Anatomical segmentectomy under uniportal video-assisted thoracoscopic surgery for early staged non-small cell lung cancer: a case report
Introduction
With the development of computed tomography (CT) technology and the consciousness of physical examination, the diagnoses of pulmonary nodules with ground-glass opacity (GGO) and part/non-solid nodules have been increasing (1). Since 1960, for early-staged non-small cell lung cancer (NSCLC), lobectomy is still the standard surgical procedure (2). However, when patients with multiple pulmonary nodules, poor cardiopulmonary function, and severe comorbidities and R0 complete resection are needed, anatomical segmentectomy is an alternative (3), and the National Comprehensive Cancer Network (NCCN) guidelines also prefer segmentectomy under these circumstances (4). Recent trials have shown that anatomic segmentectomy can achieve outcomes equivalent to lobectomy in selected patients with stage IA NSCLC (5-8), and the final results of two phase III clinical studies (JCOG0802/WJOG4607L and CALGB140503) which compare safety, mortality, and morbidity between segmentectomy and lobectomy deserve our expectation (9,10). Uniportal video-assisted thoracoscopic surgery (VATS) corresponds well with the idea of “minimally invasive”: less access to trauma, less stress response, less pain, better cosmesis, making it a popular approach to manage most thoracic surgical diseases.
Gonzalez-Rivas first reported uniportal VATS segmentectomy in 2012 (11) and briefly discussed the development of uniportal VATS in lung cancer treatment (12). After several years of development, increasingly thoracic surgeons can perform uniportal VATS segmentectomy and even subsegmentectomy. With the technique maturing, more details must be paid special attention to localization of nodules, identification of segmental structures by preoperative 3D reconstruction, identification of intersegmental plane during the operation, and other operative skills. Uniportal VATS anatomical segmentectomy needs surgical skill and patience and the effective cooperation of assistants, nurses, and anesthetists. Here we present a video of a patient undergoing S1 segmentectomy of right upper lobectomy (RUL) under uniportal VATS (Video 1), in which case, we made a 3D reconstruction and found out the variation of vessels and bronchus before performing segmentectomy. We think the diagnosis and treatment procedure of this patient is reasonable and operable. We present the following article in accordance with the CARE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1624/rc).
Case presentation
Patient
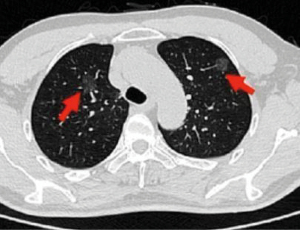
A 67-year-old male patient was admitted to our hospital with the chief complaints that two pure GGOs in the bilateral upper lobe were found by physical examination for 26 months. The high-resolution CT (HRCT) showed the GGO in RUL was about 21.1 mm in the greatest dimension, while the GGO in left upper lobectomy (LUL) was about 13.2 mm (Figure 1). He was asymptomatic and had no other comorbidities, and denied smoking history and family history. The pulmonary and cardiac function was normal, and the patient could tolerate lobectomy. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Indications
The NCCN guideline of NSCLC (2022, V3) suggests that anatomic pulmonary resection is preferred for most patients with NSCLC. However, for sublobar resection, anatomical segmentectomy is preferred over wedge resection in selected patients for the following reasons:
- Poor pulmonary reserve or another major comorbidity that contraindicates lobectomy;
- Peripheral nodule ≤2 cm with at least one of the following:
- Pure adenocarcinoma in situ (AIS) histology;
- Nodule has ≥50% ground-glass appearance on CT;
- Radiologic surveillance confirms a long doubling time (≥400 days).
Segmentectomy can be classified into two categories:
- Compromised segmentectomy: for advanced aged patients with multiple comorbidities, or those with poor pulmonary or (and) cardiac function and cannot tolerate lobectomy, or those with multiple nodules suspected early-stage cancer must be resected;
- Intentional segmentectomy: nodule ≤2 cm with low malignancy (nodule has ≥50% ground-glass appearance on CT).
The resection margins and the extent of lymph nodes (LNs) resection are preferred in NCCN guideline:
- Segmentectomy should achieve parenchymal resection margins ≥2 cm or ≥ the size of the nodule;
- Segmentectomy should also sample appropriate N1 and N2 LN stations, unless not technically feasible, without substantially increasing the surgical risk.
Strategy
The GGOs in bilateral upper lobes were pure GGOs with low malignancy, preferred to be resected by guidelines. So, although the size of the GGO in RUL is bigger than 2 cm, we plan to perform compromised segmentectomy to reserve normal lung tissue as much as possible to provide an opportunity to deal with the other GGO in LUL in the future.
3D-reconstruction
Before performing segmentectomy, we must accurately confirm where the nodules locate and realize the anatomic variation of the vessels and bronchi, especially the intersegmental vein. Transecting pulmonary veins by mistake may lead to the disorder of venous reflux, which may lead to pulmonary congestion. In our institute, we often perform 3D reconstruction with the software Mimics Medical (version 21.0).
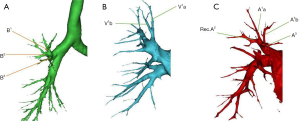
The configuration of the segmental bronchi, arteries, and veins of RUL is the most frequent type: the right upper bronchus branches into B1, B2, and B3. The A1 and A3 arteries branch from the truncus superior artery and A2a, also called Rec.A2, sometimes branch from the same artery, and we called A2b as Asc.A2 relating to the branches from the pulmonary trunk and approaches dorsal. The segmental veins branch from superior pulmonary were labeled as apical and central veins. The apical vein branches were classified as V1a, the intrasegmental vein, and V1b, the intersegmental vein between S1b and S3b.
The anatomical configuration of the bronchus and vein of S1 in this patient corresponds with what we have pointed (Figure 2A,2B), but A1a branch from a common stem with Rec.A2 instead of A1b (Figure 2C). Therefore, when we perform segmentectomy, we must expose A1a and A1b, respectively.
Anesthesia
The patient received intravenous and inhalation anesthesia through double-lumen endotracheal intubation.
Body position
Patients were placed in a left lateral decubitus position, and the arm of the operation side was fixed and kept 90° forward. We always put a small pillow at the level of the xiphoid to widen intercostal space. The surgeon stood on the ventral side of the patient, while the first and second assistants stood on the dorsal part of the patient. Changing the body position and full use of the lung’s gravity may help expose easily and obtain a better view. For example, when we dissect the anterior pulmonary hilum, we can turn the operation table dorsally. While we expose the subcarinal area, we can turn the operation table ventrally.
Incisions
Because all procedures must be performed through one port, either the size or position of the incision may affect the surgery process. In our institute, we always make a 3.5–4 cm-long incision in the 5th intercostal space, comparable with those commonly used for double- or triple-port approaches, through which we can obtain the same vision with thoracotomy will be more comfortable dissecting vessels and bronchus. Also, when conversion is necessary, we can extend the incision directly. A wound protector is always used to ease the insertion of the instruments and prevent wound contamination.
The 30° thoracoscope is usually inserted in the posterior part of the incision to avoid the interference of instruments. And the long-curved double-joint surgical instruments are employed.
Surgical technique
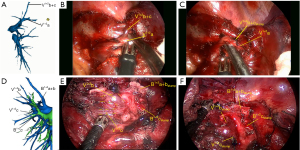
Individual dissection of segmental veins, arteries, bronchi, and intersegmental plane division are key points of segmentectomy. The sequence we deal with segmental structures is different when we perform different segmentectomies according to the anatomical relations among arteries, veins, and bronchi. The method of segmental vein dissection is different, either. We can dissect segmental veins from proximal to the distal end of the heart when the veins we plan to transect branch early from the superior or inferior pulmonary vein and run superficially (always in segmentectomy of S1, S3, and S6) (Figure 3A-3C). We can also dissect segmental veins under the stamp of targeted bronchus after transecting the targeted bronchus when the veins we plan to transect go deeply (always in segmentectomy of the lower lobe) (Figure 3D-3F).
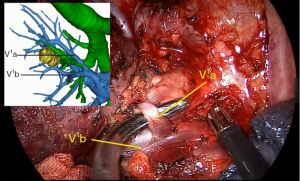
The S1 segmentectomy is simple to perform because the vessels and bronchus we plan to dissect are usually the first branches from the trunk. In our case, we should dissect and cut A1a and A1b arteries, V1a vein, and B1 bronchus, while the V1b vein should be preserved.
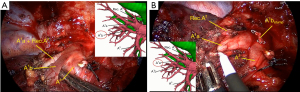
The process of the resection was as follows: we first retracted the lung, opened the right hilum adequately, and dissected the superior pulmonary vein with a combination of blunt and sharp dissection, and the LNs of station 10 were dissected. As 3D CTBA showed, the V1a vein was the first branch. Then the V1a vein was ligated by silk and transected by harmonic scalpel (Figure 4).
Then we dissected the right superior pulmonary artery, which branched into Rec.A2 + A1a + A1b + A3 (Figure 5A,5B). A 3 cm endostapler transected A1b artery. After careful dissection, the Rec.A2 and A1a were mobilized, and A 3 cm endostapler transected A1a. After the arteries and veins were divided, the B1 bronchus was exposed. We chose a 3 cm endostapler with the blue cartridge to transect B1 bronchus. Before transacting the bronchus, we asked the anesthetist to inflate the lung to check that the anterior and posterior segmental bronchus are not being transacted.
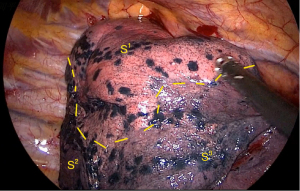
The method of inflation-deflation was used to divide the intersegmental plane. After airway suctioning, the anesthetist was asked to inflate the right lung fully with pure oxygen and collapse it. After 15 minutes, the S1 segment was inflated, while other segments deflated (Figure 6). The intersegmental plane was marked with an electric hook and divided with the 6 or 4.5 cm endostapler. However, including the arterial, venous, and bronchial stumps, when we remove the targeted segment helps set the right direction of the endostaplers, so we always continue dissecting the segmental hilum after transecting the whole structures. When we set endostaplers, we should pay special attention to avoid damaging the remaining segmental veins and bronchus. Finally, the specimen was removed, and the intraoperative frozen section diagnosis was atypical adenomatous hyperplasia (AAH) and AIS.
Because the intraoperative pathological diagnosis was AAH and AIS, we performed mediastinal LNs sampling. We divided the inferior pulmonary ligament and removed the station 9 LNs. Then we incised the posterior mediastinal pleura, and the station 7 LNs were removed. Finally, the superior mediastinal pleura was incised, and stations 2 and 4 were removed.
A 20-Fr chest tube is inserted through the same incision at the end of the procedure after checking bleeding and air leakage.
iMDT discussion
Discussion of the indication
Here, we performed compromised segmentectomy from the bilateral pulmonary GGOs, despite the tumor size. We performed compromised segmentectomy from the bilateral pulmonary GGOs, despite the tumor size. However, only when confirmed AIS histology can we perform segmentectomy for patients with solitary nodules smaller than 2 cm, according to NCCN guidelines. We cannot get an accurate intraoperative pathological diagnosis most of the time to confirm whether the nodule is AIS or minimally invasive adenocarcinoma (MIA) or invasive adenocarcinoma. Sometimes, even worse, we get an AIS diagnosis intraoperatively, then an invasive adenocarcinoma diagnosis postoperatively. Under these circumstances, could segmentectomy be a radical approach? Should we continue to perform lobectomy after segmentectomy with an intraoperative malignant diagnosis? Sublobectomy for treating early-stage lung cancer has been a controversial issue among thoracic surgeons for a long time. A recent meta-analysis showed that lobectomy in patients with stage IA disease caused a better survival rate than sublobar resection (including segmentectomy and wedge resection) (13). Dai et al. reported a similar conclusion and found that for patients with NSCLC ≤1 and 1–2 cm, sublobectomy showed worse survival than lobectomy (14). However, Tsutani et al. reported that for clinical IA lung adenocarcinoma, the 3-year recurrence-free survival and overall survival of segmentectomy were equivalent to standard lobectomy in all cohorts or propensity score-matched patients (5). However, two famous phase III clinical trials (JCOG0802/WJOG4607L and CALGB140503) compare the safety, mortality, and morbidity between segmentectomy lobectomy are under our expectation, and more practice data should be collected and analyzed (9,10). We can see the benefit of the segmentectomy with significant differences in OS is demonstrated in the phase III clinical trials JCOG0802/WJOG4607L, which enrolled 1,106 patients (lobectomy arm, n=554; segmentectomy arm, n=552) (15).
In WHO classification of tumors (5th edition, 2021), AAH and AIS are defined as Precursor lesions instead of Preinvasive lesions in 2015 edition. It means AAH and AIS are not in the category of adenocarcinoma, so the indication of surgery should be more prudent. In other words, whether AAH and AIS should be resected needs further discussion (16).
Discussion of resection margins and LNs biopsy
Resection margins and the metastasis state of No. 12 and No. 13 LNs should also be considered when discussing radical resection. Evidence shows a higher incidence of locoregional recurrence and worse patient survival when the surgical margin is inadequate (17). There are several recommendations to ensure the safe enough margins: Ginsberg and Rubenstein suggested the extension ≥ of 2 cm or margin-to-tumor ratio ≥1 (18), while Giraud et al. concluded the extension <8 and <6 mm for adenocarcinoma and squamous carcinoma respectively (19). In Wang’s segmentectomy series, segmental line always 3–5cm beyond which is spacious enough (20). Finally, according to the NCCN guideline, segmentectomy should achieve parenchymal resection margins ≥2 cm or ≥ the size of the nodule. In our case, the nodule was located exactly in the center of S1 of RUL, so the resection margin was enough. When nodules are located in the edge of one segment, we must perform “extended segmentectomy” or “combined segmentectomy or subsegmentectomy”, in which we extend the resection line beyond conventional anatomical segments by cutting into adjacent segments in a non-anatomical or anatomical manner to secure resection margins.
We often assess the metastasis state of No. 12 and No. 13 LNs of a targeted segment by intraoperative frozen sections when we perform segmentectomy. Because the No. 12 and No. 13 LNs are sentinel LNs, a higher proportion is sentinel LNs than the No. 10 and No. 11 LNs. According to his study, Nomori suggested that frozen sections intraoperatively assess No. 12 and No. 13 LNs (21,22). So only when we get a negative intraoperative pathological diagnosis of No. 12 and No. 13 LNs can we continue to perform segmentectomy? Otherwise, we must adjust our surgery plan to lobectomy (18)—however, the more LNs sampled during surgery, the more accurately we get pathologic staging. Segmentectomy is often associated with a higher number of LNs sampled to avoid misclassification of patients with LNs involvement as having stage I disease (23), another advantage of segmentectomy, and could explain the association between segmentectomy and improved survival.
Discussion of the recognition of the intersegmental plane
Recognition of the intersegmental plane is a key step in performing segmentectomy. Here, we used the method of inflation-deflation to identify the intersegmental plane. It is easy to perform and commonly used, but sometimes it may cost time to wait for the lung to deflate, especially when severe emphysema occurred in patients. In our institute, the intravenous injection of indocyanine green (ICG) under a fluorescent thoracoscope is an alternative. After resecting the vessels of the target segment, we inject ICG intravenously. After a few seconds, the target segment is visualized as a dark area, while the reserved lung perfused with ICG appears bright green. In one case, we performed a left S8 segmentectomy. After dealing with all the structures, we first performed the ICG method to confirm the intersegmental plane and marked the line (Figure 7A). Then we performed the inflation-deflation method to reconfirm (Figure 7B,7C). However, using the ICG method can verify whether we transect the correct vessels.

Discussion of the loss of pulmonary function
Since there are two to five pulmonary segments in each lobe, normal segments can be kept after removing just one or a few segments and theoretically preserving lung function. Several studies supported the opinion that segmentectomy can spare lung function better than lobectomy. Keenan et al. found that patients who accepted lobectomy experienced significant declines in forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), maximum voluntary ventilation (MVV) and diffusing capacity. However, only a decline in diffusing capacity in patients accepted segmentectomy 1 year after operation (24). The significant change of FEV1 was in the whole lung and the ipsilateral non-operated lung. Nomori suggested segmentectomy preserved the lobe and increased the function of the ipsilateral non-operated lobe 6 months after operation (25,26). One systematic review, including 16 studies, also reached the same conclusion (27).
However, despite preserving more lung tissue, other studies reported segmentectomy did not preserve lung function (28-30). However, the methods of segment division (either with electrocautery or with a stapler) and the surgical skills of segment division (whether or not protect bronchus and vessels of adjacent segment) and the time of follow-up (short- or long-term) may affect the conclusion (31). According to our experience, if multiple nodules are detected in different lobes, segmentectomy but not lobectomy can provide opportunities to resect all lesions radically.
Discussion of the technical issues of VATS
VATS was originally described by Jacobeus in 1910 and was widely adopted when Walker performed an anatomic lobectomy under VATS in 1993. Then several studies demonstrated there was no differences in short-term outcomes or mortality between VATS and thoracotomy in T1 and T2 tumors and patients, especially elderly patients, with VATS had fewer postoperative complications. In Ceppa’s study, VATS lobectomy had significantly fewer pulmonary complications compared with thoracotomy (P=0.023) (32,33).
Compared with multiportal VATS, uniportal VATS shortened the operation time and postoperative drainage period and significantly reduced the use of analgesics (34) and may be a safer and less invasive surgical procedure. Moreover, uniportal VATS can also achieve similar short- and medium-term outcomes as multiportal VATS in patients with NSCLC (35).
Although uniportal VATS can be less access trauma, less stress response, less pain, shorter hospital stays, and a lower postoperative complication rate, all procedures must be performed in one small port, and sometimes it is difficult to deal with the position of surgical instruments. Also, segmentectomy gives surgeons a higher request for anatomical knowledge and surgical skills and requires more experienced assistants, nurses, and anesthetists team. In conclusion, the uniportal VATS anatomical segmentectomy learning curve may be long, limiting this technique.
Several issues on the diagnosis and treatment of this patient were further discussed as follows
Question 1: If we cannot resect all nodules, what is the optimal treatment protocol for patients with multiple GGOs?
Expert opinion 1: Dr. Hideki Ujiie
Many patients with multiple GGOs might be intolerant to surgical resection due to impaired cardiopulmonary function or other impaired conditions. One of the options for treatment to multiple GGOs is stereotactic body radiation therapy (SBRT), also called stereotactic ablative therapy (SABR), a novel radiation modality recommended as an optional therapeutic strategy.
Expert opinion 2: Dr. Motoki Yano
It is not easy to define the protocol for multiple GGOs. We usually discuss multidisciplinary treatment in the team. The larger nodules with consolidation would be preferred to treat by resection or irradiation with priority.
Question 2: How can we distinguish multiple primary cancers or metastatic cancers when multiple nodules occur?
Expert opinion 1: Dr. Hideki Ujiie
Even though according to Martini-Melamed criteria, distinguishing multiple primary cancers or metastatic cancer is challenging. To better define the relationship between multiple lesions in the lung, alternative approaches using novel molecular and biomarker testing, including immunohistochemical and molecular analysis, have been useful methods.
Expert opinion 2: Dr. Motoki Yano
This question is also not easy to define. We usually discuss in the team of multidisciplinary treatment. If the multiple nodules include GGO, we usually diagnose multiple primary cancers. If the multiple nodules do not include GGO, we usually suspect multiple primary cancer and metastatic cancer.
Question 3: Is it essential for patients with multiple nodules to do molecular and biomarker analysis when they do not need postoperative therapy?
Expert opinion 1: Dr. Hideki Ujiie
As we mentioned above, molecular and biomarker analysis is useful for distinguishing multiple nodules without postoperative therapy.
Expert opinion 2: Dr. Motoki Yano
I want to analyze molecules and biomarkers for each nodule of multiple primary cancer should, if possible. However, it is not simple in many aspects, including health insurance or economics.
Question 4: Can it distinguish between segmentectomy and wedge resection with sufficient resection margins in the patient survival?
Expert opinion 1: Dr. Hideki Ujiie
Even though GGOs lesion, some patients have a recurrence, especially in wedge resection. There have been some reasons, including not enough margin and lung cancer subtype. For example, the micropapillary subtype with tumor spread through air spaces (STAS) has a higher recurrence rate.
Expert opinion 2: Dr. Motoki Yano
The difference may not be big. Especially, there may be no differences in GGOs.
Conclusions
Anatomical segmentectomy under uniportal VATS can be a feasible and safe procedure that reduces trauma and has equivalent oncology outcomes to lobectomy in early-stage lung cancer but need a more experienced medical center to perform.
Acknowledgments
Funding: The study was supported by the National Key Research and Development Program of China (No. 2018YFC1312100), Central Financial Fund for Promoting Medical Service and Safeguarding Capability (Capability Construction of Medical and Health Organizations)—a Subsidy to the Construction of Provincial Key Specialty, and Nature Science Foundation of China (No. 82073286).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1624/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1624/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hernandez-Arenas LA, Purmessur RD, Gonzalez-Rivas D. Uniportal video-assisted thoracoscopic segmentectomy. J Thorac Dis 2018;10:S1205-14. [Crossref] [PubMed]
- CAHAN WG. Radical lobectomy. J Thorac Cardiovasc Surg 1960;39:555-72. [Crossref] [PubMed]
- Gonzalez-Rivas D, Mendez L, Delgado M, et al. Uniportal video-assisted thoracoscopic anatomic segmentectomy. J Thorac Dis 2013;5:S226-33. [PubMed]
- NCCN clinical practice guidelines in oncology-non-small cell lung cancer (version 3, 2022). Available online: https://www.nccn.org/
- Tsutani Y, Miyata Y, Nakayama H, et al. Oncologic outcomes of segmentectomy compared with lobectomy for clinical stage IA lung adenocarcinoma: propensity score-matched analysis in a multicenter study. J Thorac Cardiovasc Surg 2013;146:358-64. [Crossref] [PubMed]
- Martin-Ucar AE, Nakas A, Pilling JE, et al. A case-matched study of anatomical segmentectomy versus lobectomy for stage I lung cancer in high-risk patients. Eur J Cardiothorac Surg 2005;27:675-9. [Crossref] [PubMed]
- Koike T, Yamato Y, Yoshiya K, et al. Intentional limited pulmonary resection for peripheral T1 N0 M0 small-sized lung cancer. J Thorac Cardiovasc Surg 2003;125:924-8. [Crossref] [PubMed]
- Smith CB, Swanson SJ, Mhango G, et al. Survival after segmentectomy and wedge resection in stage I non-small-cell lung cancer. J Thorac Oncol 2013;8:73-8. [Crossref] [PubMed]
- Suzuki K, Saji H, Aokage K, et al. Comparison of pulmonary segmentectomy and lobectomy: Safety results of a randomized trial. J Thorac Cardiovasc Surg 2019;158:895-907. [Crossref] [PubMed]
- Altorki NK, Wang X, Wigle D, et al. Perioperative mortality and morbidity after sublobar versus lobar resection for early-stage non-small-cell lung cancer: post-hoc analysis of an international, randomised, phase 3 trial (CALGB/Alliance 140503). Lancet Respir Med 2018;6:915-24. [Crossref] [PubMed]
- Gonzalez-Rivas D, Fieira E, Mendez L, et al. Single-port video-assisted thoracoscopic anatomic segmentectomy and right upper lobectomy. Eur J Cardiothorac Surg 2012;42:e169-71. [Crossref] [PubMed]
- Bulgarelli Maqueda L, García-Pérez A, Minasyan A, et al. Uniportal VATS for non-small cell lung cancer. Gen Thorac Cardiovasc Surg 2020;68:707-15. [Crossref] [PubMed]
- Liu Y, Huang C, Liu H, et al. Sublobectomy versus lobectomy for stage IA (T1a) non-small-cell lung cancer: a meta-analysis study. World J Surg Oncol 2014;12:138. [Crossref] [PubMed]
- Dai C, Shen J, Ren Y, et al. Choice of Surgical Procedure for Patients With Non-Small-Cell Lung Cancer ≤1 cm or >1 to 2 cm Among Lobectomy, Segmentectomy, and Wedge Resection: A Population-Based Study. J Clin Oncol 2016;34:3175-82. [Crossref] [PubMed]
- Saji H, Okada M, Tsuboi M, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022;399:1607-17. [Crossref] [PubMed]
- Xie H, Chen G. The Earliest Stage of Lung Adenocarcinoma: the Pathological Diagnosis and Clinical Significance of Adenocarcinoma In Situ. Zhongguo Fei Ai Za Zhi 2021;24:753-5. [PubMed]
- Sienel W, Stremmel C, Kirschbaum A, et al. Frequency of local recurrence following segmentectomy of stage IA non-small cell lung cancer is influenced by segment localisation and width of resection margins--implications for patient selection for segmentectomy. Eur J Cardiothorac Surg 2007;31:522-7; discussion 527-8. [Crossref] [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [Crossref] [PubMed]
- Giraud P, Antoine M, Larrouy A, et al. Evaluation of microscopic tumor extension in non-small-cell lung cancer for three-dimensional conformal radiotherapy planning. Int J Radiat Oncol Biol Phys 2000;48:1015-24. [Crossref] [PubMed]
- Wang G, Wang Z, Sun X, et al. Uniportal video-assisted thoracoscopic anatomic segmentectomy for small-sized lung cancer. J Vis Surg 2016;2:154. [Crossref] [PubMed]
- Nomori H, Watanabe K, Ohtsuka T, et al. In vivo identification of sentinel lymph nodes for clinical stage I non-small cell lung cancer for abbreviation of mediastinal lymph node dissection. Lung Cancer 2004;46:49-55. [Crossref] [PubMed]
- Nomori H. Segmentectomy for c-T1N0M0 non-small cell lung cancer. Surg Today 2014;44:812-9. [Crossref] [PubMed]
- Ludwig MS, Goodman M, Miller DL, et al. Postoperative survival and the number of lymph nodes sampled during resection of node-negative non-small cell lung cancer. Chest 2005;128:1545-50. [Crossref] [PubMed]
- Keenan RJ, Landreneau RJ, Maley RH Jr, et al. Segmental resection spares pulmonary function in patients with stage I lung cancer. Ann Thorac Surg 2004;78:228-33; discussion 228-33. [Crossref] [PubMed]
- Nomori H, Shiraishi A, Cong Y, et al. Differences in postoperative changes in pulmonary functions following segmentectomy compared with lobectomy. Eur J Cardiothorac Surg 2018;53:640-7. [Crossref] [PubMed]
- Tane S, Nishio W, Nishioka Y, et al. Evaluation of the Residual Lung Function After Thoracoscopic Segmentectomy Compared With Lobectomy. Ann Thorac Surg 2019;108:1543-50. [Crossref] [PubMed]
- Charloux A, Quoix E. Lung segmentectomy: does it offer a real functional benefit over lobectomy? Eur Respir Rev 2017;26:170079. [Crossref] [PubMed]
- Deng B, Cassivi SD, de Andrade M, et al. Clinical outcomes and changes in lung function after segmentectomy versus lobectomy for lung cancer cases. J Thorac Cardiovasc Surg 2014;148:1186-92.e3. [Crossref] [PubMed]
- Suzuki H, Morimoto J, Mizobuchi T, et al. Does segmentectomy really preserve the pulmonary function better than lobectomy for patients with early-stage lung cancer? Surg Today 2017;47:463-9. [Crossref] [PubMed]
- Hwang Y, Kang CH, Kim HS, et al. Comparison of thoracoscopic segmentectomy and thoracoscopic lobectomy on the patients with non-small cell lung cancer: a propensity score matching study. Eur J Cardiothorac Surg 2015;48:273-8. [Crossref] [PubMed]
- Tanvetyanon T, Keenan RJ. Recovery of lung function after segmentectomy versus lobectomy for early-stage lung cancer. J Thorac Dis 2018;10:S2144-6. [Crossref] [PubMed]
- Ceppa DP, Kosinski AS, Berry MF, et al. Thoracoscopic lobectomy has increasing benefit in patients with poor pulmonary function: a Society of Thoracic Surgeons Database analysis. Ann Surg 2012;256:487-93. [Crossref] [PubMed]
- Salfity H, Tong BC. VATS and Minimally Invasive Resection in Early-Stage NSCLC. Semin Respir Crit Care Med 2020;41:335-45. [Crossref] [PubMed]
- Matsuura N, Igai H, Yazawa T, et al. Uniportal versus Multiportal Video-assisted Thoracic Surgery for Primary Lung Cancer. Kyobu Geka 2021;74:167-71. [PubMed]
- Zhong D, Lin Q, Zhang J, et al. Short- and medium-term outcomes after uniportal and multiportal video-assisted thoracic surgery lobectomy in elderly patients with non-small cell lung cancer. J BUON 2021;26:1453-9. [PubMed]
(English Language Editor: J. Chapnick)