Update on three-dimensional image reconstruction for preoperative simulation in thoracic surgery
Introduction
Multi-detector computed tomography (CT) able to construct three-dimensional (3D) images has been developed, and this technology has been refined over time. Clinical use of 3D-CT technology has also been introduced to the field of surgery, and 3D displays of anatomic structures have currently become feasible for decision-making in surgical procedures. In the field of hepatic surgery, in particular, preoperative simulation has been widely employed (1,2). To date, several software programs have even been used internationally in the field of thoracic surgery, but the quality of images and the time required for image acquisition are key issues limiting the wider use of this technology (3). In addition, complexity in manipulating the software is another major obstacle to its use. However, a novel high-speed and high-quality 3D-CT technology has recently been developed and introduced to the field of thoracic surgery.
Minimally invasive thoracic surgery is now widely performed for early stage lung cancers, which are also detected in patients without symptoms due to the wider use of low-dose CT screening. In Japan, more than half of lung cancer surgeries are performed with video-assisted thoracoscopic surgery (VATS), and the number of VATS procedures using only a monitor for vision, often called “complete VATS”, is increasing. Precise knowledge of the anatomy of the pulmonary vessels and the bronchi is mandatory for safe VATS procedures, especially when performing complete VATS. Anatomical variations of pulmonary vessels and bronchi are inevitable, and therefore preoperative confirmation of the surgical anatomy of each patient would contribute to the safety of the surgical procedures.
A novel high-speed and high-quality 3D image analysis system has enabled thoracic surgeons to easily understand the pulmonary anatomy of each patient preoperatively, and even to simulate surgical procedures with confidence. The purpose of this manuscript is to demonstrate the use of this 3D-CT technology in various scenarios of thoracic surgery, with several typical examples.
Methods
In the department of Thoracic Surgery in Kyoto University, newly-developed high speed and high quality 3D image analysis system (Synapse Vincent, Fuji Film Co., Ltd., Tokyo, Japan) was used to obtain 3D images of the pulmonary vessels and the tracheobronchial tree. This system automatically extracts information on pulmonary structures and displays 3D images with only a few clicks within a few minutes. In various thoracic surgeries, simulation and/or navigation were performed using this 3D-CT technology. In this manuscript, we demonstrated several typical examples of these 3D-CT technologies in various scenes of thoracic surgery.
Results
VATS lobectomy for lung cancer
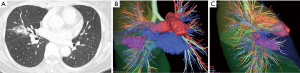
In our institution, 3D-CT simulation has been extensively performed in patients undergoing VATS. Most surgeons tend to perform complete VATS procedures and therefore precise knowledge of the anatomy of the pulmonary vessels and the bronchi in each patient has been increasingly needed. Anatomical variations of pulmonary vessels and bronchi are inevitable and have been confirmed preoperatively using a 3D-CT image. Of note, surgeons rather than radiologic specialists and technicians created 3D-CT images using the image analysis system preoperatively (Figure 1). Furthermore, preoperative 3D-CT simulation was useful in sharing anatomical information with all the doctors involved in the surgery, both preoperatively and intraoperatively.
VATS sublobar resection for small lesions
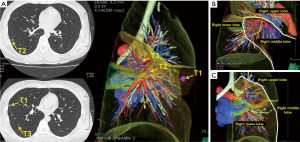
With the development and wider use of CT technology, smaller nodules or lesions with ground glass opacity have recently been detected. In such cases, it is very difficult to identify and resect targeted lesions even with VATS. Since sublobar resection is required in most cases, surgical simulation and navigation using virtual reality 3D-CT images might be useful in obtaining an adequate surgical margin due to tumor location and the number of tumors. For precise navigation of thoracoscopic sublobar lung resection, we developed a technique named virtual-assisted lung mapping (VAL-MAP), which is a bronchoscopic multi-spot dye-marking technique using virtual images (4). In a small lesion, which was supposedly neither palpable nor visible, preoperative marking was performed using 3D-CT simulation, and wedge resection and segmentectomy were then successfully performed (Figure 2).
Virtual endobronchial ultrasonography (EBUS)
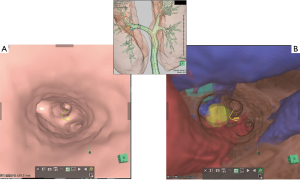
EBUS with transbronchial needle aspiration (TBNA) has been proposed as a safe, less invasive alternative to mediastinoscopy for the diagnosis of mediastinal lymph nodes and tumors. Currently, EBUS-TBNA is in widespread use since it enables less invasive cytologic evaluation of mediastinal/hilar abnormalities without general anesthesia (5). Diagnosis of mediastinal/hilar lymph nodes and tumors is often challenging in patients with a previously treated thoracic malignancy, especially with a history of thoracotomy. EBUS-TBNA has been proposed as a safe, less invasive modality for even these patients (6). In our institution, we also used 3D-CT images to perform an accurate simulation (virtual EBUS) before the actual EBUS-TBNA. Virtual EBUS created by the 3D image analyzer made it possible to show the image with or without a transparent bronchial wall (Figure 3). This technique also made EBUS safer and more reliable.
Living-donor CT angiography
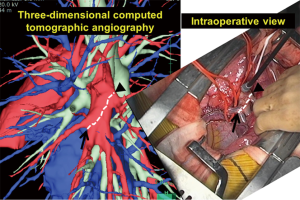
Due to a severe donor shortage, living-donor lobar lung transplantation (LDLLT) is an established treatment for severely ill patients with end-stage lung disease who cannot wait for cadaveric donors (7-10). In our institution, surgeons involved in both donor and recipient surgeries evaluated CT findings in every LDLLT case, focusing on finding incomplete fissures and evaluating the state of vessel and bronchial branches. Preoperatively, the surgical procedure for donor lobectomy was simulated by 3D-CT angiography, which also affected the surgical procedure for recipient surgery (11,12). In 58 donors (68%), small branches of the pulmonary artery were sacrificed to obtain an adequate arterial cuff for safe implantation (12). Furthermore, according to our updated database, pulmonary arterioplasties were performed with autopericardial patches or end-to-end anastomosis in 12 out of 48 donors who donated left lower lobe (LLL) (25%). In our series, intraoperative complications were noted in only 2 donors (2%), which were low compared with the number in previous reports (12). A major reason for such a low rate of intraoperative complications in our institution was that we performed preoperative anatomical evaluation with 3D-CT angiography in all donors (13). For example, branches of the interlobar left pulmonary artery depicted by 3D-CT angiography were consistent with those seen in the actual operative field (Figure 4).
New surgical technique in living-donor lobar lung transplantation (LDLLT)
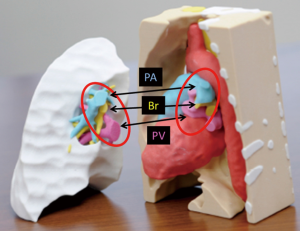
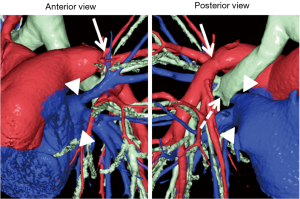
Unlike deceased-donor lung transplantation, small-for-size donor grafts are often an impediment in LDLLT for adults. Generally, the right lower lobe (RLL) is approximately 120% larger than the LLL (14). If we transplant an RLL instead of an LLL in the left thorax, we can transplant a larger donor graft in the recipient, which might potentially solve issues regarding small-for-size donor grafts. With this concept, we developed a novel surgical technique in which an inverted RLL graft can be transplanted into the left thorax. In the first patient, we successfully performed this novel procedure after careful surgical simulation using a 3D printer (13). Simulation of the positional relationship among structures was performed using 3D-CT images. However, as there was no report of such a complicated LDLLT, preoperative surgical simulation was performed using 3D models produced by a 3D printer (ZPrinter 450, 3D Systems, Andover, MA, USA; Figure 5). Accurate simulation of the positional relationship among structures showed that the bronchial anastomosis could be performed after the donor bronchus was sutured to the recipient’s left upper bronchus. The anatomic posterior aspect of the RLL graft would become anterior in the left chest of the recipient. Pulmonary artery anastomosis was created behind the bronchus. The donor pulmonary vein could be anastomosed to the recipient’s left upper pulmonary vein as usual, but it seemed more suitable to suture the donor pulmonary vein to the recipient’s left atrial appendage. In actual LDLLT, all anastomoses were performed smoothly as preoperatively planned. Because of the initial success, this procedure was performed successfully in six additional patients. Postoperative 3D-CT images in bilateral LDLLT with implantation of the inverted RLL in the left thorax are shown in Figure 6. In this case, the donor pulmonary vein was sutured to the recipient’s left upper pulmonary vein, as is usually performed in LDLLT.
Discussion
As shown by several examples in thoracic surgery, we extensively used a newly-developed high-speed and high-quality 3D image analysis system (Synapse Vincent) to obtain virtual-reality 3D images of the pulmonary vessels and the tracheobronchial tree preoperatively. Because of technological advances in software, the equipment is manipulated by mostly automatic procedures, and it takes only a few minutes to obtain appropriate 3D images of the pulmonary structures for surgical simulation (15). It is really a striking technological development because most commonly used software programs required a longer process and image guidance by professionals to obtain 3D images. In this manuscript, we demonstrated typical uses of this novel software program in various settings of thoracic surgery.
It should be emphasized that these virtual-reality 3D-CT images can be created by a surgeon without a radiology expert within less than 10 minutes. Furthermore, the constructed images can be rotated freely and visualized interactively from any angle, providing an overview of the 3D relationships of the pulmonary structures. The results of preoperative assessment of variations of pulmonary vessel branching patterns as well as short-term surgical outcomes were also reportedly satisfactory (16). In addition, this 3D-CT technology helps educate trainees and medical students in surgical anatomy. It may even be useful for preoperative informed consent.
Despite the development of this 3D-CT technology, we should keep in mind that the system still has major limitations. First, virtual visualization of the pulmonary structures consists of the inflated lung, whereas the lung is actually deflated during surgical procedures. Second, contrast-enhanced CT is required for the reconstruction of the 3D-CT images, but this system can separately reconstruct 3D images of the pulmonary arteries and veins from the data of only one conventional CT scan, and can thereby reduce radiation exposure (15).
In conclusion, preoperative simulation has been developed for various procedures following the introduction of 3D-CT technology to the field of thoracic surgery. In the near future, this technique will become more common in thoracic surgery, and frequent use of this technique by thoracic surgeons may be seen in worldwide daily practice.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Yamanaka J, Saito S, Iimuro Y, et al. The impact of 3-D virtual hepatectomy simulation in living-donor liver transplantation. J Hepatobiliary Pancreat Surg 2006;13:363-9. [PubMed]
- Mochizuki K, Takatsuki M, Soyama A, et al. The usefulness of a high-speed 3D-image analysis system in pediatric living donor liver transplantation. Ann Transplant 2012;17:31-4. [PubMed]
- Ikeda N, Yoshimura A, Hagiwara M, et al. Three dimensional computed tomography lung modeling is useful in simulation and navigation of lung cancer surgery. Ann Thorac Cardiovasc Surg 2013;19:1-5. [PubMed]
- Sato M, Omasa M, Chen F, et al. Use of virtual assisted lung mapping (VAL-MAP), a bronchoscopic multispot dye-marking technique using virtual images, for precise navigation of thoracoscopic sublobar lung resection. J Thorac Cardiovasc Surg 2014;147:1813-9. [PubMed]
- Herth FJ, Annema JT, Eberhardt R, et al. Endobronchial ultrasound with transbronchial needle aspiration for restaging the mediastinum in lung cancer. J Clin Oncol 2008;26:3346-50. [PubMed]
- Chen F, Miyahara R, Sato T, et al. Usefulness of endobronchial ultrasound in patients with previously treated thoracic malignancy. Interact Cardiovasc Thorac Surg 2012;14:34-7. [PubMed]
- Starnes VA, Barr ML, Cohen RG. Lobar transplantation. Indications, technique, and outcome. J Thorac Cardiovasc Surg 1994;108:403-10; discussion 410-1. [PubMed]
- Starnes VA, Bowdish ME, Woo MS, et al. A decade of living lobar lung transplantation: recipient outcomes. J Thorac Cardiovasc Surg 2004;127:114-22. [PubMed]
- Date H, Sato M, Aoyama A, et al. Living-donor lobar lung transplantation provides similar survival to cadaveric lung transplantation even for very ill patients†. Eur J Cardiothorac Surg 2015;47:967-72; discussion 972-3. [PubMed]
- Chen F, Yamane M, Inoue M, et al. Less maintenance immunosuppression in lung transplantation following hematopoietic stem cell transplantation from the same living donor. Am J Transplant 2011;11:1509-16. [PubMed]
- Chen F, Fujinaga T, Shoji T, et al. Short-term outcome in living donors for lung transplantation: the role of preoperative computer tomographic evaluations of fissures and vascular anatomy. Transpl Int 2012;25:732-8. [PubMed]
- Chen F, Yamada T, Sato M, et al. Postoperative pulmonary function and complications in living-donor lobectomy. J Heart Lung Transplant 2015;34:1089-94. [PubMed]
- Chen F, Miyamoto E, Takemoto M, et al. Right and left inverted lobar lung transplantation. Am J Transplant 2015;15:1716-21. [PubMed]
- Chen F, Kubo T, Shoji T, et al. Comparison of pulmonary function test and computed tomography volumetry in living lung donors. J Heart Lung Transplant 2011;30:572-5. [PubMed]
- Kitamura Y, Li Y, Ito W, et al. Data-dependent higher-order clique selection for artery-vein segmentation by energy minimization. Int J Comput Vis 2015. [PubMed]
- Hagiwara M, Shimada Y, Kato Y, et al. High-quality 3-dimensional image simulation for pulmonary lobectomy and segmentectomy: results of preoperative assessment of pulmonary vessels and short-term surgical outcomes in consecutive patients undergoing video-assisted thoracic surgery†. Eur J Cardiothorac Surg 2014;46:e120-6. [PubMed]


