
Bronchial artery revascularization in lung transplantation: a systematic review and meta-analysis
Introduction
Lung transplantation has been the standard of care for the treatment of end-stage chronic respiratory failure with the registry of the International Society for Heart and Lung transplantation following over 55,000 cases from 250 lung transplant centers since the 1990s (1). The United States performed 2,562 lung transplantations in 2018 alone contributing to a 31% increase in the number of operations performed over the preceding five years (2). Survival after lung transplant has also been improving over the years (1). Despite this, when compared to other solid-organ transplants, lung transplant survival is substantially lower (1). The survival curve following lung transplant, shows a steady drop after the first-year of transplant (3,4). This has been attributed to the development of chronic lung allograft dysfunction (CLAD), which develops in 50% of grafts at five years and has remained relatively stable over time (1,4). CLAD encompasses multiple distinct phenotypes with one of the main problematic types being bronchiolitis obliterans syndrome (BOS) (5). This CLAD phenotype has been specifically noted to be present in over 40% of lung transplant recipients within five years, has a median onset of 2.3 years, and has accounted for 27.5% of deaths in lung transplant recipients from 1990 to 2017 (6-12).
The development of BOS has been hypothesized to occur due to many factors, such as acute rejection, cytomegalovirus infection, and ischemia-reperfusion injury (13). Airway ischemia, inflammation, and subsequent necrosis due to reduced oxygenated blood supply have also been implicated in the development of progressive inflammation and fibrosis-potentially leading to BOS. This could be because following a typical lung transplantation; the lower airways are perfused via minimal retrograde flow from the pulmonary veins as the arterial flow from the bronchial arteries is sacrificed in the transplantation process. A permanent reduction in adequately oxygenated blood to the pulmonary airways could thus increase the risk of chronic ischemia and hypoxic damage (7,14-16).
Given this hypothesis, reducing lung ischemia in the early post-transplant period could be of importance in order to reduce the chances for development of late BOS; however, a clear link between ischemia related airway anastomotic problems and late BOS has not been found so far (17,18). One proposed strategy, bronchial artery revascularization (BAR), has been utilized as a surgical technique to supply the airway with oxygenated blood (19,20). After early implementation in specialized centers, BAR has demonstrated promise in delaying the onset of BOS (20-23), but has since not been implemented as standard of care given its technically demanding nature and lack of extensive experience with it. By revascularizing the bronchial arteries through anastomosis of the donor bronchial artery to the internal mammary artery or utilizing a saphenous vein graft to form a new vascular conduit from the descending aorta, direct ischemia to the airways is reduced. Early results from uncontrolled single center studies in reduction of post-operative BOS development are encouraging (20-23). In order to systematically analyze this technique, we performed a systematic search and meta-analysis of bronchial artery revascularization to assess its outcomes and success following lung transplantation. We present the following article in accordance with the PRISMA reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-213/rc).
Methods
Literature search strategy
An electronic search was performed in September 2019 using Ovid Medline, Cumulative Index of Nursing and Allied Health Literature (CINAHL), Scopus and Cochrane Controlled Trials Register (CCTR). To achieve maximum sensitivity of the search strategy, combined terms such as “lung”, “transplantation”, “transplant”, “bronchial”, “artery”, “revascularization”, “BAR”, “lung transplantation”, “bronchial artery revascularization”, “lung transplant”, “en bloc double lung transplant”, “single lung transplant”, “sequential lung transplant”, “lung transplant recipient”, “right intercostobrachial artery”, “bronchial artery”, “bronchial arteries”, “graft rejection”, “bronchiolitis obliterans syndrome”, “internal thoracic artery”, “mammary arteries”, “bronchial artery anastomosis” were used as either keywords or MeSH terms. The reference lists of all eligible studies were reviewed for further identification of potentially relevant studies and assessed using the inclusion and exclusion criteria.
Selection criteria
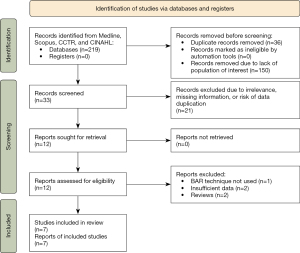
Eligible studies for the systematic review included all articles discussing lung transplantation utilizing bronchial artery revascularization. Articles were excluded if they did not contain information regarding post-transplant outcomes including development of BOS or survival. When institutions published duplicate studies with overlapping data, only the most complete reports with the longest follow-up period were included for quantitative assessment. Articles published from 1987 onwards were included. Patients under the age of 16 were excluded. Studies not published in the English language and those not involving human subjects were excluded. Abstracts, case reports, conference presentations, editorials, reviews, expert opinions, and studies without adequate extractable data were also excluded. A PRISMA diagram reflecting the search strategy is demonstrated in Figure 1. Risk of bias assessment was carried out using the Newcastle-Ottawa scale (NOS) score (Tables S1,S2). A PRISMA 2020 checklist is provided as supplementary material.
Data extraction and critical appraisal
Data were extracted from article texts, tables, and figures. Discrepancies and disagreements were resolved by discussion, consensus, and adjudication by a senior coauthor.
Statistical analysis
A binary outcome meta-analysis of proportions was conducted for the available main perioperative and postoperative variables with logit transformation. Heterogeneity was evaluated using Cochran Q and the I2 test. Survival data from each study were collected and pooled to retrieve a weighted mean and 95% confidence interval at specific time points. Such data were then graphically displayed to visualize survival over time. Meta-regression analysis was also done to assess the impact of time on mortality. R software, version 3.6.0 (R Foundation for Statistical Computing, Vienna, Austria) was used for all data analysis and visualization. The meta-analysis was performed using metafor package for R. P values less than 0.05 were considered statistically significant. This review did not have a previously published protocol nor was it registered. IRB approval was not required since publicly available deidentified data was used for the study.
Results
Baseline study and patient characteristics
Seven studies comprising 143 patients were included in this meta-analysis (20-26). Five of the studies comprising 105 patients were conducted from the year 1990 to 2000 (20-23,26). Of the remaining two, one was conducted from 1993 to 2003 (25) while the other was from 2007 to 2010 (24). Additional details are presented in the supplementary tables. The mean patient age was 47 (95% CI: 40–55) years. 61% (95% CI: 48–72%) of patients were male. Of all transplants, 73% (95% CI: 65–79%) were double lung transplants while 27% (95% CI: 21–25%) were single lung transplants. Indications for transplant included emphysema [71% (95% CI: 38–91%)], alpha-1 antitrypsin deficiency [40% (95% CI: 2–96%)], pulmonary fibrosis [26% (95% CI: 4–75%), cystic fibrosis [24% (95% CI: 0–100%)], and pulmonary hypertension [6% (95% CI: 2–17%)]. These baseline characteristics are listed in Table 1.
Table 1
| Variable | Pooled value [95% CI] | Number of studies | Events/total (n/N) | Heterogeneity, (%) |
|---|---|---|---|---|
| Age, years | 47 [40–55] | 3 | 67 | 32 |
| Male (%) | 61 [48–72] | 4 | 47/77 | 0 |
| Single lung transplant (%) | 27 [21–25] | 7 | 39/143 | 0 |
| Double lung transplant (%) | 73 [65–79] | 7 | 104/143 | 0 |
| Indications | ||||
| Emphysema (%) | 71 [38–91] | 3 | 57/79 | 36 |
| Alpha-1 AT deficiency (%) | 40 [2–96] | 3 | 38/75 | 85* |
| Pulmonary fibrosis (%) | 26 [4–75] | 4 | 22/77 | 69* |
| Cystic fibrosis (%) | 24 [0–100] | 3 | 14/76 | 87* |
| Pulmonary HTN (%) | 6 [2–17] | 3 | 5/84 | 0 |
*, indicates significant heterogeneity, P<0.05. CI, confidence interval; AT, antitrypsin; HTN, hypertension.
Perioperative characteristics
A total of 91% (95% CI: 46–99%) of bronchial artery revascularizations were performed using an internal mammary artery conduit. Seven percent (95% CI: 1–51%) of the remaining transplants were performed with a saphenous vein graft as the conduit for bronchial artery revascularization. Of the total patients, 89% (95% CI: 79–95%) underwent angiography to evaluate bronchial artery revascularization. Ninety-three percent (95% CI: 82–97%) of patients who underwent angiography demonstrated successful bronchial artery revascularization with contrast passing through the conduit. Of these, 96% (95% CI: 94–97%) of patients utilizing an internal mammary artery conduit had patent revascularization of the bronchial artery. Eighty-seven percent (95% CI: 65–96%) ultimately healed their tracheal anastomosis (Table 2).
Table 2
| Variable | Pooled value [95% CI] | Number of studies | Events/total (n/N) | Heterogeneity (%) |
|---|---|---|---|---|
| Conduit type | ||||
| Internal mammary artery (%) | 91 [46–99] | 7 | 123/143 | 72* |
| Saphenous vein (%) | 7 [1–51] | 7 | 18/143 | 73* |
| Patent angiography, any conduit (%) | 93 [82–97] | 6 | 116/133 | 7 |
| Patent angiography, IMA conduit (%) | 96 [94–97] | 5 | 103/106 | 0 |
| Fully healed tracheal anastomosis (%) | 87 [65–96] | 3 | 57/65 | 0 |
| Complications | ||||
| Re-exploration (any) (%) | 20 [9–38] | 5 | 24/125 | 50 |
| Re-exploration due to bleeding (%) | 14 [4–41] | 3 | 11/80 | 0 |
| Bleeding, all (%) | 18 [9–31] | 5 | 19/115 | 11 |
| BOS/pre-BOS (%) | 19 [8–37] | 6 | 14/93 | 31 |
| Cause of death | ||||
| Respiratory failure (%) | 8 [0–99] | 2 | 3/45 | 0 |
| Multi-organ failure (%) | 6 [3–11] | 4 | 4/77 | 0 |
*, indicates significant heterogeneity, P<0.05. CI, confidence interval; IMA, internal mammary artery; BOS, bronchiolitis obliterans syndrome.
Postoperative outcomes and complications
Re-operation for any reason was required by 20% (95% CI: 9–38%) of patients while 14% (95% CI: 4–41%) of patients required re-exploration due to bleeding complications. 18% (95% CI: 9–31%) of patients experienced bleeding complications of any kind. Development of BOS or its precursor, pre-BOS, was demonstrated in 19% (95% CI: 8–37%) of patients. Cause of death was related to respiratory failure in 8% (95% CI: 0–99%) of patients and multi-organ failure in 6% (95% CI: 3–11%) of patients.
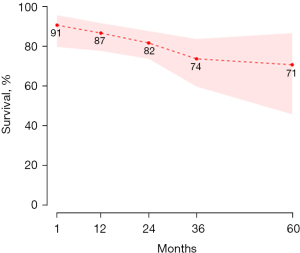
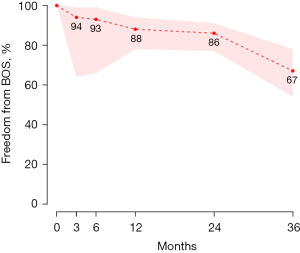
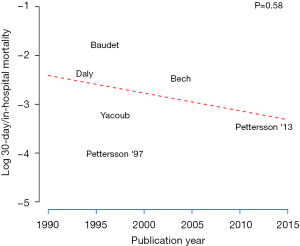
The mean follow-up time was 21 months (95% CI: 3–38 months) with a 30-day/in-hospital mortality of 6% (95% CI: 3–11%). 79% (95% CI: 63–89%) of patients were free from rejection at three months. Eighty-four percent (95% CI: 49–97%) of patients were free from anastomotic intervention at both three and six months. Eeighty-three percent (95% CI: 29–98%) of patients were free from signs of airway ischemia (assessed via bronchoscopy) at three and six months (Table 3). Pooled survival analysis, seen in Figure 2, demonstrates 87% (95% CI: 78–92%) and 71% (95% CI: 46–87%) survival at one year and five years respectively. Pooled freedom from bronchiolitis obliterans is demonstrated in Figure 3 with 86% (95% CI: 77–91%) of patients free from BOS at two years. A meta-regression analysis to assess the relationship between Log 30-day/in-hospital mortality and time (publication year), shown in Figure 4, showed no significant effect of time on the mortality outcome (P=0.58).
Table 3
| Variable | Pooled value [95% CI] | Number of studies | Events/total (n/N) | Heterogeneity, (%) |
|---|---|---|---|---|
| Follow up, months | 21 [3–38] | 4 | 70 | 94* |
| 30-day/in-hospital mortality (%) | 6 [3–11] | 6 | 8/135 | 0 |
| Freedom from airway ischemia (%) | ||||
| 3 months | 83 [29–98] | 3 | 48/55 | 48 |
| 6 months | 83 [29–98] | 3 | 48/55 | 48 |
| Freedom from anastomotic intervention (%) | ||||
| 3 months | 84 [49–97] | 4 | 57/63 | 37 |
| 6 months | 84 [49–97] | 4 | 57/63 | 37 |
| Freedom from rejection (%) | ||||
| 3 months | 79 [63–89] | 4 | 51/63 | 0 |
*, indicates significant heterogeneity, P<0.05. CI, confidence interval.
Discussion
With the increasing number of lung transplants being performed and a continual lagging median survival rate when compared to other solid-organ transplants, the field of lung transplantation has been hampered by the long-term development of CLAD. In order to strive to reach the survival rates attained by other solid-organ transplants, strategies to mitigate development of BOS must be developed. Whether through surgical technique or medications, reducing BOS is of paramount importance to lengthening the survival time of lung transplant recipients. Given the hypothesis of ischemia in the early peri-operative period potentially leading to late development of BOS, it stands to reason that improvements in surgical technique may limit its development and therefore improve patient survival. Following conventional lung transplantation without bronchial artery revascularization, the recipient’s lungs are dependent on collateral flow from the pulmonary vein in the submucosal plexus with bronchial arterioles and retrograde flow through these vessels (23,27,28). Revascularization of the donor organ by recipient bronchial arteries may take up to two to four weeks to restore flow which represents a critical period for healing. Restoring bronchial artery flow at the time of transplant could potentially result in reduced early ischemia to the bronchial anastomosis, improved overall survival, and reduced rates of BOS development.
As a contributing factor to the development of BOS, understanding airway ischemia and its effects on the lung may be helpful in outlining the evolution of BOS (6,7,13,15,16). In our study, 83% (95% CI: 29–98%) of patients did not have any signs of airway ischemia following bronchial artery revascularization at both three and six months post-transplantation. This percentage is largely consistent with the rate of airway complications following conventional lung transplantation, reported as 15.7% in a large scale study (29). The reported incidence of airway complications with conventional lung transplantation ranges from 2% to 33% and these complications vary in their requirement for intervention, ranging from simple conservative management to angioplasty, stenting, and surgical intervention (30). In the BAR cohort, 84% (95% CI: 49–97%) of patients were free from anastomotic intervention at both three and six months. When comparing this intervention rate with conventional lung transplantation, 14.6% of patients required stent insertion at an average of 76 days for anastomotic complications in a cohort of 123 conventional lung transplant patients (31). However, based on these data, BAR has yielded similar airway ischemia and airway intervention rates among lung transplant recipients compared to conventional lung transplants.
While airway ischemia rates may be similar between BAR and conventional lung transplant recipients, in order to truly evaluate the results of the BAR cohort, long-term survival needs to be examined. The United Network for Organ Sharing (UNOS) reports overall survival percentages for lung transplantation at 1-, 3-, and 5-year as 89.4%, 74.8%, and 61.2%, respectively (32). While these numbers include all types of lung transplant techniques, and given that BAR is rarely performed, these percentages are then more likely to represent conventional transplantation without BAR. When comparing these survival rates with those in our study, survival percentages at one year, three years, and five years are 87%, 74%, and 71% respectively. These numbers are similar at the one year and three year mark, yet the survival at 5 years is almost 10% better in the BAR cohort. One of the many possible reasons for this difference could be CLAD (33) which has a median onset of 2.3 years (12). It thus stands to reason that reduction in CLAD may contribute to improved long term survival. This is further supported by our data demonstrating 19% (95% CI: 8–37%) of patients who underwent BAR developed BOS or showed signs of pre-BOS over the mean follow up period of 21 months (95% CI: 3–38 months). When demonstrated as freedom from development of BOS, 67% of patients had no signs of BOS at 36 months. In comparison, approximately 43% of patients develop CLAD (without subtype distinction) at a median time of 2.3 years (12). This difference may suggest a correlation between BAR, reduction of BOS development, and improved survival outcomes. Further long-term data is needed to determine if this difference is statistically significant.
While there may be benefits to patients following BAR for lung transplantation, it is a demanding technique requiring additional focus on conduit preservation and monitoring of post-operative complications. Procuring the donor organ requires additional meticulous preservation of the bronchial artery and understanding of variant bronchial artery anatomy. This additional understanding requires further specialized training and has only been accomplished in limited number of centers with expert surgeons. Further, the additional preservation and formation of an additional arterial anastomosis increases the risk of bleeding complications and has been historically suggested as a reason for increased operative and cardiopulmonary bypass times (34). However, a propensity matched trial by Pettersson et al. indicated similar cardiopulmonary bypass time [BAR (n=20) vs. Non-BAR/Double lung (n=37): 164±32 vs. 178±78; P=0.3] and skin-to-skin times [BAR vs. Non-BAR/Double lung: 350±71 vs. 318±86; P=0.07] (24). Despite this smaller scale study, concerns persist regarding the feasibility of BAR and its effect on short-term outcomes.
When evaluating the short-term success of BAR, the two important factors to note are peri-operative mortality and bleeding complications. In evaluation of peri-operative mortality, when examining all transplants from 1989 to 2014, the UNOS lung transplantation 30-day mortality was 5.5% (35). In this cohort of BAR patients, the 30-day/in-hospital mortality was 6% (95% CI: 3–11%). These mortality rates are similar given the UNOS-reported overall rate is within the 95% confidence interval from our pooled cohort. With respect to bleeding complications in our study, 18% (95% CI: 9–31%) of patients suffered a hemorrhage of any kind with 14% (95% CI: 4–41%) requiring re-operation due to bleeding. Re-exploration for any cause was seen in 20% (95% CI: 9–38%) of patients. In comparison, a study of 224 patients undergoing conventional lung transplantation revealed a hemorrhage rate of 25.3% while re-operation for bleeding was required in 5.8% of patients. In this series, reoperation was needed in 7.2% of patients. Therefore, re-operation for bleeding and re-operation for any reason were higher in our cohort of patients with BAR, while overall hemorrhage rates were similar in both groups (36). Previous studies have thus listed risk of bleeding from revascularization sites as a potential complication from the BAR procedure and this is reflected in our systematic review too (23,24).
In evaluating BAR, surgeons should compare the increased technical requirements and peri-operative bleeding complications with the potential reduction or delay of BOS onset, which may outweigh the complications (20,24,26). It is possible that advancement in lung transplantation techniques and policies may have resulted in the improved overall survival after lung transplantation. By extension, it can be argued that BOS rates may also have improved (8,37). However, our results showed no effect of time on the 30-day/in-hospital mortality. It is also possible that offsetting the period of early ischemia following transplantation can reduce BOS and improve survival times. Despite this theoretical difference, freedom from airway ischemia and airway intervention was similar between BAR and non-BAR patients. Further long-term, large-scale analysis is needed to determine if the reduction in BOS development and improvement in long-term survival remain true for BAR.
Limitations and future directions
This systematic review and meta-analysis is limited by the available surgeons, institutions, and papers discussing a highly specialized technique. Given these limited numbers, direct evaluation via double-armed studies comparing BAR to similarly matched non-BAR patients was not possible, and therefore the technique was compared to overall numbers as cited by UNOS. Further, as the surgeons and centers who performing BAR are likely invested in its positive portrayal, they may be subject to some selection and publication bias. This systematic review indicated many positive aspects of utilizing BAR in lung transplantation and provided early signs of high survival, low development of BOS, and low interventions for airway ischemia. However, long-term studies directly comparing similar patients undergoing BAR and non-BAR lung transplantation are needed to further evaluate BAR’s effect on outcomes relative to conventional transplantation. While it is possible for double lung transplant patients to undergo a tracheal anastomosis with a left bronchial artery revascularization, analysis of included papers shows lack of sufficient granularity to differentiate that. Additionally, for the sake of this review the single-lung transplant patients were assumed to have undergone unilateral BAR with no subsequent contralateral single-lung transplant with contralateral BAR. The promising nature of this intervention will require a more granular study of sequential bilateral single-lung transplant with bilateral BAR versus double-lung transplant with bilateral BAR as well as double-lung transplant with unilateral BAR. However, it should be noted that the literature on survival differences between single vs. double lung transplantation is still conflicting (38,39). Donation after circulatory death (DCD) is another aspect that would be worth investigating. Lung transplant outcomes are generally comparable between DCD and Donation after Brainstem Death (DBD) (40); however, a higher rate of BOS following DCD lung transplantation has been reported (41). These conflicting results warrant further investigation into the relationship, if any, between airway ischemia and development of BOS. Given that, DCD has been gaining gradual acceptance, and that the studies included in this analysis span the last three decades, not much information was available to analyze this. Future comparative studies on BAR with results stratified by donor type (DCD vs. DBD) would help in answering such questions. Further, due to the technical requirements, all surgeons participating in a comparative study should be adequately trained at centers with extensive experience in BAR, otherwise, true results may be masked by imperfect technique.
Conclusions
Bronchial artery revascularization is a viable lung transplantation technique that results in high long-term survival, low long-term development of bronchiolitis obliterans, and low early anastomotic intervention due to ischemia at the cost of increased short-term bleeding complications. Further comparative analysis should be performed to evaluate this surgical technique versus conventional lung transplant.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-213/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-213/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-213/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yusen RD, Edwards LB, Dipchand AI, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-third Adult Lung and Heart-Lung Transplant Report-2016; Focus Theme: Primary Diagnostic Indications for Transplant. J Heart Lung Transplant 2016;35:1170-84. [Crossref] [PubMed]
- Valapour M, Lehr CJ, Skeans MA, et al. OPTN/SRTR 2018 Annual Data Report: Lung. Am J Transplant 2020;20:427-508. [Crossref] [PubMed]
- Baikoussis NG, Argiriou M, Argiriou O, et al. Perceval S aortic valve implantation in an achondroplastic Dwarf. Ann Card Anaesth 2016;19:166-8. [Crossref] [PubMed]
- Thabut G, Mal H. Outcomes after lung transplantation. J Thorac Dis 2017;9:2684-91. [Crossref] [PubMed]
- Gauthier JM, Hachem RR, Kreisel D. Update on Chronic Lung Allograft Dysfunction. Curr Transplant Rep 2016;3:185-91. [Crossref] [PubMed]
- Bando K, Paradis IL, Similo S, et al. Obliterative bronchiolitis after lung and heart-lung transplantation. An analysis of risk factors and management. J Thorac Cardiovasc Surg 1995;110:4-13; discussion 13-4. [Crossref] [PubMed]
- Yousem SA, Dauber JH, Griffith BP. Bronchial cartilage alterations in lung transplantation. Chest 1990;98:1121-4. [Crossref] [PubMed]
- Kulkarni HS, Cherikh WS, Chambers DC, et al. Bronchiolitis obliterans syndrome-free survival after lung transplantation: An International Society for Heart and Lung Transplantation Thoracic Transplant Registry analysis. J Heart Lung Transplant 2019;38:5-16. [Crossref] [PubMed]
- Todd JL, Palmer SM. Bronchiolitis obliterans syndrome: the final frontier for lung transplantation. Chest 2011;140:502-8. [Crossref] [PubMed]
- Hayes D Jr. A review of bronchiolitis obliterans syndrome and therapeutic strategies. J Cardiothorac Surg 2011;6:92. [Crossref] [PubMed]
- Chambers DC, Cherikh WS, Goldfarb SB, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-fifth adult lung and heart-lung transplant report-2018; Focus theme: Multiorgan Transplantation. J Heart Lung Transplant 2018;37:1169-83. [Crossref] [PubMed]
- Nykänen A, Raivio P, Peräkylä L, et al. Incidence and impact of chronic lung allograft dysfunction after lung transplantation - single-center 14-year experience. Scand Cardiovasc J 2020;54:192-9. [Crossref] [PubMed]
- Fiser SM, Tribble CG, Long SM, et al. Ischemia-reperfusion injury after lung transplantation increases risk of late bronchiolitis obliterans syndrome. Ann Thorac Surg 2002;73:1041-7; discussion 1047-8. [Crossref] [PubMed]
- Verleden GM, Glanville AR, Lease ED, et al. Chronic lung allograft dysfunction: Definition, diagnostic criteria, and approaches to treatment-A consensus report from the Pulmonary Council of the ISHLT. J Heart Lung Transplant 2019;38:493-503. [Crossref] [PubMed]
- Weigt SS, DerHovanessian A, Wallace WD, et al. Bronchiolitis obliterans syndrome: the Achilles' heel of lung transplantation. Semin Respir Crit Care Med 2013;34:336-51. [Crossref] [PubMed]
- Sharples LD, McNeil K, Stewart S, et al. Risk factors for bronchiolitis obliterans: a systematic review of recent publications. J Heart Lung Transplant 2002;21:271-81. [Crossref] [PubMed]
- Yserbyt J, Dooms C, Vos R, et al. Anastomotic airway complications after lung transplantation: risk factors, treatment modalities and outcome-a single-centre experience. Eur J Cardiothorac Surg 2016;49:e1-8. [Crossref] [PubMed]
- Sundset A, Lund MB, Hansen G, et al. Airway complications after lung transplantation: long-term outcome of silicone stenting. Respiration 2012;83:245-52. [Crossref] [PubMed]
- Couraud L, Baudet E, Martigne C, et al. Bronchial revascularization in double-lung transplantation: a series of 8 patients. Bordeaux Lung and Heart-Lung Transplant Group. Ann Thorac Surg 1992;53:88-94. [Crossref] [PubMed]
- Daly RC, McGregor CG. Routine immediate direct bronchial artery revascularization for single-lung transplantation. Ann Thorac Surg 1994;57:1446-52. [Crossref] [PubMed]
- Pettersson G, Nørgaard MA, Arendrup H, et al. Direct bronchial artery revascularization and en bloc double lung transplantation--surgical techniques and early outcome. J Heart Lung Transplant 1997;16:320-33. [PubMed]
- Yacoub M, Al-Kattan KM, Tadjkarimi S, et al. Medium term results of direct bronchial arterial revascularisation using IMA for single lung transplantation (SLT with direct revascularisation). Eur J Cardiothorac Surg 1997;11:1030-6. [Crossref] [PubMed]
- Hyytinen TA, Heikkilä LJ, Verkkala KA, et al. Bronchial artery revascularization improves tracheal anastomotic healing after lung transplantation. Scand Cardiovasc J 2000;34:213-8. [Crossref] [PubMed]
- Pettersson GB, Karam K, Thuita L, et al. Comparative study of bronchial artery revascularization in lung transplantation. J Thorac Cardiovasc Surg 2013;146:894-900.e3. [Crossref] [PubMed]
- Bech B, Pressler T, Iversen M, et al. Long-term outcome of lung transplantation for cystic fibrosis--Danish results. Eur J Cardiothorac Surg 2004;26:1180-6. [Crossref] [PubMed]
- Baudet EM, Dromer C, Dubrez J, et al. Intermediate-term results after en bloc double-lung transplantation with bronchial arterial revascularization. Bordeaux Lung and Heart-Lung Transplant Group. J Thorac Cardiovasc Surg 1996;112:1292-9; discussion 1299-300. [Crossref] [PubMed]
- Mulligan MS. Endoscopic management of airway complications after lung transplantation. Chest Surg Clin N Am 2001;11:907-15. [PubMed]
- Gao H, Zhu B, Yi J, et al. Urgent tracheal resection and reconstruction assisted by temporary cardiopulmonary bypass: a case report. Chin Med Sci J 2013;28:55-7. [Crossref] [PubMed]
- Van De Wauwer C, Van Raemdonck D, Verleden GM, et al. Risk factors for airway complications within the first year after lung transplantation. Eur J Cardiothorac Surg 2007;31:703-10. [Crossref] [PubMed]
- Mahajan AK, Folch E, Khandhar SJ, et al. The Diagnosis and Management of Airway Complications Following Lung Transplantation. Chest 2017;152:627-38. [Crossref] [PubMed]
- Herrera JM, McNeil KD, Higgins RS, et al. Airway complications after lung transplantation: treatment and long-term outcome. Ann Thorac Surg 2001;71:989-93; discussion 993-4. [Crossref] [PubMed]
- Valapour M, Lehr CJ, Skeans MA, et al. OPTN/SRTR 2020 Annual Data Report: Lung. Am J Transplant 2022;22:438-518. [Crossref] [PubMed]
- Chaparro C, Scavuzzo M, Winton T, et al. Status of lung transplant recipients surviving beyond five years. J Heart Lung Transplant 1997;16:511-6. [PubMed]
- Nørgaard MA, Olsen PS, Svendsen UG, et al. Revascularization of the bronchial arteries in lung transplantation: an overview. Ann Thorac Surg 1996;62:1215-21. [Crossref] [PubMed]
- Banga A, Mohanka M, Mullins J, et al. Incidence and variables associated with 30-day mortality after lung transplantation. Clin Transplant 2019;33:e13468. [Crossref] [PubMed]
- Paradela M, González D, Parente I, et al. Surgical risk factors associated with lung transplantation. Transplant Proc 2009;41:2218-20. [Crossref] [PubMed]
- Heng D, Sharples LD, McNeil K, et al. Bronchiolitis obliterans syndrome: incidence, natural history, prognosis, and risk factors. J Heart Lung Transplant 1998;17:1255-63. [PubMed]
- Ranganath NK, Malas J, Phillips KG, et al. Single and Double Lung Transplantation Have Equivalent Survival for Idiopathic Pulmonary Fibrosis. Ann Thorac Surg 2020;109:211-7. [Crossref] [PubMed]
- Schaffer JM, Singh SK, Reitz BA, et al. Single- vs double-lung transplantation in patients with chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis since the implementation of lung allocation based on medical need. JAMA 2015;313:936-48. [Crossref] [PubMed]
- van Suylen V, Luijk B, Hoek RAS, et al. A Multicenter Study on Long-Term Outcomes After Lung Transplantation Comparing Donation After Circulatory Death and Donation After Brain Death. Am J Transplant 2017;17:2679-86. [Crossref] [PubMed]
- Sabashnikov A, Patil NP, Popov AF, et al. Long-term results after lung transplantation using organs from circulatory death donors: a propensity score-matched analysis†. Eur J Cardiothorac Surg 2016;49:46-53. [Crossref] [PubMed]


