Morphol Embryol 2017;58:671-80. [PubMed]
- Yoshino M, Sekine Y, Koh E, et al. Pulmonary granuloma associated with non-tuberculous mycobacteriosis occurring at the staple line after segmentectomy for lung cancer: report of a case. Haigan 2014;54:790-4. [Crossref]
- Yoshida S, Matsumoto I, Saito D, et al. A case of pulmonary stapler granuloma suspected to be lung cancer. Haigan 2015;29:452-5.
- Miyauchi S, Maki Y, Ueno T, et al. Two cases of inflammatory tumor and nontuberculous mycobacteriosis around the staple line after lung cancer surgery The Journal of the Japanese Association for Chest Surgery 2017;31:598-603. [J]. [Crossref]
- Goutam M, Giriyapura C, Mishra SK, et al. Titanium allergy: a literature review. Indian J Dermatol 2014;59:630. [Crossref] [PubMed]
(English Language Editor: D. Fitzgerald)