
3D localization based on anatomical LANDmarks in the treatment of pulmonary nodules
Introduction
With the continuous improvement in people’s living standards, the increase in lung cancer screening programs and the improvement in oncological follow-up, chest computer tomography (CT) examinations have become increasingly popular, and the screening rate of lung nodules in the population has been increasing. Some of these patients require thoracoscopic surgery for lung wedge resection and biopsy to determine the pathological nature. However, small lung nodules with a diameter of ≤1 cm and a distance of ≥0.5 cm from the pleura, especially ground glass nodules, are invisible and intangible during the operation, and the probability of thoracotomy is as high as 63% (1). How to accurately locate these nodules has become a popular research topic. At present, many types of positioning methods are used by clinical doctors worldwide, including preoperative CT-guided hookwire positioning (2,3), coil positioning (4,5), lipiodol positioning (6,7), radiotracers (8-10), electromagnetic navigation bronchoscope-guided positioning (11-13), and intraoperative ultrasound-assisted positioning (14,15). However, pros and cons exist to each approach. Correspondingly, positioning-related complications can occur, such as the inability to locate the nodule, positioning failure, complicated operations, and increased exposure to CT radiation, which can increase costs.
Based on these considerations, we aim to find a safer, easier, and more acceptable positioning method. Under continuous exploration and improvement, our thoracic surgery team found that a precise three-dimensional (3D) positioning method based on anatomical landmarks can meet the above requirements. More importantly, it can minimize positioning-related complications, such as pneumothorax, bleeding or air embolism caused by hookwire positioning under CT guidance. Additionally, it does not require repeated CT location irradiation before surgery, reducing the risk of CT radiation exposure. This method is performed intraoperatively such the patient does not experience the anxiety and discomfort elicited by another invasive preoperative positioning operation. Therefore, we hope that this comparison study can provide clinical colleagues and patients with a safer and more effective choice. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-203/rc).
Methods
Clinical information
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was conducted with the formal and written approval of the Institutional Ethics Committee of the University of Hong Kong-Shenzhen Hospital (No. hkuszh2020198). Informed consent was signed by all individual participants included in this study before the surgery. This was a retrospective review of surgical data obtained from patients who were diagnosed with lung nodules and underwent thoracoscopic surgery at the Department of Thoracic Surgery, the University of Hong Kong-Shenzhen Hospital, from June 2019 to December 2021. The screening criteria included the following: age between 18 and 80 years old; normal capacity for civil conduct; ability to sign an informed consent form for the operation independently; the presence of lung nodules as determined by CT examination (the CT showed clear boundaries, diameter less than or equal to 3 cm, and lungs surrounded by air-bearing lung tissue nodular lesions); no other invasive examinations, such as fine needle aspiration, performed before thoracoscopic surgery; preoperative examination to assess cardiopulmonary function for general anesthesia; no related surgical contraindications; evolving nodules or highly suspicious nodules discussed with a multidisciplinary tumor board; and the absence of retraction of the visceral pleura on preoperative CT-scan. A total of 154 patients with pulmonary nodules who agreed to undergo thoracoscopic wedge resection between June 2019 and December 2021 were involved in the study. Thirty-four patients with pulmonary nodules visible to the naked eye during surgery were excluded by our surgical team to avoid potential bias. During the operation, 120 patients with 131 pulmonary nodules were accurately located using the precise 3D positioning method based on anatomical landmarks, and then the pulmonary nodules were accurately removed to clarify the nature of the pathology.
The data of positioning time, accuracy rate, pathological result, localization-related complication rate and length of postoperative hospital stay were retrospectively reviewed and analyzed. The follow-up assessments were performed through direct examination by a clinician at the outpatient clinic as well as through questionnaire surveys and telephone calls at 1 week and 1 month after discharge.
Positioning method
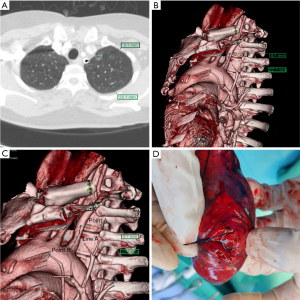
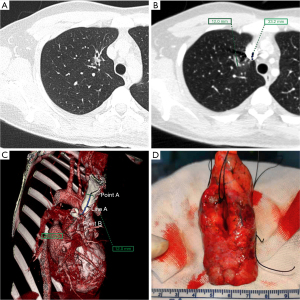
Before the operation, the image of the thin-slice CT scan of the patient with pulmonary nodules was imported into the Fuji SYNAPSE 3D image processing and analysis system, and the software was used to reconstruct and then find the location of one line (line A) and two points (point A and point B) in summary. Point A is the thoracic or mediastinal projection point of the pulmonary nodule; it refers to the projection point of the pulmonary nodule on the thoracic or mediastinum surface, which should be the closest point perpendicular to the lung nodule when the lung is inflated (Figure 1A). Line A is an anatomical marker line that refers to the linear anatomical structure (straight line or arc) adjacent to point A (Figure 1B), such as the upper edge of the rib (Figure 1C,1D), the lower edge of the rib, the superior vena cava, the blood vessels in the thorax, the subclavian artery, and the trachea. Point B is an anatomical marker point: it is located on line A and can be clearly identified during the operation, for example, the top of the transverse process of the vertebral body, the beginning of the left subclavian artery (Figure 2), the intersection of the superior vena cava and azygos vein (Figure 3) and the intersection of costal cartilage and internal thoracic vessels. Then, the length of line A between point A and point B was measured and recorded using 3D reconstruction software.
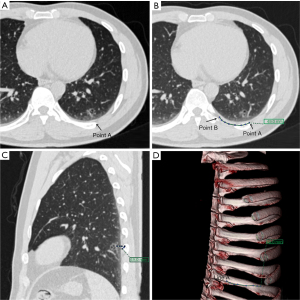
Intraoperative positioning
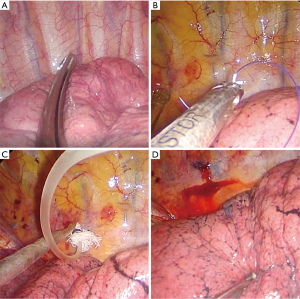
All patients received general anesthesia in the operating room and underwent the double-lumen endotracheal intubation technique as usual, and then they were placed in a 90-degree lateral decubitus position. The surgical side of the chest was prepped and draped in a sterile fashion. A 3 to 4 cm single-hole incision was made between the anterior axillary line and the mid-axillary line of the 4th or 5th intercostal space as the thoracoscopic observation hole and operation hole. When the lung on the surgical side collapsed and the wound protective sleeve was applied, a 30-degree 5 mm thoracoscope was inserted for observation. First, we looked for the relatively fixed anatomical landmarks (point B) and line A mentioned above (Figure 4A). Starting from point B, the distance along line A was measured using a sterile soft ruler to find point A. After point A was identified, a marking device, such as a small cotton ball tied around the head of a small infusion catheter (12G, produced in Shandong, China), was inserted through the surgical port and fixed to marking point A using a 4.0 Prolene thread (Figure 4B) or Kangpai glue (2 mL, produced in Beijing, China). Then, the anesthesiologist was asked to fully inflate the lungs. The airway pressure was maintained using a 40 cm water column. After the lungs were fully re-expanded, the anesthesiologists were asked to maintain this inflated state for several seconds. A 1 mL syringe with 0.05 mL melanin solution was prepared in advance and quickly injected into the small cotton ball at the front through the small infusion catheter (Figure 4C). The melanin solution on the small cotton ball was printed on the pleura of the lung, marking point A, which was the closest point perpendicular to the target lung nodule (Figure 4D). Then, the anesthesiologist was asked to ventilate the contralateral lung, and the lung on the surgical side was collapsed again. After the lung was collapsed for better exposure, we used oval forceps to clamp the pre-resected lung tissue with the melanin mark and then used fingers to feel whether a nodule could be palpated intraoperatively. Then, we removed the lung tissue containing the palpable nodules with a thoracoscopic linear cutting stapler. The specimen was placed in a sterile specimen bag and removed from the thoracic cavity, and then it was cut off at the marked point to identify the pulmonary nodule (Figure 2). The distance from the center of the marked point to the visceral pleura closest to the pulmonary nodule on the specimen was measured and recorded, and a distance less than 20 mm with a negative surgical margin was identified as accurate positioning. Once the pulmonary nodule was confirmed to be removed, the specimen was quickly frozen and sent to the pathology department for pathological examination to evaluate the nature of the pulmonary nodules to determine the next step of the operation. The target nodule localization time includes the whole operation process from the beginning of finding point B to determining point A on the pleura. Each key step of actual operation can be seen in the Video 1.
Statistical analysis
SPSS for Windows version 25.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis of the data obtained from this study. The descriptive statistics included the mean (standard deviation), median [interquartile range (IQR), 25% quantile, 75% quantile], or numbers (percentages) according to the types and distributions of the variables. The normality of the distribution was confirmed with the Kolmogorov-Smirnov test. The data of this study that did not follow a normal distribution are presented as medians (25% quantile and 75% quantile) or numbers (percentages).
Results
A total of 120 patients with 131 pulmonary nodules who underwent video-assisted thoracoscopic surgery at our department were enrolled, of whom 11 patients had two nodules in the lung. A total of 120 patients were included, with 35 males and 85 females, and the median age was 53 years (IQR, 41–63 years). All patients successfully completed the operation without death or serious complications. No mortality or major morbidity occurred within 30 days. The median localization time was 11 minutes (IQR, 8–14 minutes). The accuracy of localization was 98.5%. The median diameter of the pulmonary nodules was 8 mm (IQR, 7–13 mm), and the median distance from the visceral pleura was 6 mm (IQR, 2–10 mm). Among them, nodules were located in the right upper lobe of the lung in 36 cases (27.5%), the right middle lobe in 4 cases (3.1%), the right lower lobe in 26 cases (19.8%), the left upper lobe in 46 cases (35.1%), and the left lower lobe in 19 cases (14.5%). According to the CT images, 62 cases (47.3%) of pure ground glass nodules, 54 cases (41.2%) of mixed ground glass nodules, and 15 cases (11.5%) of solid nodules were found. No positioning-related complications occurred during the operation. No patient required a switch to a thoracotomy during surgery. The distance from the localization point to the pulmonary nodule was measured on the gross specimen. Eighty-nine patients (67.9%) had a distance of 1 to 5 mm, 37 patients (28.2%) had a distance of 6 to 10 mm, and 5 patients (3.8%) had a distance of 11 to 15 mm. The final postoperative pathology results showed that 83.2% of the nodules in this group were adenocarcinomas, including 15 cases (11.5%) of adenocarcinoma in situ (ACIS), 41 cases (31.3%) of microinvasive adenocarcinoma (MIA), and two cases that underwent further segmental pneumonectomy combined with lymph node sampling. Fifty-three patients (40.5%) had microinvasive adenocarcinoma (IA), and 28 patients underwent further lobectomy combined with radical lymphadenectomy. Furthermore, 6 cases (4.6%) of atypical adenomatous hyperplasia (AAH), 12 cases (9.2%) of benign nodules (all nodules were confirmed in the postoperative specimens), one case (0.8%) of metastatic adenocarcinoma of the rectum, 2 cases (1.5%) of inflammatory myofibroblastoma and one case (0.8%) of minute pulmonary meningeal epithelioid nodules were found. The median length of postoperative hospital stay was 5 days (IQR, 3–7 days). All patients completed outpatient follow-up at 1 week and 1 month after discharge. Details are shown in Table 1.
Table 1
| Characteristic | Values |
|---|---|
| Age, years | 53 [41–63] |
| Gender | |
| Female | 85 (70.8) |
| Male | 35 (29.2) |
| Accuracy of pulmonary nodule localization | 129 (98.5) |
| The target nodule localization time, min | 11 [8–14] |
| Diameter of lung nodule, mm | 8 [7–13] |
| Vertical distance from pleura, mm | 6 [2–10] |
| Length of postoperative hospital stay, days | 5 [3–7] |
| Surgical margin involved by the tumour | 0 (0.0) |
| Invasion of pleura | 0 (0.0) |
| Imaging performance | |
| Pure ground glass nodules | 62 (47.3) |
| Mixed ground glass nodules | 54 (41.2) |
| Solid nodule | 15 (11.5) |
| The distance from the localization point to the pulmonary nodule | |
| 0–5 mm | 89 (67.9) |
| 6–10 mm | 37 (28.2) |
| 11–15 mm | 5 (3.8) |
| Pathology | |
| AAH | 6 (4.6) |
| ACIS | 15 (11.5) |
| MIA | 41 (31.3) |
| IA | 53 (40.5) |
| No significant evidence of malignancy | 12 (9.2) |
| Metastatic adenocarcinoma of rectum | 1 (0.8) |
| Inflammatory myofibroblastoma | 2 (1.5) |
| Minute pulmonary meningeal epithelioid nodule | 1 (0.8) |
Data are expressed as the median (25th, 75th percentiles) [interquartile range] or n (%). AAH, atypical adenomatous hyperplasia; ACIS, adenocarcinoma in situ; MIA, microinvasive adenocarcinoma; IA, invasive adenocarcinoma.
Discussion
As technology has continued to advance, the application of CT has become increasingly extensive, and the screening rate of lung lesions, especially pulmonary nodules, has also increased. A pulmonary nodule is defined as a lung nodule whose diameter was ≤3 cm on CT scan. Ground glass nodules, with clear borders, are not accompanied by atelectasis, pleural effusion or mediastinal lymphadenopathy (16). However, CT cannot accurately distinguish benign from malignant pulmonary nodules (17). Percutaneous lung biopsy, bronchoscopic approaches (including robot-assisted) or thoracoscopic wedge resection biopsy is still the main way to diagnose the nature of lung nodules; however, percutaneous lung biopsy is not an all-encompassing method. Studies have found that as the diameter of lung nodules decreases, the effective rate of biopsy gradually decreases. For example, the positive rate of puncture biopsy for lung nodules larger than 1.5 cm was 73.5%, whereas the positive rate of puncture for lung nodules smaller than 1.5 cm was 51.4% (18). Fortunately, after decades of development, thoracoscopic wedge resection surgery has become available for the diagnosis and treatment of unclear pulmonary nodules and shows excellent sensitivity and specificity (19). Moreover, the advantages of a single-hole minimally invasive incision, a short operation time, light pain, and fast recovery also make these patients to accept surgery more readily (20).
Thoracoscopic surgery also faces some challenges, such as ground glass nodules with very low density on CT imaging or pulmonary nodules with a diameter of ≤1 cm or a distance of ≥0.5 cm from the pleura, which cannot be visualized or palpated during the operation, even with instruments or fingertips; thus, these results are inaccurate and unconvincing. According to relevant literature reports, lung nodules cannot be accurately located during the operation, and the probability of thoracotomy is as high as 63% (1), which undoubtedly increases the patient’s surgical risk. Methods for locating pulmonary nodules efficiently and accurately and completely removing pulmonary nodules are key technical questions. Various positioning methods can be used, such as preoperative CT-guided hookwire positioning and coil positioning as well as lipiodol positioning, electromagnetic navigation bronchoscope-guided positioning, and intraoperative ultrasound-assisted positioning. The success rate of these positioning methods is 89.6% to 100% (21-26). However, these methods have their own advantages and disadvantages. For example, preoperative CT-guided hookwire positioning, spring coil positioning, and lipiodol positioning all require a puncture needle to penetrate the chest wall and then enter the chest cavity for positioning, which can result in preoperative CT-guided hookwire positioning-related complications and pneumothorax. The incidence of pneumothorax is as high as 35%, that of intrathoracic hemorrhage is approximately 4% to 27% (27,28), and that of positioning point displacement is approximately 6%. In some severe cases, fatal air embolism or even death may occur. The probability of occurrence rate is approximately 0.061% to 0.07% (29). Electromagnetic navigation bronchoscope-guided positioning and intraoperative ultrasound-assisted positioning require expensive auxiliary equipment and experienced imaging colleagues.
Based on the above considerations, the thoracic surgery team under the leadership of Dr. Pang in our hospital has conducted a long-term investigation and found that the precise 3D positioning method based on anatomical landmarks can both satisfy the need for accurate positioning and significantly reduce the occurrence of complications. This method originally used relatively fixed body landmarks, such as the head of the transverse process of the vertebral body, the rib head, the junction of the horizontal and oblique fissure of the lung, the arch of the azygos vein, the superior vena cava, the internal thoracic artery and vein, the brachio-cephalic trunk, the common carotid artery and the subclavian artery, and the relative positioning of lung nodules on the 3D software SYNAPSE before surgery. The relative distance between the points and the fixed anatomical landmarks of the human body is measured, and then the lung nodules are accurately located during the operation. The chest wall does not need to be penetrated with a puncture needle, unlike with the hookwire positioning method. More importantly, this method can also be used to locate sites that cannot be effectively located by the hookwire positioning method, such as those that are difficult to reach due to the shielding of the scapula or ribs or those that are very close to the major blood vessels of the heart and can easily cause massive bleeding. By retrospectively analyzing the data of this group of cases, we found that intraoperative application of the precise 3D positioning method based on anatomical landmarks has a positioning accuracy of 98.5%, which is comparable with that of hookwire positioning under the guidance of pre-art CT (96%) (6), and no positioning-related complications occurred during and after the operation. All lung nodules were completely removed, and thoracotomy was not needed. At present, the hookwire positioning method commonly used in our medical center has been replaced by the precise 3D positioning method. According to the literature, some centers are trying to perform thoracoscopic wedge resection of pulmonary nodules based on 3D reconstruction pulmonary nodule localization, but the principle of positioning is different. Their method is to use relatively fixed anatomical landmarks on the lung surface after reconstruction. Then, the relative distance from these relatively fixed anatomical landmarks to the projection point of the pulmonary nodule is measured, but the lung is partially collapsed during the positioning process, which will cause displacement and deviation of the positioning (30-32). This feature makes wide adoption of this method in the clinic difficult. Our method uses relatively fixed anatomical landmarks on the chest wall, which has more landmarks than on the lung surface. When the localization of pulmonary nodules is completed, the lungs are still fully inflated, similar to two peas before surgery, which makes the positioning process more accurate, simpler and easier. Generally, the relatively fixed landmark we use for the nodules close to the back of the ribs is the top of the transverse process of the vertebral body, the relatively fixed landmark we often use for the nodules close to the front of the ribs is the internal thoracic vessels, and the relatively fixed landmark we use for the nodules close to the mediastinum is the great vessels of the heart. The relatively fixed landmark we often use for nodules close to the diaphragm is the different partition position of the diaphragm.
According to our experience in thoracic surgery, accurate 3D positioning based on anatomical landmarks has the following irreplaceable advantages: (I) this method can be performed intraoperatively, is simple to operate, and has a short positioning time; the median target nodule localization time was 11 minutes (IQR, 8–14 minutes). It is performed under direct visualization throughout the entire process, avoiding injury to intercostal blood vessels, pulmonary blood vessels, cardiac blood vessels, nerves and other important structures. Therefore, this method stands out from and is better than other methods, which may cause pneumothorax, bleeding, positioning needle fall off, and air embolism. According to our statistics, the incidence of complications in this group of cases was 0%. (II) No invasive puncture of the pulmonary nodules or visceral pleura occurred during localization; thus, needle puncture-related metastasis or pleural dissemination was not possible. (III) One-step positioning: patients do not need to travel between the CT room and operating room and do not need to be exposed to additional radiation. At the same time, this reduces the workload of radiologists and ultrasound doctors and simplifies the overall process. (IV) As this positioning method is performed after general anesthesia during the operation, the patient will not feel discomfort from the needle puncture, unlike with the hookwire puncture positioning, which is guided by CT before the operation and elicits anxiety and discomfort in patients. Thus, this method can reduce the patient’s psychological stress and improve patient satisfaction. (V) Finally, it has a wide range of applications. This method is also applicable for locations such as the ribs, scapula, close to the heart and great blood vessels, close to the diaphragm, and close to the breast in women. At the same time, it is cost effective and does not require additional high-value consumables, which is in line with the concept of “green surgery” advocated by the country.
Of course, this method also has some limitations. In patients with severe pleural adhesions, due to the intraoperative separation of pleural adhesions, the anatomical landmarks on the chest wall surface are affected by blood and become blurred, which can affect clinical judgment; in some patients, the visceral pleural surface has obvious signs. Pigmentation is easily confused with melanin deposits. Positioning failed in two of the patients in this group because massive adhesions were found in the thoracic cavity during the operation. After the adhesions were separated, the local anatomy became difficult to clearly identify, which led to deviations in positioning. In these two patients, the first case was a 70-year-old female patient with an 8 mm nodule in the left lower lobe and 13 mm from the pleura. No nodules were found in the first wedge resection. After the second extended wedge resection, the target nodule was found 2 cm away from the cutting margin. The frozen pathological results showed IA. The second case was a 39-year-old female patient with 7 mm pure ground glass nodules in the right upper lobe and 13 mm from the pleura. No nodules were found in the first wedge resection. After the second extended wedge resection, the target nodule was found 1.5 cm away from the cutting margin. The frozen pathological result showed suspected ACIS. After informing the patient’s family of the frozen pathological result, the family members of the two patients decided that further lobectomy or segmentectomy was not needed. In view of the results described for the aforementioned patients, our positioning method has clearly improved over time. In the aforementioned situation, we found that we could change to Kangpai glue for positioning to prevent this situation from recurring. In addition, another disadvantage of this localization method compared with ultrasound or radiotracers is that sometimes whether the nodule is included in the specimen or whether to evaluate the resection margins intraoperatively cannot be determined (14,33).
Limitations
This study has some limitations that should be noted. First, this study was retrospective in nature with a relatively small sample size and lacked a comparison group. Second, this was a single-center retrospective study, and further randomized controlled trials are needed in the future.
Conclusions
The precise 3D positioning method based on anatomical landmarks is accurate, safe and feasible for selected patients with pulmonary nodules. Compared with other preoperative and intraoperative positioning methods, it can significantly reduce localization-related complications.
Acknowledgments
We would like to thank Thomas Barbour for his help in polishing our paper. This abstract was submitted to the IASLC 2021 World Conference on Lung Cancer | Worldwide Virtual Event (WCLC 2021) held on September 8–14th, 2021, and was accepted as a poster presentation on June 2nd, 2021 (abstract ID: #872).
Funding: This study was financially supported by the Sanming Project of Medicine in Shenzhen “Guangzhou Institute of Respiratory Health, Professor He Jianxing Thoracic Tumor and Surgery Group” (No. SZSM202011002) and Shenzhen Science and Technology Innovation Commission (grant No. KQJSCX20170331104516).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-203/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-203/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-203/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-203/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was conducted with the formal and written approval of the Institutional Ethics Committee of the University of Hong Kong-Shenzhen Hospital (No. hkuszh2020198). Informed consent was signed by all individual participants included in this study before the surgery.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Suzuki K, Nagai K, Yoshida J, et al. Video-assisted thoracoscopic surgery for small indeterminate pulmonary nodules: indications for preoperative marking. Chest 1999;115:563-8. [Crossref] [PubMed]
- Wang T, Ma S, Yan T, et al. Computed Tomography Guided Hook-wire Precise Localization and Minimally Invasive Resection of Pulmonary Nodules. Zhongguo Fei Ai Za Zhi 2015;18:680-5. [PubMed]
- Hanauer M, Perentes JY, Krueger T, et al. Pre-operative localization of solitary pulmonary nodules with computed tomography-guided hook wire: report of 181 patients. J Cardiothorac Surg 2016;11:5. [Crossref] [PubMed]
- Han X, Li Z, Gu H, et al. Preoperative computed tomographic-guided percutaneous coil localization of pulmonary nodules: 184 Cases in Single-center. J Cancer Res Ther 2021;17:671-5. [Crossref] [PubMed]
- Liu X, Cao W, Xu QS. Computed tomography-guided coil localization for scapula-blocked pulmonary nodules: A trans-scapular approach. Medicine (Baltimore) 2021;100:e24333. [Crossref] [PubMed]
- Park CH, Han K, Hur J, et al. Comparative Effectiveness and Safety of Preoperative Lung Localization for Pulmonary Nodules: A Systematic Review and Meta-analysis. Chest 2017;151:316-28. [Crossref] [PubMed]
- Park CH, Lee SM, Lee JW, et al. Hook-wire localization versus lipiodol localization for patients with pulmonary lesions having ground-glass opacity. J Thorac Cardiovasc Surg 2020;159:1571-9.e2. [Crossref] [PubMed]
- Ricciardi S, Davini F, Manca G, et al. Radioguided Surgery, a Cost-Effective Strategy for Treating Solitary Pulmonary Nodules: 20-Year Experience of a Single Center. Clin Lung Cancer 2020;21:e417-22. [Crossref] [PubMed]
- Dailey WA, Frey GT, McKinney JM, et al. Percutaneous Computed Tomography-Guided Radiotracer-Assisted Localization of Difficult Pulmonary Nodules in Uniportal Video-Assisted Thoracic Surgery. J Laparoendosc Adv Surg Tech A 2018;28:1451-7. [Crossref] [PubMed]
- Starnes SL, Wolujewicz M, Guitron J, et al. Radiotracer localization of nonpalpable pulmonary nodules: A single-center experience. J Thorac Cardiovasc Surg 2018;156:1986-92. [Crossref] [PubMed]
- Zhang J, He J, Chen J, et al. Application of indocyanine green injection guided by electromagnetic navigation bronchoscopy in localization of pulmonary nodules. Transl Lung Cancer Res 2021;10:4414-22. [Crossref] [PubMed]
- Shi J, He J, He J, et al. Electromagnetic navigation-guided preoperative localization: the learning curve analysis. J Thorac Dis 2021;13:4339-48. [Crossref] [PubMed]
- Jeong JH, Park H, Choi CM, et al. Preoperative electromagnetic navigation bronchoscopy-guided one-stage multiple-dye localization for resection of subsolid nodules: A single-center pilot study. Thorac Cancer 2022;13:466-73. [Crossref] [PubMed]
- Kondo R, Yoshida K, Hamanaka K, et al. Intraoperative ultrasonographic localization of pulmonary ground-glass opacities. J Thorac Cardiovasc Surg 2009;138:837-42. [Crossref] [PubMed]
- Londero F, Castriotta L, Grossi W, et al. VATS-US1: Thoracoscopic ultrasonography for the identification of nodules during lung metastasectomy. Future Oncol 2020;16:85-9. [Crossref] [PubMed]
- Fraser RG, Sanders C, Barnes GT, et al. Digital imaging of the chest. Radiology 1989;171:297-307. [Crossref] [PubMed]
- Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e93S-120S.
- Kothary N, Lock L, Sze DY, et al. Computed tomography-guided percutaneous needle biopsy of pulmonary nodules: impact of nodule size on diagnostic accuracy. Clin Lung Cancer 2009;10:360-3. [Crossref] [PubMed]
- Hirai S, Hamanaka Y, Mitsui N, et al. Role of video-assisted thoracic surgery for the diagnosis of indeterminate pulmonary nodule. Ann Thorac Cardiovasc Surg 2006;12:388-92. [PubMed]
- Dai F, Meng S, Mei L, et al. Single-port video-assisted thoracic surgery in the treatment of non-small cell lung cancer: a propensity-matched comparative analysis. J Thorac Dis 2016;8:2872-8. [Crossref] [PubMed]
- Suzuki K, Shimohira M, Hashizume T, et al. Usefulness of CT-guided hookwire marking before video-assisted thoracoscopic surgery for small pulmonary lesions. J Med Imaging Radiat Oncol 2014;58:657-62. [Crossref] [PubMed]
- Gruber-Rouh T, Naguib NNN, Beeres M, et al. CT-guided hook-wire ocalization prior to video-assisted thoracoscopic surgery of pulmonary lesions. Clin Radiol 2017;72:898.e7-898.e11. [Crossref] [PubMed]
- Huang HZ, Wang GZ, Xu LC, et al. CT-guided Hookwire localization before video-assisted thoracoscopic surgery for solitary ground-glass opacity dominant pulmonary nodules: radiologic-pathologic analysis. Oncotarget 2017;8:108118-29. [Crossref] [PubMed]
- Tian Y, Wang C, Yue W, et al. Comparison of computed tomographic imaging-guided hook wire localization and electromagnetic navigation bronchoscope localization in the resection of pulmonary nodules: a retrospective cohort study. Sci Rep 2020;10:21459. [Crossref] [PubMed]
- Tang X, Jian HM, Guan Y, et al. Computed tomography-guided localization for multiple pulmonary nodules: a meta-analysis. Wideochir Inne Tech Maloinwazyjne 2021;16:641-7. [Crossref] [PubMed]
- Yan G, Cheng X, Wu S, et al. Clinical value and application of preoperative CT-guided hookwire localization of solitary pulmonary nodules for video-assisted thoracic surgery. Technol Health Care 2022;30:459-67. [Crossref] [PubMed]
- Yeow KM, Su IH, Pan KT, et al. Risk factors of pneumothorax and bleeding: multivariate analysis of 660 CT-guided coaxial cutting needle lung biopsies. Chest 2004;126:748-54. [Crossref] [PubMed]
- Khan MF, Straub R, Moghaddam SR, et al. Variables affecting the risk of pneumothorax and intrapulmonal hemorrhage in CT-guided transthoracic biopsy. Eur Radiol 2008;18:1356-63. [Crossref] [PubMed]
- Tomiyama N, Yasuhara Y, Nakajima Y, et al. CT-guided needle biopsy of lung lesions: a survey of severe complication based on 9783 biopsies in Japan. Eur J Radiol 2006;59:60-4. [Crossref] [PubMed]
- Zhang G, Xu D, Yu Z, et al. Preoperative non-invasive visual localization of synchronous multiple lung cancers using three-dimensional computed tomography lung reconstruction. J Cardiothorac Surg 2021;16:273. [Crossref] [PubMed]
- Zhao L, Yang W, Hong R, et al. Application of three-dimensional reconstruction combined with dial positioning in small pulmonary nodules surgery. J Cardiothorac Surg 2021;16:254. [Crossref] [PubMed]
- Zhao X, Lu H, Zhang Z. Preliminary Study of CT Three-dimensional Reconstruction Combined with Ground Glass Nodules of Natural Lung Collapse in Thoracoscopic Pulmonary Segmental Resection. Zhongguo Fei Ai Za Zhi 2021;24:683-9. [PubMed]
- Vollmer I, Sánchez-Izquierdo N, Martínez D, et al. Role of a portable gamma-camera with optical view for margins assessment of pulmonary nodules resected by radioguided surgery. Eur J Nucl Med Mol Imaging 2021;49:361-70. [Crossref] [PubMed]


