Survival prognostic factors in unresectable/advanced primary thoracic sarcomas
Introduction
Soft tissue sarcomas are highly aggressive malignancies of mesenchymal origin that account for approximately 1% of cancers in adults (1,2). These tumors have an increases heterogeneity making their diagnosis and treatment complicated. Due to their low incidence, elevated complexity and tremendous heterogeneity, diagnosis is complicated even for expert pathologists. According to the World Health Organization (WHO) Classification of Tumors of Soft Tissue and Bone there are more than 100 different histologic subtypes (3,4). The most common histology in adult soft tissue sarcomas regardless of location include angiosarcoma, liposarcoma, leiomyosarcoma and undifferentiated sarcoma, however these may differ depending on location (5). Regarding location, the most common site of soft tissue sarcomas include the limbs (60%), followed by retro or intraperitoneal sites (20%) and trunk (10%) (6). Location is considered an important prognostic factor as sarcomas located in the extremities have a better survival (34 months) compared to retroperitoneal (21 months) and trunk sarcomas (20 months) (7). Management depends on location and disease stage, in localized tumors, treatment is based on surgery with or without radiotherapy, however, in non resectable tumors multiple chemotherapeutic regimens based on doxorubicin are recommended (8-10). Overall, the current chemotherapeutic response rate is of 20% with a median overall survival (OS) of less than 2 years (11).
Primary chest wall sarcomas (CWS) account for <5% of all thoracic neoplasms, and more than 50% of cases are malignant (12,13). Primary CWS are subdivided in soft tissue and bony origin. Usually, the most common histologic subtypes include Ewing sarcoma, primitive neuroectodermal, chondrosarcoma, malignant fibrous histiocytoma, osteosarcoma, synovial sarcoma and fibrosarcoma (14). Various prognostic factors have been studied including sex, histopathologic subtype, but the most important ones are resection margin status in which R0 margins are recommended as well as tumor size in which those <5 cm have a better prognosis (15-17).
Primary pulmonary sarcoma (PPS) is an extremely rare and aggressive tumor with an incidence ranging between 0.1–0.5% of all lung malignant neoplasia (18,19). PPS can originate from mesenchymal elements of bronchial walls, vessels, or pulmonary stroma. The most common subtypes identified in PPS are synovial sarcomas, undifferentiated sarcomas, fibroblastic and leiomyosarcomas (3,20). Unfortunately, like CWS most of these tumors are diagnosed in advance stages as symptoms present only when anatomical structures including the heart, thoracic wall or esophagus are infiltrated. The optimal treatment is complete surgical resection, however in many cases of advanced or metastatic disease it is impossible to perform, and surgery is left to alleviate symptoms (18,21). PPS generally have low OS; however, survival outcomes differ significantly between surgical versus non-surgical PPS. In resected PPS median OS ranges from 22–39.6 months, versus unresectable cases with a 4.9-month OS (18,22). When comparing PPS versus extremity sarcomas a 5-year OS of 35% was reported (20).
This retrospective study focuses on advanced/unresectable soft tissue primary thoracic sarcomas (PTS), including PPS and primary CWS with the goal of identifying prognostic factors which have not been clearly identified in unresectable scenarios compared to resectable disease. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-472/rc).
Methods
This was a descriptive, observational, retrospective cohort study that included 157 patients from the last 5 years (2016–2021) with confirmed diagnosis of advanced/unresectable primary thoracic sarcoma, including primary pulmonary sarcomas (n=50) and CWS (n=107) in which patient clinical characteristics and tumor characteristics were evaluated with the goal of identifying prognostic factors in PTS of the lung.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Instituto Nacional de Enfermedades Respiratorias Institutional Ethics Board with committee approval number C28-21 and individual consent for this retrospective analysis was waived.
The study population met the following criteria: patients older than 18 years of age with a confirmed histopathologic diagnosis of primary thoracic sarcoma, unresectable tumors, and Eastern Cooperative Oncology Group (ECOG) status 0–4. The following clinical and pathologic variables were reviewed: age, sex, smoking status, asbestos exposure, ECOG 0–4, type 2 diabetes mellitus, systemic arterial hypertension, histopathologic diagnosis, signs and symptoms at diagnosis, number of treatment lines, chemotherapy regimen used, adverse effects, progression-free survival (PFS), OS and response as per Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST 1.1) using computed tomography scan. These variables were analyzed and correlated with PFS and OS. Biased analysis of results were avoided by only studying those cases of unresectable/advanced thoracic sarcomas that met all inclusion criteria in the specific period of time (2016–2021).
Statistical analysis
SPSS software version 24.0 (IBM, Armonk, NY, USA) was used for analysis, including the univariate and multivariate analysis. The variables were expressed as median values, together with total values and percentages. A univariate and multivariate analysis was performed using the Cox-regression model. PFS was measured in months from date of treatment initiation to date of progression and OS was measured in months from date of diagnosis to date of death. Both PFS and OS were graphed using a Kaplan-Meier plot. The criterion for statistical significance was P<0.05. Missing data was excluded when performing the statistical analysis for that specific variable. All financially related issues from the study were absorbed by the investigation group.
Results
Demographic information
During the 5-year period, a total of 157 patients were identified with advanced/unresectable PTS, 50 patients diagnosed with primary pulmonary sarcomas (PPS) and 107 patients with CWS. The mean age of patients was 51.8±16.9 years (range, 18–90 years), with 119 patients (75.8%) being under 65 years of age. Regarding gender, 87 patients were female (55.4%) and 70 patients male (44.6%). Comorbidities that patients had included systemic hypertension in 29 patients (18.5%) and type 2 diabetes mellitus in 25 patients (15.9%). A total of 30 patients (19.1%) were either current or former smokers with a mean pack-years of 16.78±16.6 (range, 1–55) and 17 patients (10.8%) had asbestos exposure.
PTS have an unspecific clinical presentation making their diagnosis difficult and generally in advanced stages. In our population the most common symptom was cough with 92 patients (58.4%) followed by thoracic pain with 87 patients (55.4%) and dyspnea with 71 patients (45.2%). Additional symptoms included weight loss with 42 patients (26.8%), fatigue with 38 patients (24.2%) and 37 patients (23.6%) presented hemoptysis. Based on clinical presentation at the time of diagnosis, the ECOG system is used to evaluate performance status. The most common ECOG at time of diagnosis was ECOG 1 with 61 patients (38.9%), followed by ECOG 0 with 56 patients (35.7%), 33 patients (21.0%) as ECOG 2 and only 7 patients with ECOG 3. In summary, 117 patients (74.5%) had an ECOG <2 and 40 patients (25.5%) had an ECOG ≥2. The full patients’ demographic characteristics are described in Table 1.
Table 1
| Characteristics | Values |
|---|---|
| Gender, n (%) | |
| Male | 70 (44.6) |
| Female | 87 (55.4) |
| Age (years) | |
| Mean ± SD | 51.8±16.9 |
| Range | 18–90 |
| ≥65, n (%) | 38 (24.2) |
| <65, n (%) | 119 (75.8) |
| Comorbidities, n (%) | |
| Systemic hypertension | 29 (18.5) |
| Type 2 diabetes mellitus | 25 (15.9) |
| Tobacco exposure, n (%) | 30 (19.1) |
| Pack-years, mean ± SD | 16.78±16.6 |
| Range | 1–55 |
| Asbestos exposure, n (%) | 17 (10.8) |
| ECOG, n (%) | |
| 0 | 56 (35.7) |
| 1 | 61 (38.9) |
| 2 | 33 (21.0) |
| 3 | 7 (4.4) |
| Clinical presentation, n (%) | |
| Cough | 92 (58.6) |
| Dyspnea | 71 (45.2) |
| Thoracic pain | 87 (55.4) |
| Fatigue | 38 (24.2) |
| Weight loss | 42 (26.8) |
| Hemoptysis | 37 (23.6) |
SD, standard deviation; ECOG, Eastern Cooperative Oncology Group.
Tumor characteristics
The diagnosis of PTS requires a histopathological diagnosis and therefore a biopsy is required. Multiple types of biopsy methods can be performed, and its decision is based on location, performance status and medical professional experience. The most common method of biopsy performed was ultrasound guided with a total of 41 patients (26.1%), followed by thoracoscopy with 33 patients (21.0%), guided via computed tomography biopsy with 24 patients (15.3%) and 21 patients via bronchoscopy (13.4%).
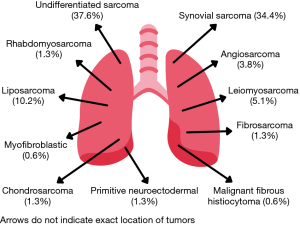
According to the 2020 WHO Classification of Soft Tissue Tumors, when a definitive histologic type could be determined, the most common histologic types were synovial sarcoma, 54 of 157 (34.4%) and liposarcoma, 16 of 157 (10.2%). However, the largest group were undifferentiated sarcomas with 59 patients (37.6%). Microscopic examination of tissue allowed us to sub differentiate synovial sarcomas and undifferentiated sarcomas. Regarding synovial sarcomas, 21 (13.4%) were monophasic, 17 (10.8%) biphasic and 16 (10.2%) were unable to be sub-classified. On the other hand, subclassification of undifferentiated sarcomas identified 10 (6.4%) cases of pleomorphic and fusiform sarcomas each, 33 patients (21.0%) as high-grade sarcomas and 5 patients (3.2%) as low-grade sarcomas. Figure 1 displays the different types of sarcomas histologies with their respective percentage and Table 2 the specific tumor characteristics.
Table 2
| Characteristics | Values |
|---|---|
| Biopsy method, n (%) | |
| USG guided | 41 (26.1) |
| CT guided | 24 (15.3) |
| Bronchoscopy | 21 (13.4) |
| Thoracoscopy | 33 (21.0) |
| Mediastinoscopy | 17 (10.8) |
| Surgical | 19 (12.1) |
| Unknown | 2 (1.3) |
| Histopathological diagnosis, n (%) | |
| Synovial sarcoma | 54 (34.4) |
| Monophasic synovial | 21 (13.4) |
| Biphasic synovial | 17 (10.8) |
| Not classified | 16 (10.2) |
| Angiosarcoma | 6 (3.8) |
| Liposarcoma | 16 (10.2) |
| Leiomyosarcoma | 8 (5.1) |
| Fibrosarcoma | 2 (1.3) |
| Primitive neuroectodermal | 2 (1.3) |
| Epithelioid sarcoma | 4 (2.5) |
| Myofibroblastic sarcoma | 1 (0.6) |
| Chondrosarcoma | 2 (1.3) |
| Malignant fibrous histiocytoma | 1 (0.6) |
| Rhabdomyosarcoma | 2 (1.3) |
| Undifferentiated sarcoma | 59 (37.6) |
| High grade sarcoma | 33 (21.0) |
| Low grade sarcoma | 5 (3.2) |
| Pleomorphic sarcoma | 10 (6.4) |
| Fusiform sarcoma | 10 (6.4) |
| Not specified | 1 (0.6) |
| Number of chemotherapy cycles, n (%) | |
| Two | 5 (3.2) |
| Three | 15 (9.6) |
| Four | 43 (27.4) |
| Five | 31 (19.7) |
| Six | 59 (37.6) |
| Eight | 4 (2.5) |
| Best RECIST, n (%) | |
| Partial response | 90 (57.3) |
| Complete response | 6 (3.8) |
| Stable disease | 34 (21.7) |
| Progressive disease | 27 (17.2) |
| ORR, n (%) | 96 (61.1) |
USG, ultrasound; CT, computed tomography; RECIST, Response Evaluation Criteria in Solid Tumors; ORR, overall response rate.
Treatment characteristics
All cases of PTS in our population were classified as metastatic tumors or irresectable tumors due to mediastinal pleural, costal of muscle infiltration which corresponds to a stage T4 based on the tumor-node-metastasis (TNM) staging system therefore, treatment was chemotherapy based in all cases. It is important to mention that patients in our population did not receive any radiation therapy in the management of their disease because tumors had a voluminous mass or were metastatic which made them non-candidates for radiotherapy or surgery therefore, treatment was fully based on chemotherapeutic regimens. Depending on patient response, patients received different number of chemotherapy cycles. Most patients received six cycles 59 of 157 (37.6%), followed by four cycles, 43 of 157 (27.4%), 31 patients (19.7%) received five cycles and 15 patients (9.6%) three cycles. Treatment response was evaluated via the RECIST, partial response was observed in 90 patients (57.3%), stable disease in 34 patients (21.7%), progressive disease in 27 patients and only 6 patients (3.8%) had complete response. Based on these results, 96 patients (61.1%) had an overall response rate (ORR) (partial response plus complete response) and 82.8% of patients had clinical benefit (partial response + complete response + stable disease). Table 2 displays treatment results and response. The chemotherapeutical regimens used in our population were 92 patients treated with epirubicin + cisplatin + ifosfamide (E/C/I), 5 patients with gemcitabine + paclitaxel, 17 patients with doxorubicin + cisplatin, 1 patient with gemcitabine + carboplatin, 31 patients with doxorubicin + ifosfamide, 10 patients with etoposide + carboplatin and 1 patient with gemcitabine + docetaxel. It is important to mention that results analyzing efficacy between regimens is not the main objective of the current study and this analysis will be evaluated in an additional study from our research group.
PFS prognostic factors
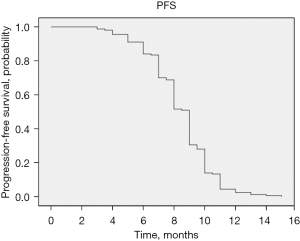
The median PFS of patients with unresectable PTS was 9 months [95% confidence interval (CI): 8.717–9.283 months], displayed in Figure 2. Based on clinical characteristics and tumor histology, our goal was to identify prognostic factors associated with an increased PFS. Therefore, a univariable analysis including all previously mentioned variables was performed and multivariable analysis including ECOG ≥2, RECIST and responders to chemotherapy (number of cycles) was performed.
Based on these analyses an ECOG ≥2, with a hazard ratio (HR) of 1.659 (95% CI: 1.148–2.397; P=0.007), chemotherapy responders HR of 0.527 (95% CI: 0.446–0.622; P<0.001), RECIST HR 1.376 (95% CI: 1.189–1.593; P<0.001) and the ORR HR of 0.550 (95% CI: 0.396–0.763; P<0.001), worked as prognostic factors in the univariable analysis. In the multivariable analysis chemotherapy responders with a HR of 0.561 (95% CI: 0.475–0.662; P<0.001), RECIST HR of 1.277 (95% CI: 1.108–1.473; P<0.001) and an ECOG ≥2 HR of 1.523 (95% CI: 1.051–2.207; P=0.026) all impacted PFS. A further survival analysis dividing patients in two groups ECOG 0–1 and ≥2 was performed (no patients with ECOG 4 were included as this status contraindicates a chemotherapeutic treatment) being significant (P=0.0017) with a median PFS of 9 months with a HR of 0.6152 (95% CI: 0.4071–0.9296) for patients with an ECOG of 0–1, and a median PFS of 8 months with a HR of 1.626 (95% CI: 1.076–2.456) for those with an ECOG 2–3 (Figure 3).
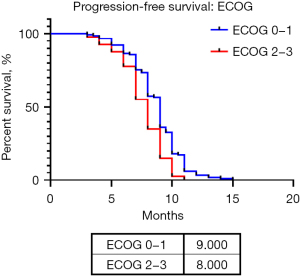
These results associate a longer progression-free time with responders to chemotherapy, ECOG and an adequate response (RECIST). It is important to mention that RECIST was included in the multivariable analysis instead of ORR as both focus on treatment response. Based on these results, we demonstrate that patients that respond better to chemotherapy have a better control of the disease if an adequate ECOG is maintained during the treatment course. Finally, based on the chemotherapeutic cycles received by our population we observe the following median months for PFS: epirubicin + cisplatin + ifosfamide (9 months), gemcitabine + paclitaxel (8 months), doxorubicin + cisplatin (9 months), gemcitabine + carboplatin (6 months), doxorubicin + ifosfamide (8 months), etoposide + carboplatin (6.5 months) and gemcitabine + docetaxel (5 months).
OS prognostic factors
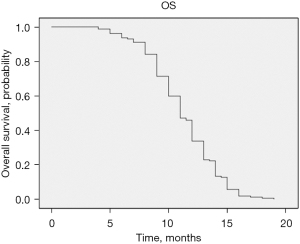
The median OS of patients with unresectable PTS was 11 months (95% CI: 10.402–11.958), displayed in Figure 4. With the goal of identifying prognostic factors associated with an increased OS, the same approach as the one performed for PFS was done. In the univariable analysis we found similar results with a HR for ECOG ≥2 being 1.767 (95% CI: 1.225–2.547; P=0.002), responders to chemotherapy HR of 0.655 (95% CI: 0.572–0.752; P<0.001), RECIST HR of 1.272 (95% CI: 1.103–1.467; P<0.001) and the ORR HR of 0.624 (95% CI: 0.451–0.863; P=0.004). In the multivariable analysis both better response to chemotherapy with a HR of 0.689 (95% CI: 0.596–0.798; P<0.001) and an ECOG ≥2 HR of 1.728 (95% CI: 1.96–2.496; P=0.004) impacted OS.
The same survival analysis as the one performed for PFS regarding ECOG was performed for OS. The analysis was significant (P=0.0006) with a median OS of 12 months with a HR of 0.5761 (95% CI: 0.3778–0.8785) for patients with an ECOG of 0–1, and a median OS of 10 months with a HR of 1.736 (95% CI: 1.138–2.647) for those with an ECOG ≥2 (Figure 5).
Based on these results maintaining an adequate ECOG under 2 together with a better response to chemotherapy regimens (receiving an adequate number of chemotherapeutic cycles) have an impact in patients’ survival. Even though RECIST focuses on treatment response, in the multivariable analysis it does not have an impact in OS compared to PFS. Concluding that patient’s survival is not impacted by treatment response (RECIST) but is impacted by an ECOG <2 and responders to chemotherapy. Table 3 displays the univariate and multivariate analysis for each variable and its impact in both PFS and OS. Regarding the chemotherapies used and their impact in OS the following median survival in months were obtained: epirubicin + cisplatin + ifosfamide (12 months), gemcitabine + paclitaxel (9 months), doxorubicin + cisplatin (12 months), gemcitabine + carboplatin (8 months), doxorubicin + ifosfamide (11 months), etoposide + carboplatin (9 months) and gemcitabine +docetaxel (14 months).
Table 3
| Survival predictor | PFS | OS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariable | Multivariable | Univariable | Multivariable | ||||||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | ||||
| >65 years old | 1.057 (0.731–1.530) | 0.767 | – | – | 1.120 (0.773–1.623) | 0.548 | – | – | |||
| Gender | 1.100 (0.800–1.513) | 0.557 | – | – | 1.037 (0.754–1.426) | 0.824 | – | – | |||
| ECOG <2 | 1.211 (1.010–1451) | 0.038 | 1.282 (1.071–1.534) | 0.007 | |||||||
| ECOG ≥2 | 1.659 (1.148–2.397) | 0.007 | 1.523 (1.051–2.207) | 0.026 | 1.767 (1.225–2.547) | 0.002 | 1.728 (1.196–2.496) | 0.004 | |||
| Hypertension | 1.018 (0.679–1.529) | 0.932 | – | – | 1.131 (0.755–1.695) | 0.550 | – | – | |||
| Type 2 diabetes mellitus | 1.279 (0.833–1.964) | 0.261 | – | – | 1.377 (0.895–2.117) | 0.145 | – | – | |||
| Tobacco exposure | 1.183 (0.792–1.768) | 0.411 | – | – | 1.072 (0.718–1.603) | 0.733 | – | – | |||
| Asbestos exposure | 0.893 (0.539–1.479) | 0.660 | – | – | 1.030 (0.622–1.707) | 0.908 | – | – | |||
| Cough | 0.895 (0.650–1.232) | 0.496 | – | – | 0.879 (0.639–1.211) | 0.431 | – | – | |||
| Dyspnea | 0.795 (0.575–1.100) | 0.166 | – | – | 0.770 (0.557–1.065) | 0.114 | – | – | |||
| Thoracic pain | 0.983 (0.717–1.349) | 0.916 | – | – | 0.912 (0.663–1.254) | 0.570 | – | – | |||
| Fatigue | 0.864 (0.598–1.247) | 0.435 | – | – | 1.026 (0.711–1.480) | 0.892 | – | – | |||
| Weight loss | 1.163 (0.812–1.666) | 0.410 | – | – | 1.243 (0.869–1.777) | 0.233 | – | – | |||
| Hemoptysis | 0.877 (0.605–1.272) | 0.490 | – | – | 0.827 (0.571–1.199) | 0.316 | – | – | |||
| Type of biopsy | 0.972 (0.890–1.062) | 0.534 | – | – | 0.966 (0.882–1.057) | 0.448 | – | – | |||
| Pathology diagnosis | 0.990 (0.919–1.067) | 0.794 | – | – | 0.992 (0.917–1.073) | 0.838 | – | – | |||
| Responders to chemotherapy | 0.527 (0.446–0.622) | <0.001 | 0.561 (0.475–0.662) | <0.001 | 0.655 (0.572–0.752) | <0.001 | 0.689 (0.596–0.798) | <0.001 | |||
| RECIST | 1.376 (1.189–1.593) | <0.001 | 1.277 (1.108–1.473) | <0.001 | 1.272 (1.103–1.467) | <0.001 | 1.143 (0.990–1.320) | 0.068 | |||
| ORR | 0.550 (0.396–0.763) | <0.001 | NA | NA | 0.624 (0.451–0.863) | 0.004 | NA | NA | |||
PFS, progression-free survival; OS, overall survival; HR, hazard ratio; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; RECIST, Response Evaluation Criteria in Solid Tumors; ORR, overall response rate; NA, not analyzed.
Discussion
PTS including primary pulmonary and CWS are extremely rare malignancies, with limited literature evidence specially in unresectable/advanced scenarios. Our study analyzed 157 cases with diagnosis of unresectable/advanced soft tissue PTS treated at a national reference institute in a 5-year period. To our knowledge, our series represents the highest number of cases of PTS in an advanced/unresectable setting reported in the literature. Location of PTS is confusing as they may appear in the lung or chest wall and sometimes are not well defined, additionally their analysis can also be confused with metastases.
When comparing our epidemiological results with published literature studies focused on PPS and primary CWS report similar incidence between male and female ranging from 44–64% being either male or female with a mean age of 43–62 years old, demonstrating that these tumors do not have a specific age and sex predisposition (18,20,21,23-25).
Thoracic tumors including PPS and primary CWS do not have a specific clinical presentation, with symptomatology similar to other pulmonary neoplasms including cough, chest pain, dyspnea, and in some cases pulmonary embolism. In our study, the most common symptom reported was cough in 58.4% of cases followed by thoracic pain (55.4%) and dyspnea (45.2%), similar to what has been reported in the literature. A study by Gołota et al. (22), focused on PPS reported that hemoptysis had a significant impact in patient’s outcome. In our population, none of these symptoms impacted patient survival, probably due to the stage of the disease.
Histopathological analysis and classification of sarcomas is difficult, even for expert pathologists. The most recent classification used for sarcomas is the WHO Classification of Soft Tissue Tumors. When considering both PPS and primary CWS our study identifies that the most common histology is divided between synovial sarcomas and undifferentiated sarcomas. Previous studies have reported synovial sarcoma as the most common histology specially in PPS similar to what has been reported in our present study (34.4%) with 10.8% being biphasic (18,20). In cases where histology is not reported, undifferentiated sarcomas is the most common histology, similar to what was reported in our current study (37.6%) with other common histologies reported in the literature specially in CWS being primitive neuroectodermal, chondrosarcoma and dermatofibrosarcoma (18,20,21,23-27). Of these undifferentiated sarcomas, our population had 21% cases of high grade sarcomas and 6.4% pleomorphic and fusiform, respectively.
PTS are a heterogenous group of tumors that present diverse biological behaviors, therefore having an aggressive course and poor prognosis. Our study, which focused on advanced tumors did not find a correlation between histological type and PFS or OS. Differently, studies done in resectable PPS a superior survival was reported in grade 1 and grade 2 tumors with a 60-month survival and a 1- and 5-year OS of 93.2% and 88.5%, respectively in resectable CWS (23,28). Recent studies have demonstrated similar results to our study, in which tumor histology or grade are not prognostic factors, even in locally advanced or early stages. It is important to mention that factors associated with a better outcome in locally resectable disease include tumor size/volume and complete resection, factors that do not apply to our population (24,25,29,30).
Regarding the main objective of the current study, which was identifying prognostic factors in advanced/unresectable PTS, for PFS responders to chemotherapy (analyzed as the number of chemotherapeutic cycles received) a poor performance status classified as an ECOG ≥2 and an increase in RECIST (which evaluates treatment response) were identified. These results indicate that patients who receive a higher number of chemotherapeutic cycles which translates to a better response to chemotherapy, with an ECOG <2 and an adequate response are associated with a longer progression-free period. On the other hand, for OS, responders to chemotherapy and an ECOG ≥2 were identified as prognostic factors. The response to treatment either by evaluating RECIST or ORR was not associated with an increased survival, different from what was observed for PFS. ORR was not associated with a better prognosis because the response rate is not a surrogate variable. Patients with this type of tumor have a poor prognosis and the fact that they respond to chemotherapy does not indicate that they will live longer because the moment they progress they generally and unfortunately pass away in a few months. Patients that have a longer OS are those with an ECOG <2 and respond to chemotherapy, therefore receiving a higher number of chemotherapeutic cycles, regardless of treatment response via RECIST.
It is important to mention that even though it could be self-explanatory that the number of chemotherapeutic cycles received correlates with a longer survival outcome, the analysis performed, and results obtained demonstrate that those patients that receive more chemotherapeutic cycles are those that are responding better to the chemotherapeutical regimen received and this is the response that translates to a better survival outcome.
Given that PTS are tumors with poor prognosis where no standardized treatments are established for advanced/unresectable disease, our findings suggest that offering chemotherapy-based regimens early in the disease offer an adequate control of the disease with a 61.1% ORR and a better prognosis. Our study offers an answer regarding the insights offered by studies in which additional information were necessary to define optimal chemotherapeutic regimens for thoracic sarcomas with our results demonstrating that a correct number of cycles, an adequate status performance and response rate are associated with a better prognosis (21,24,25).
The present study demonstrates the greatest number of patients reported with soft tissue PTS in advanced/unresectable scenarios treated fully with chemotherapeutical regimens and is the first study to evaluate prognostic factors intervening in disease progression and OS. A comparison of the efficacy between chemotherapeutical regimens used will be reported in a different study of our authorship. Even though the study is focused on the Latino population, due to the rarity of these tumors in the general population our results can be used as predictive factors in the management of these tumors and recommends the analysis of these predictive factors in prospective randomized studies.
Conclusions
PTS are rare thoracic tumors with a poor prognosis specially in advanced/unresectable scenarios. The most common histologic type when identified is synovial sarcoma. PFS in advanced/unresectable PTS is of 9 months and OS of 11 months. In our current study focused on advanced/unresectable PTS, patients age, gender, comorbidities, tobacco exposure, clinical presentation, type of biopsy and histopathological diagnosis do not impact a patient’s outcome. On the other hand, patients’ good performance status (ECOG <2) and a good response to chemotherapy are associated with a better response, both in progression-free and in OS. Regarding OS, treatment response evaluated either by RECIST or ORR does not offer a prognostic factor.
Although patients have a poor prognosis, an adequate and personalized systemic therapy taking in consideration the prognostic factors previously mentioned, offers a better treatment response with a 61.1% ORR.
The inherent limitations of any retrospective series which were assessed by establishing precise and specific inclusion criteria. This is a study performed in a national center for respiratory diseases and tumors for the Latino population, each medical center that studies sarcomas should focus on identifying the most common histologic subtypes as these may vary between populations and affect patient outcome.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-472/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-472/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-472/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-472/coif). JRRC and JAAA have received research grants for other research protocols/projects, consulting fees working as speaker bureau and participating as advisory board for Astra Zeneca, Pfizer, Bayer, Roche, Roche Diagnostics, Novartis, Merch Sharp and Dohme (MSD), Bristol Myers Squibb (BMS), Takeda, Celltrion, Daiichi Sankyo, Novartis, GSK, Amgen, Eli Lilly. However, none of these are associated to the current study. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Instituto Nacional de Enfermedades Respiratorias Institutional Ethics Board with committee approval number C28-21 and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Brennan MF, Antonescu CR, Moraco N, et al. Lessons learned from the study of 10,000 patients with soft tissue sarcoma. Ann Surg 2014;260:416-21; discussion 421-2. [Crossref] [PubMed]
- Gamboa AC, Gronchi A, Cardona K. Soft-tissue sarcoma in adults: An update on the current state of histiotype-specific management in an era of personalized medicine. CA Cancer J Clin 2020;70:200-29. [Crossref] [PubMed]
- Sbaraglia M, Bellan E, Dei Tos AP. The 2020 WHO Classification of Soft Tissue Tumours: news and perspectives. Pathologica 2021;113:70-84. [Crossref] [PubMed]
- Fletcher CDM, Unni KK, Mertens F. World Health Organisation classification of tumours: pathology and genetics of tumours of soft tissue and bone. Lyon: IARC Press, 2002.
- Blay JY, Honoré C, Stoeckle E, et al. Surgery in reference centers improves survival of sarcoma patients: a nationwide study. Ann Oncol 2019;30:1143-53. [Crossref] [PubMed]
- Clark MA, Fisher C, Judson I, et al. Soft-tissue sarcomas in adults. N Engl J Med 2005;353:701-11. [Crossref] [PubMed]
- Singer S, Corson JM, Demetri GD, et al. Prognostic factors predictive of survival for truncal and retroperitoneal soft-tissue sarcoma. Ann Surg 1995;221:185-95. [Crossref] [PubMed]
- Martin-Tellez KS, van Houdt WJ, van Coevorden F, et al. Isolated limb perfusion for soft tissue sarcoma: Current practices and future directions. A survey of experts and a review of literature. Cancer Treat Rev 2020;88:102058. [Crossref] [PubMed]
- Larrier NA, Czito BG, Kirsch DG. Radiation Therapy for Soft Tissue Sarcoma: Indications and Controversies for Neoadjuvant Therapy, Adjuvant Therapy, Intraoperative Radiation Therapy, and Brachytherapy. Surg Oncol Clin N Am 2016;25:841-60. [Crossref] [PubMed]
- Meyer M, Seetharam M. First-Line Therapy for Metastatic Soft Tissue Sarcoma. Curr Treat Options Oncol 2019;20:6. [Crossref] [PubMed]
- Du XH, Wei H, Zhang P, et al. Heterogeneity of Soft Tissue Sarcomas and Its Implications in Targeted Therapy. Front Oncol 2020;10:564852. [Crossref] [PubMed]
- Nakahashi N, Emori M, Tsuchie H, et al. Treatment outcome of chest wall soft tissue sarcomas: Analysis of prognostic factors. J Surg Oncol 2019;120:1235-40. [Crossref] [PubMed]
- Lawrence W Jr, Donegan WL, Natarajan N, et al. Adult soft tissue sarcomas. A pattern of care survey of the American College of Surgeons. Ann Surg 1987;205:349-59. [Crossref] [PubMed]
- Gladish GW, Sabloff BM, Munden RF, et al. Primary thoracic sarcomas. Radiographics 2002;22:621-37. [Crossref] [PubMed]
- Gross JL, Younes RN, Haddad FJ, et al. Soft-tissue sarcomas of the chest wall: prognostic factors. Chest 2005;127:902-8. [Crossref] [PubMed]
- Wald O, Islam I, Amit K, et al. 11-year experience with Chest Wall resection and reconstruction for primary Chest Wall sarcomas. J Cardiothorac Surg 2020;15:29. [Crossref] [PubMed]
- Mesko NW, Bribriesco AC, Raymond DP. Surgical Management of Chest Wall Sarcoma. Surg Oncol Clin N Am 2020;29:655-72. [Crossref] [PubMed]
- Robinson LA, Babacan NA, Tanvetyanon T, et al. Results of treating primary pulmonary sarcomas and pulmonary carcinosarcomas. J Thorac Cardiovasc Surg 2021;162:274-84. [Crossref] [PubMed]
- Mjid Meriem, Blibech Hana, Toujani Sonia, et al. Rare primary pulmonary tumors. Eur Respir J 2015;46:4307.
- Spraker MB, Bair E, Bair R, et al. An analysis of patient characteristics and clinical outcomes in primary pulmonary sarcoma. J Thorac Oncol 2013;8:147-51. [Crossref] [PubMed]
- Li AX, Resio BJ, Canavan ME, et al. Outcomes of surgically managed primary lung sarcomas: a National Cancer Database analysis. J Thorac Dis 2021;13:3409-19. [Crossref] [PubMed]
- Gołota J, Osowiecka K, Orłowski T. Primary pulmonary sarcoma — long-term treatment outcomes and prognostic factors. Kardiochir Torakochirurgia Pol 2018;15:162-9. [Crossref] [PubMed]
- Tsukushi S, Nishida Y, Sugiura H, et al. Soft tissue sarcomas of the chest wall. J Thorac Oncol 2009;4:834-7. [Crossref] [PubMed]
- Bagheri R, Haghi SZ, Kalantari MR, et al. Primary malignant chest wall tumors: analysis of 40 patients. J Cardiothorac Surg 2014;9:106. [Crossref] [PubMed]
- Kachroo P, Pak PS, Sandha HS, et al. Single-institution, multidisciplinary experience with surgical resection of primary chest wall sarcomas. J Thorac Oncol 2012;7:552-8. [Crossref] [PubMed]
- Duran-Moreno J, Kokkali S, Ramfidis V, et al. Primary Sarcoma of the Lung — Prognostic Value of Clinicopathological Characteristics of 26 Cases. Anticancer Res 2020;40:1697-703. [Crossref] [PubMed]
- Gangopadhyay A, Nandy K, Puj K, et al. Primary chest wall sarcoma; a single institution experience of 3 years. Cancer Treat Res Commun 2021;27:100326. [Crossref] [PubMed]
- Janssen JP, Mulder JJ, Wagenaar SS, et al. Primary sarcoma of the lung: a clinical study with long-term follow-up. Ann Thorac Surg 1994;58:1151-5. [Crossref] [PubMed]
- Huwer H, Kalweit G, Straub U, et al. Pulmonary carcinosarcoma: diagnostic problems and determinants of the prognosis. Eur J Cardiothorac Surg 1996;10:403-7. [Crossref] [PubMed]
- Petrov DB, Vlassov VI, Kalaydjiev GT, et al. Primary pulmonary sarcomas and carcinosarcomas—postoperative results and comparative survival analysis. Eur J Cardiothorac Surg 2003;23:461-6. [Crossref] [PubMed]