Robotic sleeve lobectomy: technical details and early results
Introduction
Sleeve lobectomy as described in other parts of this journal’s special edition avoids the morbidity of the resection of another lobe of the lung. More importantly, it often avoids the vastly increased morbidity of pneumonectomy. There are few reports of sleeve lobectomy performed via minimally invasive techniques. Zhou and colleagues in 2015 reported on 10 patients (1) and showed that sleeve lobectomy can be performed safely with similar early and late outcomes compared to a sleeve lobectomy performed via thoracotomy. We are aware of no published reports of robotic sleeve lobectomy except for a few case reports, yet we know several surgeons who have performed a handful safely. This chapter we will focus on the specific technical aspects of robotic sleeve resections of the airway and also briefly outline our early results in 8 patients which maybe the largest series of robotic sleeves.
Detailed technical port placement
We prefer the completely portal robotic lobectomy using four arms (CPRS-4) as we have previously described (2,3).
Since the most common sleeve is a right-sided right upper lobectomy we will describe it and on the most common robotic system a daVinci Si (Intuitive Surgical, Sunnyvale ca., USA).
Operative details
If mediastinoscopy is to be performed it is important to do it no more than 1 or 2 days before the definitive sleeve or at the same time and rely on frozen section analysis. This allows for decreasing tension on the anastomosis, by freeing up the left and main stem bronchus and ensuring there is neither N2 nor N3 metastatic disease.
The right pleural space is entered over the top of the 8th rib for sleeve lobectomy of the upper and middle lobes. We enter the pleural space using a 5-mm port exactly 21 cm from the spinous process in the right chest and 23 cm from the spinous process in the left chest. This serves as the camera port.
To initiate the operation we use a 5-mm VATS camera, but this is eventually replaced by a 12-mm robotic zero-degree camera. We only use a 0-degree camera because we believe it reduces injury to the intercostal nerve by having less torque than a 30-degree down camera and importantly it provides significantly more room for the bedside assistant.
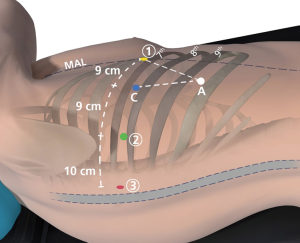
A 5-mm VATS camera is used to ensure entry into the pleural space via the camera port as shown in the diagram below. Warmed humidified CO2 is then insufflated in the chest to drive the diaphragm inferiorly. Under direct vision, a paravertebral block from ribs 3 to 11 is performed using bupivicaine with epinephrine. The next port placed is the most posterior port, which is the site of robotic arm 3. The location for this port is identified by using a long 21-gauge needle through which the bupivacaine is administered. The location chosen is at least two ribs below the major fissure and as far posterior in the chest as possible, just anterior to the spinal processes of the vertebral body. A small 5-mm incision is made and a 5-mm reusable metal da Vinci trocar is placed. This will serve as robotic arm 3.
Robotic arm two is placed next. It is located 10 cm anteriorly to the most posterior incision and along the same rib (most commonly rib 8 for upper lobe segmentectomy and rib 9 for lower lobes segmentectomy). An 8-mm metal reusable da Vinci trocar is used. Recently we have switched to a 5-mm trocar here to reduce pain. Robotic arm 2 is docked to this trocar.
The last two incisions are carefully planned with pen marks on the skin prior to making them. The VATS camera is now placed via robotic arm 2’s port in order to see the seeking needle that helps guides these port placements. The location for robotic arm 1 is at least 9 cm anteriorly to the camera port. It should be as inferior and anteriorly in the chest as possible. It is usually much further away from the camera than 9 cm. It is commonly placed along the same rib as the previously placed ports, as shown in Figure 1, but can be placed a rib or two higher if needed. It should be located to maximize the depth and width of the triangle made by the camera port, the access port and robotic arm 1. Prior to placing the access port, we ensure that the CO2 insufflation is active, to depress the diaphragm as low as possible. Once these two port locations are chosen a 12-mm plastic disposable port is placed in the access port and an 8-mm metal robotic reusable port is placed for robotic arm 1.
The robot is driven over the patient’s shoulder on a 15-degree angle and attached to the four ports. In general, only four robotic instruments are used for the lobectomy and we add tow more for sleeve resection: a 5-mm lung grasper in robotic arm 3, a 5-mm Schertel in robotic arm 2 (if an 8-mm trocar is used then a Cadiere forceps is used), a 12-mm zero degree camera in the camera port and an 8-mm bipolar curved tip dissector in robotic arm 1. For the anastomosis we use the small robotic needle driver and the robotic Debakey forceps.
Operative technique
After the pleural surface is inspected to confirm the absence of metastases, we proceed with mediastinal lymph node dissection:
Right-sided right upper lobectomy sleeve resection
The inferior pulmonary ligament is divided and lymph node station 9 is removed followed by lymph nodes from station 8 and then 7. Robotic arm 3 is used to retract the lower lobe medially and anteriorly in order to remove lymph nodes from station 7. Care is taken to control the two feeding arteries to the subcarinal lymph node. For a sleeve of the right upper lobectomy the triangle between the bronchus intermedius (BI) and the right upper lobe bronchus is identified. The number 11 lymph node is removed and the posterior segmental artery to the right upper lobe is identified. This can be taken with a vascular stapler now. Robotic arm 3 is then used to retract the upper lobe inferiorly while robotic arms 1 and 2 are used to dissect out stations 2R and 4R, clearing the space between the SVC anteriorly and the azygos vein. We prefer to ligate the azygous on sleeve resection and it can be taken now. The 10R lymph node between the right main stem bronchus and the pulmonary artery is then removed. The appropriate inter-lobar lymph nodes are removed especially the ones that are adjacent to the bronchus to be removed.
Once the posterior segmental artery is stapled or clipped and divided the RUL bronchus can be dissected. If the tumor in the airway prevents this move then the lung should be retracted posteriorly and the anterior hilum viewed.
The right superior pulmonary vein (sparing the right middle lobe vein) and the anterior apical pulmonary arterial branch are identified, encircled and stapled and divided. The rest of the pulmonary artery should be carefully inspected to ensure there are no other small PA braches left going to the RUL.
The fissure is then stapled next and the bronchus is cut last. We prefer to staple the fissure from the back to the front and not in the more traditionally manner from anterior to posterior.
If needed a bronchoscope can be inserted to help mark the best part for the bronchotomy. Perhaps one of the greatest advantages of the robot is the 10 times magnified 3 dimensional view of the operative field. For this reason we prefer to cut the BI first to be able to then look inside the airway to see the optimal location to cut the right main stem bronchus to obtain a negative margin but leave as much of the right mains stem as possible. If the tumor is large and bulky we often will cut the right upper lobe bronchus flush at its origin, which allows us to remove the specimen and obtain more room in the operative field.
Preparing for the anastomosis
The ideal instrument used to cut the bronchus is a monopolar shears scissors. We prefer to cut the BI margin first as described above and then the right main stem margin. Separate margins are sent to ensure they are both negative on frozen section but we do not wait for pathology if they appear normal on visualization using the 10 magnified view. We start the anastomoses while the pathologists are cutting and freeze it. It’s important to ensure that the distal BI does not twist. An important technique that we have used to avoid any twisting is to place a suture in the BI and cartilage at the 12 o’clock position. We then retract the BI posteriorly and use blunt dissection to obtain more lengthens as we dissect it off of the main pulmonary artery going to the middle and lower lobes. This is a nice maneuver that affords length of the BI in the same way that mediastinoscopy affords length of the right and left main stem and trachea. It is critical to keep the posterior part of the airway (both the right main stem and the BI) posteriorly so they stay aligned. It should run parallel to the vertebral bodies
As shown in the video (Figure 2). We then lift up the stapled and divided azygous vein and retract it posteriorly and then dissect out the tissue off the membranous mainstream part of the trachea. In addition, we remove all of the subcarinal lymph node to clearly see the right left main stem. This provides length and reduces tension on the anastomosis.

The anastomosis
We use the small robotic needle driver with which has a scissors in its heal to cut after tying. We prefer 3-0 Vicrly suture on an RV 1 needle that is cut to be 8 cm in length. The first suture is critical and the knot should be outside the airway. We preferred to place our first suture at 6 o’clock on the right main stem bronchus (from out to in) as shown in the video to show on the figure and then from in to out on the BI at the corresponding 12 o’clock. This places the knot in the cartilage close to the membranous part of the airway and tie without tearing the tissue. It is important not to place the first suture in the membranous part of the airway since it can tear as it is tied. We then place interrupted sutures with knots on the outside staying on the cartilage part of the airway to slowly bring the two cut ends together. Although a running suture line is what we prefer for open cases (using 3-0 or 4-0 PDS on an RV 1 needle) the robotic instruments can fray sutures especially earlier in one experience and the PDS is difficult to work with on the robot. For these reasons we prefer multiple interrupted Vicryl’s and it allows the resident and fellow to sew and tie. Since the right main stem bronchus has a larger caliber than the BI this is taken into consideration, as the sutures are placed. We do telescope the anastomosis.
Once the entire cartilaginous aspect of the airway anastomosis is completed the lung is then retracted anteriorly and inferiorly, which allows one to view the entire membranous part of the anastomosis as shown below. This can be easily closed with a running PDS or Vicrly suture with knots again outside the airway. The anastomosis can be covered on the right side with the part of the thymus as we have used for over ten years now or the intercostal muscle or pleura can be used. We do not routinely wrap the anastomoses anymore unless there was preoperative radiation and or the patient has risks for a leak such as steroids etc. We do not circumferentially wrap an anastomosis but rather just the anterior aspect of it. Once the anastomosis is completed warm water should be placed in the chest, the CO2 should be turned off (which is an important step that many seem to forget) and the anesthesiologist is asked to deliver a few small breaths to ensure the anastomosis does not leak. Will use a single 20 French chest tube that is placed anteriorly.
Results
Table 1 shows our results of these eight patients. It shows that six had a right upper lobectomy, one had left upper lobectomy sleeve and one had a resection of a neuroendocrine tumor of the BI. There was one conversion to thoracotomy for bleeding that occurred in an attempted right upper lobectomy sleeve because of injury to the apical segment of the pulmonary artery. The artery was adherent to the bronchus. The injury was packed and elective conversion to thoracotomy was performed without the need for blood transfusion. The patient did well and underwent right upper lobe sleeve resection.

Full table
There were no 30 or 90-day mortality and no major morbidity. One patient had a short burst of atrial fibrillation for 3 hours. All patients except one are at least 6 months out with no evidence of recurrence on CT scan. All patients have had at least one post-operative surveillance bronchoscopy and there is no significant stricture or recurrent cancer at the anastomosis.
Acknowledgements
None.
Footnote
Robert J. Cerfolio: proctor and teacher for Intuitive Surgical, grant support—Precision, proctor and teacher for Ethicon and Covidian, consultant for Community Health Services, consultant for Bovie, consultant for KCL, and consultant for C-SATS.
References
- Zhou S, Pei G, Han Y, et al. Sleeve lobectomy by video-assisted thoracic surgery versus thoracotomy for non-small cell lung cancer. J Cardiothorac Surg 2015;10:116. [PubMed]
- Cerfolio RJ. Total port approach for robotic lobectomy. Thorac Surg Clin 2014;24:151-6. v. [PubMed]
- Cerfolio RJ, Bryant AS, Skylizard L, et al. Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J Thorac Cardiovasc Surg 2011;142:740-6. [PubMed]
- Cerfolio RJ. Robotic right upper lobe sleeve lobectomy for a 72-year-old patient. Asvide 2016;3.068. Available online: http://www.asvide.com/articles/820


