
Improving outcomes in malignant pleural mesothelioma in an integrated health care system
Introduction
Malignant pleural mesothelioma (MPM) is a rare cancer that is linked to asbestos exposure and has a long latency period of up to 40 years (1,2). MPM is associated with a median survival of 4–12 months without treatment (3). There are three main histological subtypes of MPM: epithelioid, sarcomatoid, and biphasic, which determine prognosis.
There is no consensus for optimal treatment of MPM due to limited number of randomized controlled trials given the rarity of this disease. National Comprehensive Cancer Network (NCCN) guidelines recommend patients with MPM should be managed by a skilled multidisciplinary team with experience in MPM management (4). Multidisciplinary review and regionalization, the shifting of patient care to designated centers within a certain region, improves outcomes in patients with non-small cell lung cancer (5-7). Little is known about regionalization of MPM surgeries to a specialized surgeon.
In 2014, Kaiser Permanente Northern California (KPNC), an integrated health care system, regionalized surgical care for MPM to increase specialization and standardization of surgery. In 2017, the authors introduced a regional bi-disciplinary tumor board to increase specialization and standardization of diagnosis and systemic therapy. The aim of this study was to investigate whether patients with MPM received more treatment after regionalizing MPM surgeries and institution of a MPM bi-disciplinary review. In surgical patients, we evaluated complication rates before and after regionalization. We hypothesized that eligible patients with MPM would receive increased treatment in the later cohort. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-427/rc).
Methods
Through this cohort study, electronic medical records of 368 adult patients with histologically confirmed MPM from 1/1/2009 to 12/31/2020 were retrospectively reviewed at KPNC. The number of patients included was determined by the number of new diagnoses of MPM during the study period. We compared patient demographics, clinical characteristics, and outcomes between two periods: 2009–2014 and 2015–2020. The reasons for choosing these two periods are regionalization of MPM surgeries to one surgeon at one surgical center starting in 2014 and initiation of a weekly bi-disciplinary team review starting in 2017 at KPNC. Prior to 2014, mesothelioma surgeries were performed at two sites by two surgeons with a low volume of annual mesothelioma cases. Surgical selection criteria were similar in the two cohorts. Select patients with bi-phasic histology underwent surgery, in addition to patients with epithelioid histology. Sample size limited an effective comparison among three periods. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional review board of KPNC (No. 00001045) and individual consent for this retrospective analysis was waived.
The bi-disciplinary team consisted of medical oncology and thoracic surgery representatives. Representatives from pathology and radiology were consulted by the tumor board as needed. All mesothelioma cases were obtained through our institution’s internal data management division. Cases were ascertained starting in 2017, using natural language processing to develop an algorithm that mines free-text pathology reports to identify new cases of mesothelioma on a weekly basis. The pathology reports were subsequently verified, and the cases were reviewed during the weekly bi-disciplinary tumor board. The mesothelioma team worked with the programmers to optimize the algorithm over the first 6 months. Eligibility criteria include current KPNC membership, at least 18 years of age at diagnosis, and recent diagnosis of MPM. During the bi-disciplinary virtual review, clinical stage was confirmed based on CT scan with subsequent PET/CT scan performed as needed for surgical consideration. Pathology was reviewed with clear documentation on specific histology (epithelioid, biphasic, or sarcomatoid) on the pathologic report, and referrals were made to the designated mesothelioma surgeon, medical oncology and/or radiation oncology when appropriate, based on NCCN guidelines. Patient’s undergoing surgery were intended to receive adjuvant systemic therapy, while radiation was not commonly administered as part of adjuvant treatment. We chart reviewed to confirm MPM stage and histology. Twelve patients had missing MPM stage due to incomplete staging imaging. Patients with missing stage were excluded from the final analyses.
For surgical patients, we compared their characteristics by early and later periods. More extrapleural pneumonectomies (EPP) were performed during the early period, and more pleurectomy/decortications (PD) were performed in the later period due to changes in practice at our institution, related to influences from the MARS trial results (8). EPP was performed in the standard fashion with exploratory thoracotomy, complete EPP, resection of the pericardium with reconstruction with Gore-Tex mesh, resection of the diaphragm with reconstruction with Gore-Tex mesh, and complete mediastinal lymph node dissection. PD consisted of exploratory thoracotomy, extended radical parietal and visceral pleurectomy, resection of the pericardium with reconstruction with bovine pericardium mesh, complete resection of the diaphragm with reconstruction using porcine-derived acellular dermal matrix (STRATTICE Reconstructive Tissue Matrix) mesh, and complete mediastinal lymph node dissection. At the time of cytoreductive surgery, betadine scrub was used as an adjunctive treatment. Patients are discharged home after surgery with Blake drains to proactively prevent prolonged effusion or complications arising from air leak. Both, EPP and PD operations performed in both study periods consisted of planned gross complete macroscopic resection (R1). Operations involving extensive tumor invasion or partial resections (R2) were included in this study and went on to receive systemic therapy. Post-operative complications within 30 days of EPP or PD were defined as anemia, atrial fibrillation/atrial flutter, chyle leak, prolonged supplemental oxygen, hypotension, mucous plug, pneumonia, pericardial effusion, remained intubated post-operatively, prolonged initial intubation (>48 hours), stroke, acute respiratory failure, cardiac arrest, empyema, venous thromboembolism, urinary retention, urinary tract infection, wound infection, reintubation, pleural effusion, tracheostomy, bronchopleural fistula, and re-operation. Post-operative complications were reviewed and confirmed by manual review of electronic medical records.
Statistical analysis
We conducted a multivariable logistic regression to examine the associations with receiving any treatment and period with adjustments for age, gender, histology, stage, and Charlson comorbidity index (CCI) at time of cancer diagnosis (9,10). We used the Kaplan-Meier method and log-rank test to compare survival rates by period, treatment type, and stage. The endpoint was death from all causes. Patients were followed from cancer diagnosis date until they died or end of study follow-up (May 31, 2021), whichever occurred first. Ten patients who left our institution prior to the study end point were censored from the study. We also conducted Cox proportional hazards regression model to examine the associations between overall survival (OS) and period with adjustments for any treatment, age, gender, histology, stage, and CCI at cancer diagnosis. Comparisons of patient characteristics were assessed in bivariate analyses using Chi-square or Fisher exact tests for categorical variables and the Student t-test for continuous variables. All analyses were performed using SAS v.9.4 (Cary, NC, USA).
Results
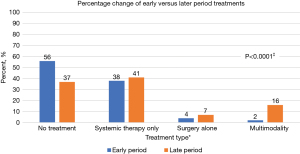
Demographic data showed similar patient characteristics from the early to later periods for the included 368 patients with MPM except for histology and CCI, which was higher in the later period cohort (Table 1). We note the difference in CCIs may have been affected by the ICD coding switch from ICD-9 to ICD-10 in September 2015, with the ICD-10 using additional codes. There was no missing data for variables, other than stage. Despite similar age, staging and inclusion of patients with greater CCIs, more patients received any MPM directed treatment in the later period from 2015–2020 (n=124, 63.0%) compared with those patients in the early period from 2009–2014 (n=75, 43.9%, P<0.0001, Table 1). When evaluating patients with stage I-II epithelioid MPM, we found a 39% increase in any treatment offered to these eligible patients from the early to later periods. We did not see an increase in administration of systemic therapy with or without radiation from the early period to the later period. Thirty eight percent of patients received systemic therapy +/− radiation in early period compared to 41% in the later period (Figure 1). A significant difference was noted in surgical management with 6% of patients receiving surgery in the early period, whereas 22% of patients received surgery during the later period, P<0.0001. Specifically, there was an increase in patients who received PD from 2015–2020 (n=42, 21.3%) compared to those who received PD from 2009–2014 (n=6, 3.5%) (P<0.0001) (Table 1). For appropriate surgical candidates, PD was the most utilized surgery in the later period, due to institutional change in practice, given lower mortality with PD compared with EPP (1).
Table 1
| Variables | 2009–2014 (N=171) | 2015–2020 (N=197) | P value |
|---|---|---|---|
| Age at cancer diagnosis, years | 0.649† | ||
| Mean ± SD | 74.7±9.7 | 75.2±10.2 | |
| Min–Max | 45.0–95.0 | 43.0–98.0 | |
| Median (IQR) | 75.0 (69.0-82.0) | 76.0 (70.0–81.0) | |
| Charlson comorbidity index | <0.0001† | ||
| Mean ± SD | 3.5±2.9 | 4.9±3.2 | |
| Median (IQR) | 3.0 (2.0–6.0) | 4.0 (3.0–7.0) | |
| Charlson comorbidity index, No. (%) | <0.0001‡ | ||
| 0–3 | 101 (59.1) | 78 (39.6) | |
| 4–6 | 43 (25.1) | 57 (28.9) | |
| 7+ | 27 (15.8) | 62 (31.5) | |
| Gender, No. (%) | 0.863‡ | ||
| Male | 128 (74.9) | 149 (75.6) | |
| Female | 43 (25.1) | 48 (24.4) | |
| Race/ethnicity, No. (%) | 0.435‡ | ||
| White | 126 (73.7) | 134 (68.0) | |
| African-American | 6 (3.5) | 13 (6.6) | |
| Hispanic non-Black | 18 (10.5) | 28 (14.2) | |
| Asian/Pacific islander | 9 (5.3) | 12 (6.1) | |
| Native American/multiracial/other/unknown | 12 (7.0) | 10 (5.1) | |
| Language, No. (%) | 0.860‡ | ||
| English | 163 (95.3) | 187 (94.9) | |
| Non-English | 8 (4.7) | 10 (5.1) | |
| Stage (AJCC 8th Edition), No. (%) | 0.090‡ | ||
| I | 53 (31.0) | 41 (20.8) | |
| II | 29 (17.0) | 37 (18.8) | |
| III | 41 (24.0) | 61 (31.0) | |
| IV | 40 (23.4) | 54 (27.4) | |
| Unavailable | 8 (4.7) | 4 (2.0) | |
| Histology, No. (%) | <0.0001‡ | ||
| Epithelioid | 72 (42.1) | 114 (57.9) | |
| Biphasic | 17 (9.9) | 27 (13.7) | |
| Sarcomatoid | 18 (10.5) | 30 (15.2) | |
| Other | 64 (37.4) | 26 (13.2) | |
| Smoking status, No. (%) | 0.951‡ | ||
| Current | 6 (3.5) | 8 (4.1) | |
| Former | 93 (54.4) | 105 (53.3) | |
| Never | 72 (42.1) | 84 (42.6) | |
| BMI, No. (%) | 0.638‡ | ||
| <25 | 76 (44.4) | 79 (40.1) | |
| 25.0–29.9 | 65 (38.0) | 84 (42.6) | |
| ≥30 | 30 (17.5) | 34 (17.3) | |
| Any treatment, No. (%) | <0.0001‡ | ||
| No | 96 (56.1) | 73 (37.0) | |
| Yes | 75 (43.9) | 124 (63.0) | |
| Surgery, No. (%) | <0.0001‡ | ||
| No surgery | 161 (94.2) | 153 (77.7) | |
| EPP | 4 (2.3) | 2 (1.0) | |
| PD | 6 (3.5) | 42 (21.3) |
†, two-sample t-test; ‡, Chi-square test. ICD coding switched from ICD-9 to ICD-10 in September 2015, with the ICD-10 using additional codes. This affects comparison of the Charlson comorbidity index. MPM, malignant pleural mesothelioma; SD, standard deviation; IQR, interquartile range; EPP, extrapleural pneumonectomies; PD, pleurectomy/decortications.
Ten MPM surgeries were performed in the early period, compared with 44 MPM surgeries performed in the later period. The CCIs and Eastern Cooperative Oncology Group (ECOG) Performance Status Scale (11) were similar in both the early and later surgical cohorts. CCI was 7 or greater in 29.5% of patients who received surgery during the later period and in 20.0% of patients who received surgery during the early period. Length of stay, emergency department visit or readmission rate within 30 days due to surgical complication, and 30- and 90-day mortality were similar in both early and later cohorts (Table 2). Seven patients (70.0%) in the early period and 26 patients (59.1%) in the later period were found to have any complication, P=0.723. Major complications were defined as acute respiratory failure, cardiac arrest, and death within 30 days. No patients in the early period had major complications, while 5 patients (11.4%, P=0.571) in the later period had major complications (Figure S1). All patients who underwent PD developed prolonged air leak, air leak lasting for greater than 5 days, that was managed with a Blake drain.
Table 2
| Variables | 2009–2014 (N=10) | 2015–2020 (N=44) | P value |
|---|---|---|---|
| Age at cancer diagnosis, years | 0.145† | ||
| Mean ± SD | 68.6±4.2 | 69.5±9.2 | |
| Min–Max | 60.0–74.0 | 43.0–84.0 | |
| Median (IQR) | 69.5 (67.0–71.0) | 71.0 (65.5–76.0) | |
| Charlson comorbidity index, No. (%) | 0.473‡ | ||
| 0–3 | 7 (70.0) | 20 (45.5) | |
| 4–6 | 1 (10.0) | 11 (25.0) | |
| 7+ | 2 (20.0) | 13 (29.5) | |
| ECOG, No. (%) | 0.114‡ | ||
| 0 | 2 (20.0) | 23 (52.3) | |
| 1 | 4 (40.0) | 14 (31.8) | |
| 2 | 1 (10.0) | 3 (6.8) | |
| Not available | 3 (30.0) | 4 (9.1) | |
| Preoperative testing | |||
| Echocardiogram LVEF <50%, No. (%) | 0 | 1 (2.3) | 0.442‡ |
| Pulmonary hypertension, No. (%) | 1 (10.0) | 1 (2.3) | 0.339‡ |
| FEV1 (mean ± SD) | 64.2±16.6 | 73.7±17.5 | 0.170† |
| DLCO (mean ± SD) | 90.2±33.6 | 77.8±21.1 | 0.531† |
| Length of stay | 0.395† | ||
| Mean ± SD | 9.4±3.9 | 11.0±6.7 | |
| Min–Max | 5.0–18.0 | 4.0–40.0 | |
| Median (IQR) | 8.0 (7.0–10.0) | 9.5 (7.5–12.0) | |
| Number of ED visits within 30 days due to surgical complication, No. (%) | 0.408‡ | ||
| No | 7 (70.0) | 36 (81.8) | |
| Yes | 3 (30.0) | 8 (18.2) | |
| Number of hospital readmissions within 30 days due to surgical complication, No. (%) | 1.000‡ | ||
| No | 9 (90.0) | 39 (88.6) | |
| Yes | 1 (10.0) | 5 (11.4) | |
| Complication* within 30 days, No. (%) | 0.723‡ | ||
| No | 3 (30.0) | 18 (40.9) | |
| Yes | 7 (70.0) | 26 (59.1) | |
| 30-day mortality, No. (%) | 1.000‡ | ||
| Alive at least 30 days after surgery | 10 (100) | 41 (93.2) | |
| Died within 30 days after surgery | 0 | 3 (6.8) | |
| 90-day mortality, No. (%) | 1.000‡ | ||
| Alive at least 90 days after surgery | 9 (90.0) | 37 (84.1) | |
| Died within 90 days after surgery | 1 (10.0) | 7 (15.9) | |
†, two-sample Wilcoxon rank-sum test; ‡, Fisher’s exact test; *, post-operative complications were defined as anemia, chyle leak, hypotension, pleural effusion, wound infection, pneumonia, empyema, pulmonary embolus/deep vein thrombosis, pericardial effusion, atrial fibrillation, prolonged air leak, bronchopleural fistula, prolonged intubation, reintubation, tracheostomy, prolonged supplemental oxygen, mucous plug, atelectasis, urinary retention, UTI, stroke, acute respiratory failure, cardiac arrest. ECOG, Eastern Cooperative Oncology Group; LVEF, left ventricular ejection fraction; FEV1, forced expiratory volume in first second; DLCO, diffusion capacity for carbon monoxide; SD, standard deviation; IQR, interquartile range; ED, emergency department; UTI, urinary tract infection.
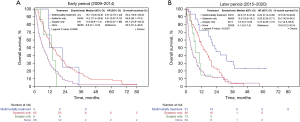
Median survival in patients who received multi-modality treatment (surgery, systemic therapy, +/− radiation) during 2009–2014 was 16.7 months [95% confidence interval (CI), 10.8–34.8 months] compared to 6.9 months (95% CI, 5.1–10.0 months) in patients who received no treatment (Figure 2A). During 2015–2020, median survival for patients who received multi-modality therapy was 22.6 months (95% CI, 17.6–37.8 months) compared to median survival of 4.1 months (95% CI, 3.0–7.5 months) in those who received no treatment (Figure 2B).
Patients in the later period cohort were three times more likely to receive treatment than patients in the early period cohort (adjusted odds ratio 3.25, 95% CI, 1.93–5.49, P<0.0001) after adjusting for age, gender, CCI, histology, and stage at cancer diagnosis. Patients with epithelioid histology were twice as likely to receive treatment compared to patients with other histologies (adjusted odds ratio 1.78, 95% CI, 1.08–2.93, P=0.023). Patients greater than 75 years old and with CCI ≥7 were less likely to receive any treatment (Table 3).
Table 3
| Variables | Adjusted odds ratio (95% CI) | P value |
|---|---|---|
| Study period | ||
| 2015–2020 | 3.25 (1.93–5.49) | <0.0001 |
| 2009–2014 | Reference | |
| Age at cancer diagnosis, years | ||
| 18–64 | Reference | |
| 65–74 | 1.29 (0.58–2.86) | 0.533 |
| 75–84 | 0.32 (0.15–0.65) | 0.002 |
| ≥85 | 0.10 (0.04–0.25) | <0.0001 |
| Male vs. female | 1.77 (1.00–3.14) | 0.048 |
| CCI | ||
| 0–3 | Reference | |
| 4–6 | 0.54 (0.30–0.99) | 0.046 |
| 7+ | 0.38 (0.20–0.70) | 0.002 |
| Histology | ||
| Epithelioid | 1.78 (1.08–2.93) | 0.023 |
| Biphasic, sarcomatoid, other | Reference | |
| Stage | ||
| I/II | 1.04 (0.63–1.70) | 0.888 |
| III/IV | Reference |
CI, confidence interval; CCI, charlson comorbidity index.
In the overall population, factors associated with improved survival were receipt of any treatment compared with no treatment (adjusted hazard ratio 0.57, 95% CI, 0.44–0.73, P<0.0001), early stage compared with late stage (adjusted hazard ratio 0.62, 95% CI, 0.49–0.78, P<0.0001), and epithelioid histology compared with other histologies (adjusted hazard ratio 0.71, 95% CI, 0.56–0.89, P<0.001). Cox proportional hazards model revealed age older than 75, histology other than epithelioid, and later stage was associated with greater risk of death (Table 4).
Table 4
| Variables | Adjusted hazard ratio (95% CI) | P value |
|---|---|---|
| Study period | ||
| 2015–2020 | 0.99 (0.77–1.26) | 0.916 |
| 2009–2014 | Reference | |
| Any treatment vs. none | 0.57 (0.44–0.73) | <0.0001 |
| Age at cancer diagnosis, years | ||
| 18–64 | Reference | |
| 65–74 | 1.21 (0.84–1.74) | 0.308 |
| 75–84 | 1.86 (1.30–2.67) | <0.001 |
| ≥85 | 2.33 (1.51–3.59) | <0.001 |
| Male vs. female | 1.02 (0.78–1.33) | 0.893 |
| CCI | ||
| 0–3 | Reference | |
| 4–6 | 0.86 (0.65–1.14) | 0.293 |
| 7+ | 1.27 (0.94–1.71) | 0.122 |
| Histology | ||
| Epithelioid | 0.71 (0.56–0.89) | <0.001 |
| Biphasic, sarcomatoid, other | Reference | |
| Stage | ||
| I/II | 0.62 (0.49–0.78) | <0.0001 |
| III/IV | Reference |
CI, confidence interval; CCI, charlson comorbidity index.
Discussion
Regionalization of MPM surgeries and bi-disciplinary review can lead to increased utilization of surgery as part of multimodal therapy in an older population with multiple comorbidities within a large integrated health care system without higher rates of surgical complications. We observed a correlation between patients who received tri- or bi-modality MPM directed therapies in the later period and improved survival. Our findings are consistent with other studies suggesting a relationship between treatment and improved survival (12).
Regionalization of surgical practice has been associated with improved outcomes for patients with non-small cell lung cancer and other thoracic malignancies (5,13). An Australian study of patients with MPM showed that improved survival was associated with greater experience of the surgeon (>100 cases) (14), but further evaluation of centralization of MPM surgeries has yet to be determined. We were able to increase our overall number of MPM surgeries performed by a dedicated, specialized surgical team over time, performing at a rate similar to that demonstrated through review of the SEER database and a retrospective review at Memorial Sloan-Kettering Cancer Center (3,15,16). With increased use of surgery, we did not find a significant increase in length of stay, nor rates of postoperative complications. Prolonged air leak was not considered a major complication as all of our PD patients before and after regionalization had prolonged air leak, thus we considered this more of a normal side effect from PD. There was a non-significant increase in 30-day surgical mortality from 0% prior to regionalization to 6.8% after regionalization, which was likely due to the limited number of surgeries performed during the early period and the higher CCI observed in patients receiving multimodality surgical treatment in the later period. Through surgical regionalization, eligible patients have increased access to specialized surgical care, which may have contributed to the improvement in survival seen in this observational study.
Historically in community practices, rates of surgical interventions for patients with MPM are low compared with tertiary care centers (15). With an experienced surgical team, we were able to increase rates of cytoreductive surgery in a community setting. In the early cohort, we found that over half of patients received no treatment, despite similar age and higher CCI in the later cohort. We suspect that medical oncologist did not commonly recommend chemotherapy over supportive care due to a perception of low benefit of chemotherapy in our population. This is a practice pattern reflective of a real-world community setting and has been demonstrated in other institutions (17). Our study showed successful implementation and increased use of multimodal therapy within a patient population that is representative of the greater population of California (18).
One of the main highlights of our regionalization program, was that significantly more eligible patients for multimodality treatment did indeed undergo optimal treatment compared to pre-regionalization. With experienced surgeons and a bi-disciplinary team, increased guideline based, multimodality treatment can be offered to appropriate patients. From the early to the later period, we saw an increase in use of any treatment from 44% to 65%, respectively, despite higher CCIs in the later period cohort. There was a significant increase in the number of surgeries performed from 6% to 22% after surgeries were performed by a specialized surgical team. We suspect that the establishment of bi-disciplinary review also contributed to the increase in number of surgeries performed over time as more surgeries were offered appropriately to eligible patients. Due to institutional preference to perform PD instead of EPP, the number of PDs increased from the early to later periods. In an NCI study, 71% of patients received any treatment with 10% of patients receiving surgery, systemic therapy, and radiation, and 9% receiving surgery and systemic therapy. Patients who received surgery and systemic therapy had a median OS of 25 months (12), while patients in our population who received tri- or bimodality therapies had a similar median OS of 23 months in the later period.
Bi-disciplinary review has been demonstrated to improve outcomes in non-small cell lung cancer (19,20). In MPM, bi-disciplinary review can improve histologic diagnosis, increase accurate staging of MPM, assure evidence-based treatment recommendations, and increase enrollment in clinical trials, but improvement in outcomes have yet to be reported (21). As a result of the MPM bi-disciplinary review, we found a significant difference in histological subtype between the early and later period cohorts. The tumor board interacted directly with pathologists and radiologists to determine each patient’s histological subtype and staging, leading to a more accurate diagnosis, prognosis, and appropriate treatment plan. We note that there may have been a nondifferential misclassification present in histologic subtypes in the early period due to non-specific pathological diagnoses. There were no changes in patient demographics over time that contributed to change in histological subtype. For 12 patients, imaging for staging was not obtained due to patient preference to proceed with supportive care. By accurately determining each patient’s histology, we were able to increase standardized, guideline-based care, recognize more patients who were eligible for multimodality therapies, and subsequently increase the number of treatment types received in the later period cohort. Although this study was not a prospective evaluation to determine if our tumor board improved outcomes, we did see that bi-disciplinary review of patients with MPM was associated with increased utilization of multimodality treatments.
Because of the rarity of MPM and the difficulty of conducting RCTs, questions remain about the role of surgery in MPM management (22). The MARS trial was a limited, randomized controlled trial that showed no benefit of EPP in MPM (8). Results from the MARS2 trial comparing PD and chemotherapy compared with chemotherapy alone are awaited (23). Retrospective review of the International Association for the Study of Lung Cancer mesothelioma database and other retrospective reviews showed multimodality therapy, surgery plus chemotherapy and/or radiation, compared to surgery alone improved survival (24-27). After regionalization, patients in our population who received multimodal therapies had median OS of 22.6 months (95% CI, 17.6–37.8 months). While we obtained similar median survival after multimodal treatment reported by published studies, we did not observe an improvement in OS in all patients.
Limitations of our study include its observational nature and an overall small sample size, especially for certain subsets of the total population. Our small sample size limited our ability to compare cohorts among three periods: before surgical regionalization, after surgical regionalization, and after institution of a bi-disciplinary patient review. We also were not able to account for unmeasured confounding factors including changes in surgical technique and changes in systemic therapies over time. However, despite our data review over an eleven-year period, we did not see appreciable differences in survival when comparing the overall population by period (data not shown), showing that despite improvements in other factors that may have changed over time, mesothelioma remains a challenging cancer to treat with very poor prognosis overall. Similarly, although immunotherapy is a newer MPM systemic treatment option, very few cases received any adjuvant immunotherapy, and standard of care chemotherapy options among our cohort did not change over time. Regarding surgical outcomes, we were limited in our review of survival and quality of life outcomes for PD versus EPP due to small sample size and the retrospective nature of our study. Although our study suggested that treatment was associated with improved survival, other confounding factors that also impact survival such as improvements in surgical technique and postoperative management, as well as modifications in systemic therapy could not be controlled for given the retrospective nature of this review. However, when comparing two matched groups for age and CCI in patients with early stage, epithelioid MPM, the OS benefit was seen only in patients who received multimodality treatment.
We are currently examining additional details of types and quantities of systemic therapies administered to determine the impact of bi-disciplinary guidance. Although we saw a reduction in the number of patients receiving no MPM directed therapies, further work is needed to increase treatment options for older patients, patients with multiple comorbidities, or non-epithelioid histology who are less likely to receive treatment. Checkmate 743 and CONFIRM studies have shown that immunotherapy is beneficial in all patients with MPM, while the benefit appeared to be greater in non-epithelioid histologies (28,29). Our study shows the importance of bi-disciplinary review as treatments to these subgroups increase over time.
Conclusions
In summary, consolidating mesothelioma surgery to specialized surgeons and weekly bi-disciplinary review of MPM cases to determine appropriate multimodality therapy allows eligible patients to receive increased treatments. By rapid case ascertainment and multidisciplinary review, more patients who may benefit from surgery and systemic therapy can be identified. Our study suggests using expert review of MPM cases and cytoreductive surgery performed by an experienced surgical team leads to increased availability and receipt of multimodality treatment options
Acknowledgments
The abstract was submitted to the International Association for the Study of Lung Cancer World Conference on Lung Cancer in September 2021 and accepted in abstract form for publication in Journal of Thoracic Oncology.
Funding: This work was supported by the Kaiser Permanente Northern California Graduate Medical Education Program, Kaiser Foundation Hospitals.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-427/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-427/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-427/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-427/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional review board of Kaiser Permanente Northern California (No. 00001045) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ricciardi S, Cardillo G, Zirafa CC, et al. Surgery for malignant pleural mesothelioma: an international guidelines review. J Thorac Dis 2018;10:S285-92. [Crossref] [PubMed]
- Hajj GNM, Cavarson CH, Pinto CAL, et al. Malignant pleural mesothelioma: an update. J Bras Pneumol 2021;47:e20210129. [PubMed]
- Taioli E, Wolf AS, Camacho-Rivera M, et al. Determinants of Survival in Malignant Pleural Mesothelioma: A Surveillance, Epidemiology, and End Results (SEER) Study of 14,228 Patients. PLoS One 2015;10:e0145039. [Crossref] [PubMed]
- National Comprehensive Cancer Network. (2021). Malignant Pleural Mesothelioma (version 1/2021).
- Ely S, Jiang SF, Patel AR, et al. Regionalization of Lung Cancer Surgery Improves Outcomes in an Integrated Health Care System. Ann Thorac Surg 2020;110:276-83. [Crossref] [PubMed]
- Boxer MM, Vinod SK, Shafiq J, et al. Do multidisciplinary team meetings make a difference in the management of lung cancer? Cancer 2011;117:5112-20. [Crossref] [PubMed]
- Forrest LM, McMillan DC, McArdle CS, et al. An evaluation of the impact of a multidisciplinary team, in a single centre, on treatment and survival in patients with inoperable non-small-cell lung cancer. Br J Cancer 2005;93:977-8. [Crossref] [PubMed]
- Treasure T, Lang-Lazdunski L, Waller D, et al. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomised feasibility study. Lancet Oncol 2011;12:763-72. [Crossref] [PubMed]
- Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613-9. [Crossref] [PubMed]
- Kallogjeri D, Gaynor SM, Piccirillo ML, et al. Comparison of comorbidity collection methods. J Am Coll Surg 2014;219:245-55. [Crossref] [PubMed]
- Azam F, Latif MF, Farooq A, et al. Performance Status Assessment by Using ECOG (Eastern Cooperative Oncology Group) Score for Cancer Patients by Oncology Healthcare Professionals. Case Rep Oncol 2019;12:728-36. [Crossref] [PubMed]
- Enewold L, Sharon E, Thomas A. Patterns of care and survival among patients with malignant mesothelioma in the United States. Lung Cancer 2017;112:102-8. [Crossref] [PubMed]
- Ely S, Alabaster A, Ashiku SK, et al. Regionalization of thoracic surgery improves short-term cancer esophagectomy outcomes. J Thorac Dis 2019;11:1867-78. [Crossref] [PubMed]
- Yan TD, Cao CQ, Boyer M, et al. Improving survival results after surgical management of malignant pleural mesothelioma: an Australian institution experience. Ann Thorac Cardiovasc Surg 2011;17:243-9. [Crossref] [PubMed]
- Flores RM, Riedel E, Donington JS, et al. Frequency of use and predictors of cancer-directed surgery in the management of malignant pleural mesothelioma in a community-based (Surveillance, Epidemiology, and End Results [SEER]) population. J Thorac Oncol 2010;5:1649-54. [Crossref] [PubMed]
- Flores RM, Zakowski M, Venkatraman E, et al. Prognostic factors in the treatment of malignant pleural mesothelioma at a large tertiary referral center. J Thorac Oncol 2007;2:957-65. [Crossref] [PubMed]
- Warby A, Dhillon HM, Kao S, et al. Managing malignant pleural mesothelioma: experience and perceptions of health care professionals caring for people with mesothelioma. Support Care Cancer 2019;27:3509-19. [Crossref] [PubMed]
- Gordon N, Lin T. The Kaiser Permanente Northern California Adult Member Health Survey. Perm J 2016;20:15-225. [Crossref] [PubMed]
- Boxer MM, Vinod SK, Shafiq J, et al. Do multidisciplinary team meetings make a difference in the management of lung cancer? Cancer 2011;117:5112-20. [Crossref] [PubMed]
- Forrest LM, McMillan DC, McArdle CS, et al. An evaluation of the impact of a multidisciplinary team, in a single centre, on treatment and survival in patients with inoperable non-small-cell lung cancer. Br J Cancer 2005;93:977-8. [Crossref] [PubMed]
- McNair AG, Choh CT, Metcalfe C, et al. Maximising recruitment into Randomized controlled trials: the role of multidisciplinary cancer teams. Eur J Cancer 2008;44:2623-6. [Crossref] [PubMed]
- Woodard GA, Jablons DM. Surgery for pleural mesothelioma, when it is indicated and why: arguments against surgery for malignant pleural mesothelioma. Transl Lung Cancer Res 2020;9:S86-91. [Crossref] [PubMed]
- Lim E, Darlison L, Edwards J, et al. Mesothelioma and Radical Surgery 2 (MARS 2): protocol for a multicentre randomised trial comparing (extended) pleurectomy decortication versus no (extended) pleurectomy decortication for patients with malignant pleural mesothelioma. BMJ Open 2020;10:e038892. [Crossref] [PubMed]
- Rusch VW, Giroux D, Kennedy CIASLC Staging Committee, et al. J Thorac Oncol 2012;7:1631-9. [Crossref] [PubMed]
- Vicidomini G, Della Corte CM, Noro A, et al. A Trimodality, Four-Step Treatment including Chemotherapy, Pleurectomy/Decortication and Radiotherapy in Early-Stage Malignant Pleural Mesothelioma: A Single-Institution Retrospective Case Series Study. Cancers (Basel) 2021;14:142. [Crossref] [PubMed]
- Thompson AB, Quinn TJ, Siddiqui ZA, et al. Addition of radiotherapy to surgery and chemotherapy improves survival in localized malignant pleural mesothelioma: A Surveillance, Epidemiology, and End Results (SEER) study. Lung Cancer 2020;146:120-6. [Crossref] [PubMed]
- Alnajar A, Rodriguez E, Razi S, et al. Survival benefit of multiagent chemotherapy with and without curative surgery for malignant pleural mesothelioma. J Thorac Oncol 2021;16:S897-8. [Crossref]
- Baas P, Scherpereel A, Nowak AK, et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet 2021;397:375-86. [Crossref] [PubMed]
- Fennell DA, Ewings S, Ottensmeier C, et al. Nivolumab versus placebo in patients with relapsed malignant mesothelioma (CONFIRM): a multicentre, double-blind, randomised, phase 3 trial. Lancet Oncol 2021;22:1530-40. [Crossref] [PubMed]


