Impact of correcting the lymphocyte count to improve the sensitivity of TB antigen-specific peripheral blood-based quantitative T cell assays (T-SPOT.®TB and QFT-GIT)
Introduction
Interferon gamma release assays (IGRAs) utilizing RD-1 antigen-specific peripheral blood T-lymphocyte IFN-γ responses have been developed as more specific diagnostic tests, compared to the TST, for presumed latent tuberculosis infection (1-4). The two commercially available IGRAs: T-SPOT.®TB (Oxford Immunotec Ltd, Oxford) and QuantiFERON®-TB Gold In-Tube assay (Cellestis Ltd., Carnegie) have different technical and performance characteristics. The T-SPOT.®TB is an ELISPOT assay that requires same day processing (if not using the proprietary T cell Xtend) and isolation of peripheral blood mononuclear cells (PBMC), which are seeded into antigen-containing wells at a pre-determined concentration (2.5×105 mononuclear cells/well). The assay is therefore enriched for lymphocytes.
By contrast, the QuantiFERON®-TB Gold In-Tube (QFT-GIT) assay, which is more user-friendly, uses 1 mL of whole blood per tube. There is no standardization of the lymphocyte count, which can vary up to 2 to 3 fold in healthy individuals (1) and even more in immunocompromized individuals (2). This reduced proportion and number of IFN-γ-secreting lymphocytes may influence test performance. Indeed, meta-analyses have shown that the QFT-GIT assay has significantly lower sensitivity than the T-SPOT.®TB assay (3-6). Furthermore, the rate of indeterminate reactions between the assays varies between recent meta-analyses and may have a similar genesis (4-8).
Nevertheless, several other reasons may explain the difference in performance characteristics between the assays. These include selection of the assay cut-point (9-11), technical differences inherent in the type of immunological technique, and antigens used (ELISPOT vs. ELISA) including the length of the peptides, and the composition of the antigen cocktail used (QFT-GIT uses TB 7.7 in addition to RD-1 antigens). However, here we address the variability in lymphocyte count alone. We hypothesized that adjusting the lymphocyte count, either by volume adjustment or by lymphocyte enrichment, while maintaining the volume at 1 mL in the QFT-GIT tubes would increase the proportion of IFN-γ-producing effector cells and hence improve the sensitivity of the QFT-GIT. According to the updated CDC IGRA guidelines (12) and a review of research priorities for IGRAs (13), addressing this question is considered to be important.
Materials and methods
Blood was drawn from participants enrolled in a health care worker screening study (n=26) and HIV positive subjects attending clinics for TB screening (n=10). Ethical approval was granted by the University of Cape Town Health Sciences Faculty Research Ethics Committee. The T-SPOT.®TB test, an unadjusted QFT-GIT assay (1ml drawn directly into vacuum collection tubes), and QFT-GIT assay adjusted for lymphocyte count were performed according to the manufacturer’s instructions. Categorical variables were compared using the χ2 test and continuous variables were compared using Student’s t-test where appropriate, with Mann-Whitney used for non-parametrically distributed continuous variables (Graphpad Prism, Version 5.03 and Open Epi, Version 2.3.1 were used).
To directly evaluate the effect of variation in lymphocyte count on the QFT-GIT assay, blood was drawn from 5 participants known to be QFT-GIT positive, and their lymphocytes were isolated and concentrated. The concentrated lymphocytes were diluted in RPMI to a total volume of 1ml in QFT-GIT tubes to get adjusted lymphocyte counts of 1×106, 2×106, 2.5×106, 3×106 and 4×106 per tube respectively.
Optimization experiments were performed in 5 subjects, to ensure that vacuum-filling vs. syringe-filling did not impact on the results. To directly evaluate the effect of antigen dilution on IFN-γ responses 1 ml of whole blood was diluted (1.6, 2.0, and 2.5 fold) with AIM-V (GIBCO) in three participants. To assess antigen dilution without volume change, aliquots of a solution containing ESAT-6 overlapping peptides (Oxford Immunotec) were added to a fixed number of cells (250,000 per well) in 150 μL of serum free medium (1× and 0.4× concentration of antigen). In parallel the T-SPOT.®TB assay was performed according to the manufacturer’s instructions using 250,000 PBMCs per well.
To meaningfully compare within-test results (multiple samples taken from the same patient at the same time) we conducted experiments to evaluate test-retest variability in four subjects (in each subject 3 antigen, mitogen and nil tubes were sequentially taken at the same time). Thus there were 24 observations in the data set. Within-test variability was calculated by determining the mean ± standard deviation (SD) for all subjects. Expressing this SD value as a percentage enabled calculation of the 95% confidence intervals (2SD) and hence test variability. The same experienced technician who was blinded to the patient identities performed all the QFT-GIT and T-SPOT.®TB assays.
Increasing lymphocyte count by concentration requires a dedicated laboratory. We therefore assessed volume-based lymphocyte adjustment in QFT-GIT, by adding an appropriate volume of whole blood, to each tube to achieve a standardized lymphocyte count of 2.5×106 lymphocytes per tube based on the lymphocyte count from a specimen drawn at the same time of day (9 am). Volume based corrections would be practically easier. For example if the whole blood lymphocyte count was 2×106/mL then the final volume was adjusted to 1.25 mL by using a syringe to add the required volume to the QFT tube.
To determine the effect of adjusting the lymphocyte count in QFT-GIT tubes while keeping the volume at ~1 mL (the manufacturer recommends that the tube may be filled with between 0.8 and 1.2 mL of blood), lymphocytes were isolated, concentrated and then added to each QFT-GIT tube to bring up the count to an arbitrarily-selected total count of 2.5×106 per tube in 16 participants. This means that the experiment could only be performed on those participants who had a 9 am total lymphocyte count of less than 2.5×106/mL.
Results
Test-retest variability
To meaningfully interpret downstream results test-retest variability was first quantified. All subjects (n=4) were QFT-GIT positive with an IFN-γ level (antigen minus nil) ranging from 1.04 to 2.03 IU/mL. The variability (SD) of each within-test triplicate was first determined. The mean of the four SD (expressed as a %) was 22.5%. Therefore the 95% CI (2SD) of the mean variability was ±45% of any given IFN-γ value (Figure 1).
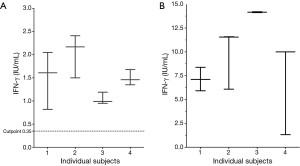
Effect of in-vitro lymphocyte enrichment (within a ~1 mL volume) in QFT positive samples
Three out of 5 subjects showed an increasing IFN-γ concentration with increasing lymphocyte count. Overall, there was an increase in IFN-γ production up to a lymphocyte count of 2.5×106 /mL (P=0.03 compared to 1×106/mL), which appeared to plateau at higher lymphocyte counts (Figures 2,3).
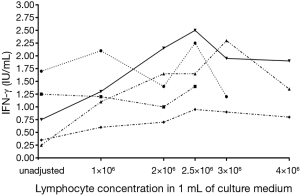
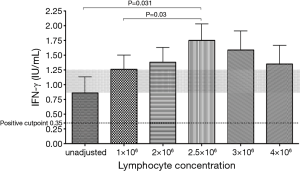
Impact of different tube-filling methods, volume adjustment and reduction in antigen load
The impact of syringe vs. vacuum-based filling of the QFT-GIT tubes was evaluated. Readouts were similar 0.82 (0–14.20) vs. 0.92 (0–14.00) IU/mL in 1ml directly vacuum (during phlebotomy) vs. syringe-filled tubes. Next the impact of volume dilution and antigen load was assessed. Over a range of dilutions (1 to 2.5 fold dilution of 1 mL of blood with medium) there was a roughly 2.5 fold reduction in IFN-γ concentration: 0.99 (0.31–3.20) vs. 0.39 (0.18–0.40) IU/mL but this did not reach statistical significance (P=0.25). When cell number and volume were maintained, but only antigen load (using serial dilutions of ESAT-6) IFN-γ remained constant 0.17 (0.03–0.79) at (1×) of antigen and 0.19 (0.07–0.78) at (0.4×) of antigen (P=0.4).
Lymphocyte enrichment by blood volume expansion
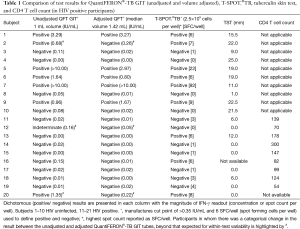
The median (range) peripheral blood white cell count (WCC) was 6.9 (3.8–14.8) ×109/L (Siemens ADVIA 2120 automated cell counter). The median (range) adjusted volume of the QFT-GIT assay to achieve a lymphocyte count of 2.5×106/mL was 1.42 (0.96–3.33) mL per tube. This was significantly higher in the HIV positive compared to the HIV negative group 1.82 (1.08–3.33) vs. 1.17 (0.96–1.62) mL (P<0.001). Seven of the 20 subjects (35%) were QFT-GIT positive and 9/20 (45%) T-SPOT.®TB positive. Two subjects “converted” across the 0.35 IU/mL cut-point from positive to negative after volume adjustment (Table 1); both results decreased by more than the 45% i.e., than what would be expected by “test-retest variability” (Figure 4).
Full table

After adjusting for the lymphocyte count, the median (range) IFN-γ concentration (IU/mL) was significantly lower in the adjusted samples 0.02 (0–12.93) vs. 0.09 (0–14.23) IU/mL (P=0.008); when this result was stratified by HIV status, no difference was seen. The total IFN-γ production (IFN-γ per tube), however, was unchanged in the volume-adjusted tubes. The median (range) of IFN-γ per lymphocyte (IU/lymphocyte) was significantly lower in the adjusted samples 1.38×10−8vs. 5.5×10−8 IU/lymphocyte (P=0.008) (Figure 4). In summary, lymphocyte adjustment by volume increase did not increase the IFN-γ concentration or total IFN-γ per tube.
Lymphocytes enrichment whilst keeping the volume of blood constant (~1 mL)
The median (range) laboratory WCC was 7.0 (4.8–12.4) ×109/L and the median (range) laboratory total lymphocyte count (TLC) was 2.15 (1.30–3.39) ×109/L. Five participants (#24, #30, #33, #34, #36) had TLC >2.5×109/L and could not be used for this experiment. The median [range] volume of concentrated lymphocytes that was added to the QFT-GIT tubes was 261 [82–621] µL per tube. When unadjusted for lymphocyte count 2/10 subjects were QFT positive. One subject (#21) “converted” across the 0.35 IU/mL cut-point from negative to positive after lymphocyte count adjustment. There was no significant difference in the median (range) IFN-γ concentration (IU/mL) between the adjusted and the unadjusted samples 0.02 (0–4.41) vs. 0.10 (0–2.40) IU/mL (P=0.64) (Table 2).
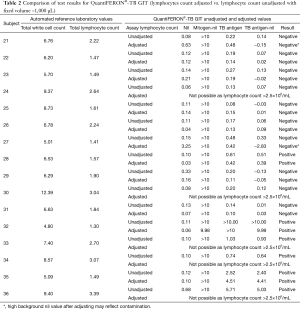
Full table
Discussion
The apparently poorer sensitivity (and higher indeterminate rate) of the QFT-GIT assay compared to T-SPOT.®TB, now confirmed in meta-analyses, may compromise its utility in clinical practice and, in particular, in immunocompromized patients (4,8,14,15). Although several explanations have been proposed for the poorer sensitivity of the QFT-GIT assay there are few data investigating these possibilities. We hypothesized that the poorer sensitivity of the QFT-GIT is due to relatively reduced numbers of IFN-γ-secreting lymphocytes related to the more than 2 fold natural variation in individual blood mononuclear cell counts. Thus, the sensitivity could potentially be improved by adjusting the lymphocyte counts in QFT tubes. We serially enriched the lymphocyte count in confirmed QFT positive participant samples to demonstrate that higher numbers of sensitized T cells result in more IFN-γ. We then enriched the lymphocyte counts either by adding blood (a more practical option) with a resultant volume increase or by lymphocyte enrichment while maintaining the volume in QFT-GIT tubes to ~1 mL (requiring a specialized laboratory).
There was an increase in IFN-γ production peaking at a lymphocyte count of 2.5×106 when increasing numbers of lymphocytes were added to the QFT tubes. This suggests that 2.5×106/mL may be the optimum lymphocyte concentration for the QFT-GIT assay. Lymphocyte adjustment, in a cohort of patients undergoing TB screening, either by addition of whole blood or concentration of lymphocytes, did not increase the number of positive subjects significantly. Thus, lymphocyte-independent factors seem to be more likely to explain the lower sensitivity of the QFT-GIT assay. These might include the inherent nature of the technique [ELISPOT can detect IFN-γ at cellular level (16)], the assay-specific antigen concentration, the antigen cocktail used (10,13), or a combination of these factors. Indeed, this may explain the discordant results for the 2 assays (positive vs. negative and the magnitude of positivity as shown in Table 1). The most likely explanation, however, and given our results, is selection of assay cut-point. Thus, lowering the QFT-GIT cut-point may improve sensitivity with a slight reduction in specificity (17,18), improve inter-assay agreement (10) and impact on the proportion of conversions and reversions (19). However, choosing the optimal cut-point will depend on clinical context e.g., avoiding unnecessary treatment in latent TB infection (LTBI) screening programs may require greater specificity whilst screening for active TB requires a higher sensitivity. Thus, further work is required to evaluate the effect of cut-point selection on test outcomes in different clinical contexts (13).
To ensure that the observed changes were not due to random variability we first quantified test-retest variability (testing repeated in the same subject at a single time-point). There are hardly any independent published data about within person (20) and test-retest (instrument) variability (20) and, to our knowledge, none about test-retest (operator) variability. Our preliminary data suggest that ±45% of any given IFN-γ value explains 95% of the within-test variability if the test is repeated immediately (same blood draw). Significant variability is also documented where automated vs. manual processing is undertaken (21) repeat testing is done on the same samples or over a period of time (22-24). These studies also demonstrate the significant variability over time, especially around the cut point in serially tested individuals (22-24).
The volume-adjusted IFN-γ concentration and IFN-γ per lymphocyte was paradoxically reduced despite higher lymphocyte counts per tube. In separate experiments changes in antigen dilution or volume, and test-retest variability did not satisfactorily explain these results. We speculate that some of these paradoxical changes may be explained by a lower level of receptor saturation in the adjusted tubes, which may result in a failure to turn off inhibitory immunological pathways including modulators of IFN-γ production such as IL-9, IL-10, TGF-β, or regulatory T cells (25). Nevertheless, these changes often occurred well below the assay cut-point, were in many cases within the limits of test-retest variability, and the effect disappeared when results were stratified by HIV status. Thus, although statistically significant, they are unlikely to be biologically meaningful or clinically significant.
In the study design, we initially chose a ‘correction’ method that would potentially be feasible in clinical practice and hence a protocol that did not require separation of PBMCs. Volume adjustment is a convenient method allowing the addition of whole blood to the QFT tubes within an hour of blood sampling. The drawback to this approach is that we were unable to increase the numbers of cells without a significant increase in volume.
There are several limitations of our study. The serial lymphocyte enrichment experiments were conducted using RPMI and the results when using whole blood, which contains immunosuppressive cells, may have been different. Another limitation is the small number of test subjects evaluated and hence type 2 error. It is possible that a larger cohort of patients would have shown improvement in sensitivity with adjustment of lymphocyte count. We feel this is unlikely for two reasons. The IFN-γ level (total or concentration) did not increase significantly in a single individual despite up to a more than 3 fold correction of lymphocyte count. Secondly, 1 mL of blood in the QFT tube has more PBMCs (~1 million PBMC) and hence lymphocytes, even with a 2 fold variation in counts, than the T-SPOT TB well (250,000 PBMC).
In summary, the reduced sensitivity of the QFT-GIT assay is likely to be due to factors other than lymphocyte count alone. Larger studies in different settings are now required to confirm and clarify our findings.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Dheda K, Barry CE 3rd, Maartens G. Tuberculosis. Lancet 2015. [Epub ahead of print].
- Carmichael KF, Abayomi A. Analysis of diurnal variation of lymphocyte subsets in healthy subjects in the Caribbean, and its implication in HIV monitoring and treatment. Afr J Med Med Sci 2006;35:53-7. [PubMed]
- Menzies D, Pai M, Comstock G. Meta-analysis: new tests for the diagnosis of latent tuberculosis infection: areas of uncertainty and recommendations for research. Ann Intern Med 2007;146:340-54. [Crossref] [PubMed]
- Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med 2008;149:177-84. [Crossref] [PubMed]
- Diel R, Loddenkemper R, Nienhaus A. Evidence-based comparison of commercial interferon-gamma release assays for detecting active TB: a metaanalysis. Chest 2010;137:952-68. [Crossref] [PubMed]
- Sester M, Sotgiu G, Lange C, et al. Interferon-γ release assays for the diagnosis of active tuberculosis: a systematic review and meta-analysis. Eur Respir J 2011;37:100-11. [Crossref] [PubMed]
- Ferrara G, Losi M, D'Amico R, et al. Use in routine clinical practice of two commercial blood tests for diagnosis of infection with Mycobacterium tuberculosis: a prospective study. Lancet 2006;367:1328-34. [Crossref] [PubMed]
- Richeldi L. An update on the diagnosis of tuberculosis infection. Am J Respir Crit Care Med 2006;174:736-42. [Crossref] [PubMed]
- Harada N, Higuchi K, Yoshiyama T, et al. Comparison of the sensitivity and specificity of two whole blood interferon-gamma assays for M. tuberculosis infection. J Infect 2008;56:348-53. [Crossref] [PubMed]
- Arend SM, Thijsen SF, Leyten EM, et al. Comparison of two interferon-gamma assays and tuberculin skin test for tracing tuberculosis contacts. Am J Respir Crit Care Med 2007;175:618-27. [Crossref] [PubMed]
- Higuchi K, Kawabe Y, Mitarai S, et al. Comparison of performance in two diagnostic methods for tuberculosis infection. Med Microbiol Immunol 2009;198:33-7. [Crossref] [PubMed]
- Mazurek GH, Jereb J, Vernon A, et al. Updated guidelines for using Interferon Gamma Release Assays to detect Mycobacterium tuberculosis infection - United States, 2010. MMWR Recomm Rep 2010;59:1-25. [PubMed]
- Pai M, Dheda K, Cunningham J, et al. T-cell assays for the diagnosis of latent tuberculosis infection: moving the research agenda forward. Lancet Infect Dis 2007;7:428-38. [Crossref] [PubMed]
- Raby E, Moyo M, Devendra A, et al. The effects of HIV on the sensitivity of a whole blood IFN-gamma release assay in Zambian adults with active tuberculosis. PLoS One 2008;3:e2489. [Crossref] [PubMed]
- Cattamanchi A, Smith R, Steingart KR, et al. Interferon-gamma release assays for the diagnosis of latent tuberculosis infection in HIV-infected individuals: a systematic review and meta-analysis. J Acquir Immune Defic Syndr 2011;56:230-8. [Crossref] [PubMed]
- Dheda K, Udwadia ZF, Huggett JF, et al. Utility of the antigen-specific interferon-gamma assay for the management of tuberculosis. Curr Opin Pulm Med 2005;11:195-202. [Crossref] [PubMed]
- Lee JY, Choi HJ, Park IN, et al. Comparison of two commercial interferon-gamma assays for diagnosing Mycobacterium tuberculosis infection. Eur Respir J 2006;28:24-30. [Crossref] [PubMed]
- Mori T, Sakatani M, Yamagishi F, et al. Specific detection of tuberculosis infection: an interferon-gamma-based assay using new antigens. Am J Respir Crit Care Med 2004;170:59-64. [Crossref] [PubMed]
- Pai M, Joshi R, Dogra S, et al. Serial testing of health care workers for tuberculosis using interferon-gamma assay. Am J Respir Crit Care Med 2006;174:349-55. [Crossref] [PubMed]
- Veerapathran A, Joshi R, Goswami K, et al. T-cell assays for tuberculosis infection: deriving cut-offs for conversions using reproducibility data. PLoS One 2008;3:e1850.
- Goodwin DJ, Mazurek GH, Campbell BH, et al. Automation of an interferon-γ release assay and comparison to the tuberculin skin test for screening basic military trainees for Mycobacterium tuberculosis infection. Mil Med 2014;179:333-41. [Crossref] [PubMed]
- Metcalfe JZ, Cattamanchi A, McCulloch CE, et al. Test variability of the QuantiFERON-TB gold in-tube assay in clinical practice. Am J Respir Crit Care Med 2013;187:206-11. [Crossref] [PubMed]
- Tagmouti S, Slater M, Benedetti A, et al. Reproducibility of interferon gamma (IFN-γ) release Assays. A systematic review. Ann Am Thorac Soc 2014;11:1267-76. [Crossref] [PubMed]
- Thanassi W, Noda A, Hernandez B, et al. Delineating a retesting zone using receiver operating characteristic analysis on serial QuantiFERON tuberculosis test results in US healthcare workers. Pulm Med 2012;2012:291294.
- Guyot-Revol V, Innes JA, Hackforth S, et al. Regulatory T cells are expanded in blood and disease sites in patients with tuberculosis. Am J Respir Crit Care Med 2006;173:803-10. [Crossref] [PubMed]


