Quality assessment and its influencing factors of lung cancer clinical research registration: a cross-sectional analysis
Introduction
Clinical research refers to scientific investigation involving human subjects and is broadly divided into interventional and observational studies (1). Details of clinical research must be recorded and shared to improve transparency, minimize biases, and avoid duplication of studies (2,3). Clinical trial registration is a key part of this, and calls for a clinical trial registry were first made in 1986 (4). The United States formally proposed the concept of clinical trial registration in 1970 (4), and ClinicalTrials.gov, a registry website of clinical trials maintained by the National Library of Medicine (NLM)-National Institutes of Health, was developed in 1997 (5,6). Since then, ClinicalTrials.gov has become the largest database for clinical research registration authorized by World Health Organization’s (WHO) International Clinical Trials Registry Platform, and provides the most comprehensive information about ongoing and completed clinical studies (7). Initially, ClinicalTrials.gov was generally used to report the details of study protocols. In 2007, the U.S. Food and Drug Administration (FDA) required timely reporting of basic summary results on this database by the study sponsor(s) within 1 year of completion of data collection (for the pre-specified primary outcome) or within 1 year of early termination (8,9). An increasing number of clinical studies have since been registered on ClinicalTrials.gov and there is growing awareness of registration as institutions increasingly require its use (10). However, there are still many studies with untimely or inaccurate registration and unavailable results (10,11).
To standardize clinical trial registration, the WHO issued a registration standard in May 2007, the Trial Registration Data Set (TRDS), which specified a minimum of 20 registration items as the international standard (12). The TRDS was updated to version 1.3.1 in November 2017, which contains 24 items (13). In November 2018, three optional items were also added (14). The TRDS regulates the registration criteria for interventional studies and some studies had modified TRDS to evaluate the registration quality of clinical trials (10,15). To improve the efficiency and accuracy of registration, ClinicalTrials.gov also issued guidance documents, including the ‘ClinicalTrials.gov Protocol Registration Data Element Definitions for Interventional and Observational Studies’ (16) and the ‘ClinicalTrials.gov Results Data Element Definitions for Interventional and Observational Studies’. The latest versions of these were released in December 2020 (17).
According to the latest WHO data, oncology trials represent the largest proportion of all clinical research (18). Moreover, lung cancer, which is the second most commonly diagnosed cancer, was still the leading cause of cancer death according to GLOBOCAN 2020 (19). Collecting information on the quality of clinical studies in lung cancer research is key to summarizing the existing treatment technologies and informing future research. However, to date, few studies have explored the characteristics of lung cancer clinical research. One article, published in 2013, assessed the features in lung cancer clinical research registration since 2009 (20). Although a study has commented on the relationship between the publication rate and characteristics of lung cancer clinical trials (21), none have investigated the registration quality and influencing factors.
Complete records of clinical research details and sharing of clinical research results are conducive to improving the transparency, minimize biases, and avoid duplication of research. In this study, we carried out a comprehensive investigation of interventional and observational studies to summarize the general features of lung cancer clinical research registration (22). Additionally, we modified the TRDS in combination with the registration requirements of ClinicalTrials.gov, then extracted completed or terminated clinical studies that were first posted between 1 July 2007 and 7 July 2020 to appraise the registration quality. We also explored the factors affecting the quality of registrations. A better understanding of the quality and its influencing factors of lung cancer clinical research registration is important for further standardizing the registration.
Methods
Search and selection of relevant registered trials
A cross-sectional design was used in this study. On 7 July, 2021, we searched the ClinicalTrials.gov (https://clinicaltrials.gov/) database for relevant trials using the search term ‘Pulmonary Neoplasms OR Neoplasms, Lung OR Lung Neoplasm OR Neoplasm, Lung OR Neoplasms, Pulmonary OR Neoplasm, Pulmonary OR Pulmonary Neoplasm OR Lung Cancer OR Cancer, Lung OR Cancers, Lung OR Lung Cancers OR Pulmonary Cancer OR Cancer, Pulmonary OR Cancers, Pulmonary OR Pulmonary Cancers OR Cancer of the Lung OR Cancer of Lung.’ The inclusion criteria were as follows: (I) studies that were registered on ClinicalTrials.gov before 7 July 2021; and (II) clinical research on lung cancer, including interventional and observational studies. The exclusion criteria were as follows: (I) research involving solid tumors rather than lung cancer; and (II) research targeting multiple cancers (i.e., not limited to lung cancer).
Data extraction
Two investigators independently screened the clinical studies against the predefined inclusion and exclusion criteria. Any disagreements were resolved by discussion. Two authors independently extracted the following data from included clinical studies: (I) basic study information: registry number, title, start date, date when first posted, source of funding, and recruitment status; (II) participant information: sex, age, and sample size; (III) study design: allocation, masking, model, and primary purpose for interventional studies; model and time perspective for observational studies; and (IV) study type and results.
Quality evaluation
Two investigators independently appraised the quality of completed or terminated clinical studies on lung cancer, which were originally registered between 1 July 2007 and 7 July 2020. The quality assessment standard (Appendix 1) was developed according to the TRDS (V.1.3.1) (13), where the explanatory text of each item referred to the ‘ClinicalTrials.gov Protocol Registration Data Element Definitions for Interventional and Observational Studies’ (16) and ‘ClinicalTrials.gov Results Data Element Definitions for Interventional and Observational Studies’ documents (17). The modified TRDS included 22 points and was applicable to both interventional and observational studies; the sub-items for observational studies, including eligibility and study design, were also added. Additionally, item 22 on the ‘data monitoring committee’ was used to assess whether such a committee had been appointed for the study, especially given the importance of data management in clinical studies.
Information on the modified TRDS was provided on ClinicalTrial.gov. Complex items (i.e., Arm, Groups, and Interventions) were divided into different sub-items for evaluation, and each item/sub-item was given a score of 1 score if it was fully reported, or 0 if incompletely reported or missed. The total maximum score was 54, and the detailed scoring methods of each item are presented in Table 1. To increase the accuracy of scoring, the predefined rules were first tested on 50 registered studies and then subsequently applied to all records, and all investigators were trained on the scoring rules. Two researchers (QY and ZC) independently evaluated the trials, the results were double-checked, and problems or ambiguities were resolved by discussion with a third investigator (LC).
Table 1
| Category | Specifics | Number of records | Percentage of records (%) | |
|---|---|---|---|---|
| Study type* | Interventional study | 5,280 | 81.9 | |
| Observational study | 1,168 | 18.1 | ||
| Source of funding | Industry only | 1,751 | 27.2 | |
| Academic institutions only | 2,958 | 45.9 | ||
| Government only | 184 | 2.9 | ||
| Other | 1,555 | 24.1 | ||
| Study results | Has results | 1,019 | 15.8 | |
| No results available | 5,429 | 84.2 | ||
| Intervention type | Drug | 3,701 | 57.4 | |
| Device | 196 | 3.0 | ||
| Biological/vaccine | 429 | 6.7 | ||
| Procedure/surgery | 382 | 5.9 | ||
| Radiation | 325 | 5.0 | ||
| Behavioral | 157 | 2.4 | ||
| Genetic | 52 | 0.8 | ||
| Dietary supplement | 41 | 0.6 | ||
| Combination product | 28 | 0.4 | ||
| Diagnostic test | 103 | 1.6 | ||
| Other | 465 | 7.2 | ||
| Not reported | 569 | 8.8 | ||
| Information of trial recruitment | ||||
| Target sample size | 0–100 | 3,897 | 60.4 | |
| 101–500 | 1,789 | 27.8 | ||
| 501–1,000 | 370 | 5.7 | ||
| 1001–5,000 | 200 | 3.1 | ||
| 5001–99,999 | 51 | 0.8 | ||
| ≥100,000 | 3 | 0.1 | ||
| Not reported | 138 | 2.1 | ||
| Age | Children (0–17 y) | 2 | 0.0 | |
| Adults (18–65 y) | 17 | 0.3 | ||
| Older adults (66+ y) | 91 | 1.4 | ||
| Children, adults | 2 | 0.0 | ||
| Adults, older adults | 6,026 | 93.5 | ||
| All ages | 56 | 0.9 | ||
| Not reported | 254 | 3.9 | ||
| Gender | Male | 10 | 0.2 | |
| Female | 20 | 0.3 | ||
| Both | 6,411 | 99.4 | ||
| Not reported | 7 | 0.1 | ||
| Study design of interventional study (N=5,280) | ||||
| Allocation | Randomized | 2,211 | 41.9 | |
| Non-randomized | 825 | 15.6 | ||
| N/A** | 2,085 | 39.5 | ||
| Not reported | 159 | 3.0 | ||
| Intervention model | Single group | 2,538 | 48.1 | |
| Parallel | 2,289 | 43.4 | ||
| Crossover | 61 | 1.2 | ||
| Factorial | 32 | 0.6 | ||
| Sequential | 125 | 2.4 | ||
| Not reported | 235 | 4.5 | ||
| Masking | Single blind | 151 | 2.9 | |
| Double blind | 257 | 4.9 | ||
| Triple blind | 111 | 2.1 | ||
| Quadruple blind↑ | 226 | 4.3 | ||
| Open label | 4,375 | 82.9 | ||
| Not reported | 160 | 3.0 | ||
| Primary purpose | Treatment | 4,462 | 84.5 | |
| Prevention | 132 | 2.5 | ||
| Diagnostic | 260 | 4.9 | ||
| Supportive care | 178 | 3.4 | ||
| Screening | 69 | 1.3 | ||
| Health services research | 24 | 0.5 | ||
| Basic science | 32 | 0.6 | ||
| Device feasibility | 4 | 0.1 | ||
| Other | 90 | 1.7 | ||
| Not reported | 29 | 0.6 | ||
| Study design of observational study (N=1,168) | ||||
| Observational model | Cohort | 690 | 59.1 | |
| Case-control | 108 | 9.3 | ||
| Case-only | 221 | 18.9 | ||
| Case-crossover | 8 | 0.7 | ||
| Ecologic or community studies | 11 | 0.9 | ||
| Family-based | 4 | 0.3 | ||
| Other | 75 | 6.4 | ||
| Not reported | 51 | 4.4 | ||
| Time perspective | Retrospective | 249 | 21.3 | |
| Prospective | 805 | 68.9 | ||
| Cross-sectional | 45 | 3.9 | ||
| Other | 46 | 3.9 | ||
| Not reported | 23 | 2.0 | ||
*, prospective refers to when the date of the ‘date of registration’ field is prior to the date of the ‘date of first enrolment’ field (according to the ICTRP standards) and was otherwise considered retrospective; **, N/A (not applicable): for a single-arm trial; ↑, quadruple blind: the participants, care providers, investigators, and outcomes assessors were prevented from having knowledge of the interventions assigned to individual participants.
We performed a systematic search of the PubMed, Google Scholar, and EMBASE databases to determine the publication status of the assessed studies, The search was conducted in the following order and terms: (I) national clinical trial identifier; (II) name of applicant/investigator; (III) trial title; and (IV) study methods/PICO (Population, Intervention, Comparison, Outcome) components. We retrieved the full texts of the articles to assess the eligibility of each article.
Statistical analysis
Descriptive statistics were used to analyze the characteristics of clinical research on lung cancer, including: (I) the number of clinical studies conducted from 1999 to 2021; (II) the increase in the number of registrations from 1999 to 2021; (III) recruitment status; (IV) time of registration, prospectively or retrospectively; (V) source of funding; (VI) methodology of study design; (VII) information on trial recruitment, including the target sample size, age, and sex of participants; (VIII) study type; (IX) intervention type; and (X) study results.
All quality scores were recorded in Microsoft Office Excel (V.2016, Redmond, USA), and the analyses included the following: (I) categorical data and quality scores, presented as absolute numbers and percentages; and (II) multivariate logistic regression, adjusted for time of registration, number of centers, study duration, primary sponsor, and publication on registration quality. Registration quality was a binary variable. An above-average registration quality score represented a high registration quality, while lower-than-average scores signified a low registration quality. The statistical significance level was set at P<0.05. Statistical analyses were performed using SPSS software (SPSS Inc., Version 26.0, Chicago, USA).
Results
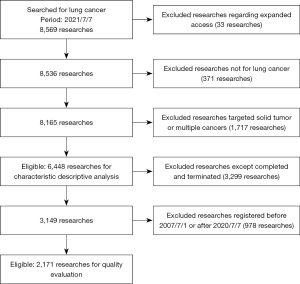
Our initial search identified 8,569 records. We then excluded 33 studies regarding expanded access. Of the remaining 8,536 clinical studies, 371 were excluded because they did not focus on lung cancer. A further 665 studies that targeted solid tumors and 1,052 studies that targeted multiple cancers were also excluded. Finally, a total of 6,448 studies were included for descriptive analysis. Of 3,149 completed and terminated clinical studies, we excluded 978 that were registered before 1 July 2007 or after 7 July 2020. Finally, a total of 2,171 studies were included for quality evaluation (Figure 1).
Registration of clinical studies over time
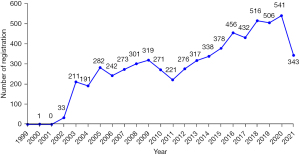
A total of 6,448 clinical studies on lung cancer were registered from 1 Jan 1999 to 7 July 2021. The number of registrations increased from 0 in 1999 to 541 in 2020; this number increased rapidly in 2003 and grew steadily for the next 19 years. Registrations between 2016 and 2021 accounted for 43.33% of all registered studies (Figure 2).
Recruitment status of registered studies
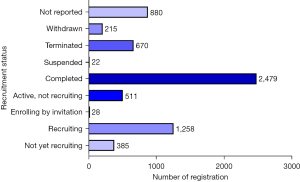
Most studies had completed recruitment 38.45% (2,479/6,448), while 19.51% (1,258/6,448) were still recruiting participants. A few studies had been suspended (0.34%, 22/6,448) or were enrolling participants by invitation (0.43%, 28/6,448). Furthermore, 5.97% (385/6,448) of studies did not report on the recruitment status (Figure 3).
“Withdrawn”: study halted prematurely, prior to enrollment of the first participant; “Terminated”: study halted prematurely and will not resume; participants are no longer being examined or receiving intervention; “Suspended”: study halted prematurely but will potentially resume; “Completed”: the study has concluded normally, participants are no longer receiving an intervention or being examined (that is, the last participant’s final visit has occurred); “Active, not recruiting”: study is continuing, meaning that participants are receiving an intervention or being examined, but new participants are not currently being recruited or enrolled; “Enrolling by invitation”: participants are being (or will be) selected from a predetermined population; “Recruiting”: participants are currently being recruited, regardless of whether any participants have yet been enrolled; “Not yet recruiting”: participants are not yet being recruited.
Prospective and retrospective study registration
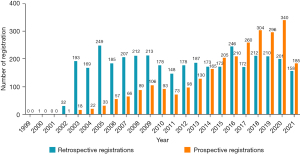
Of the 6,448 studies that were eligible for analysis, 3,697 (57.34%) were retrospective registrations, and their numbers were higher than those of prospective registrations between 2002 and 2013. In contrast, the number of prospective registrations has gradually increased over the years and exceeded the number of retrospective registrations between 2017 and 2021 (Figure 4).
Study types and funding sources
Most studies were interventional by design (81.89%, 5,280/6,448), with drugs being the most common interventions used (57.40%, 3,701/6,448). Funding sources included industry (27.16%), government (2.85%), academic institutions (45.87%), and others (24.12%). Among all of the registered studies, only 1,019 (15.80%) registered their results, of which 931 (91.36%) were completed or terminated studies (Table 1).
Characteristics of recruitment and study design
Most studies reported a target sample size between 0–100 participants (60.44%, 3,897/6,448), followed by 101–500 (27.75%, 1,798/6,448), while a few (9.68%, 624/6,448) enrolled >500 participants. There were three studies with a sample size of >100,000: one interventional and two observational studies. The participant age groups varied widely, but children and adults were the most frequently represented groups, accounting for 93.46% (6,026/6,448). The most commonly included ages were 18–75 years, and the majority (99.43%, 6,411/6,448) of studies included both sexes (Table 1).
According to the study design of interventional studies, randomized trials were most common (41.88%, 2,211/5,280), followed by non-randomized trials (15.63%, 825/5,280). Interventions typically involved a single group (48.07%, 2,538/5,280), but a significant proportion had parallel arms (43.35%, 2,289/5,280). Open-label was the most frequently reported masking type (82.86%, 4,375/5,280), followed by single, double, triple, or quadruple-blinded designs (14.11%, 745/5,280). Treatment was the primary purpose of most studies (84.51%, 4,462/5,280), followed by diagnostics (4.92%, 260/5,280).
Among the observational studies, cohort studies were the most frequent (59.08%, 690/1,168), while 18.92% (221/1,168) were case-only. Prospective studies were more common (68.92%, 805/1,168) than retrospective designs (21.32%, 249/1,168), while 1.97% (23/1,168) of studies did not report information on this.
Registration quality of lung cancer clinical research
A total of 2,171 completed or terminated studies that were first registered between 1 July 2007 and 7 July 2020 were included for quality evaluation, including 1,711 interventional studies and 460 observational studies.
The average quality score of these 2,171 studies was 36.76±5.69 out of a maximum score of 54. The score for interventional studies was 38.20±5.14 and for observational studies was 31.40±4.27. All (100%) studies reported the primary registration details, including a unique identification number, date of registration, study sponsor, and recruitment status. Most (>90%) studies presented the secondary identifying numbers, scientific title, and health condition. Public titles and country of recruitment were mentioned in >90% of interventional studies and <90% of observational studies. Overall, less than half of the studies provided information on collaborators and the dates of first enrolment and completion. Overall, 43.95% of interventional and 18.04% of observational studies reported involving a data monitoring committee (Table 2).
Table 2
| No. | Items | Specifics | Interventional studies (N=1,711, %) | Observational studies (N=460, %) |
|---|---|---|---|---|
| 1 | Primary registry and trial identifying number | – | 1,711 (100.0) | 460 (100.0) |
| 2 | Date of registration in primary registry | – | 1,711 (100.0) | 460 (100.0) |
| 3 | Secondary identifying numbers | – | 1,658 (96.9) | 434 (94.4) |
| 4 | Study sponsor | – | 1,711 (100.0) | 460 (100.0) |
| 5 | Collaborators | – | 638 (37.3) | 161 (35.0) |
| 6 | Contact for principal investigators | Name | 1,190 (69.6) | 332 (72.2) |
| Email address | 8 (0.5) | 1 (0.2) | ||
| Telephone number, postal address | 625 (36.5) | 40 (8.7) | ||
| Affiliation | 1,515 (88.5) | 373 (81.1) | ||
| Degree | 987 (57.7) | 278 (60.4) | ||
| Title | 1,497 (87.5) | 377 (82.0) | ||
| Total average | 970 (56.7) | 234 (50.8) | ||
| 7 | Public title | – | 1,559 (91.1) | 379 (82.4) |
| 8 | Scientific title | – | 1,660 (97.0) | 421 (91.5) |
| 9 | Countries of recruitment | – | 1,639 (95.8) | 412 (89.6) |
| 10 | Health condition or and problem studied | – | 1,704 (99.6) | 453 (98.5) |
| 11 | Arm, groups, and Interventions | Arm title | 1,659 (97.0) | – |
| Arm type | 1,616 (94.5) | – | ||
| Arm description | 1,662 (97.1) | – | ||
| Group/cohort label | – | 265 (57.6) | ||
| Group/cohort description | – | 264 (57.4) | ||
| Interventions | 1,570 (91.8) | 205 (44.6) | ||
| Total average | 1,627 (95.1) | 245 (53.2) | ||
| 12 | Key inclusion and exclusion criteria | Sex/gender | 1,705 (99.7) | 459 (99.8) |
| Age limits | 1,710 (99.9) | 445 (96.7) | ||
| Accepts healthy volunteers | 1,710 (99.9) | 452 (98.3) | ||
| Inclusion criteria | 1,709 (99.9) | 457 (99.4) | ||
| Exclusion criteria | 1,619 (94.6) | 378 (82.2) | ||
| Study population description | – | 432 (93.9) | ||
| Sampling method | – | 451 (98.0) | ||
| Total average | 1,691 (98.8) | 439 (95.5) | ||
| 13 | Study type | Type of study | 1,711 (100.0) | 460 (100.0) |
| Interventional study model | 1,696 (99.1) | – | ||
| Primary purpose | 1,694 (99.0) | – | ||
| Study phase | 1,711 (100.0) | – | ||
| Masking | 1,703 (99.5) | – | ||
| Allocation | 1,698 (99.2) | – | ||
| Allocation concealment | 7 (0.4) | – | ||
| Observational study model | – | 443 (96.3) | ||
| Time perspective | – | 447 (97.2) | ||
| Biospecimen retention | – | 132 (28.7) | ||
| Biospecimen description | – | 132 (28.7) | ||
| Target follow-up duration | – | 31 (6.7) | ||
| Total average | 1,460 (85.3) | 274 (59.6) | ||
| – | – | – | ||
| 14 | Date of first enrollment | – | 556 (32.5) | 166 (36.1) |
| 15 | Sample size | Number of participants that the trial plans to enroll in total | 1,710 (99.9) | 460 (100.0) |
| Number of participants that the trial has enrolled | 1,711 (100.0) | 460 (100.0) | ||
| Total average | 1,711 (100.0) | 460 (100.0) | ||
| 16 | Recruitment status | – | 1,711 (100.0) | 460 (100.0) |
| 17 | Primary outcome (s) | The name of the outcome (do not use abbreviations) | 1,707 (99.8) | 440 (95.7) |
| The metric or method of measurement used (be as specific as possible) | 1,133 (66.2) | 226 (49.1) | ||
| The timepoint(s) of primary interest | 1,399 (81.8) | 326 (70.9) | ||
| Total average | 1,413 (82.6) | 331 (71.9) | ||
| 18 | Key secondary outcomes | The name of the outcome (do not use abbreviations) | 1,481 (86.6) | 285 (62.0) |
| The metric or method of measurement used (be as specific as possible) | 999 (58.4) | 154 (33.5) | ||
| The timepoint(s) of primary interest | 1,202 (70.3) | 219 (47.6) | ||
| Total average | 1,227 (71.7) | 219 (47.7) | ||
| 19 | Completion date | – | 659 (38.5) | 191 (41.5) |
| 20 | Summary results | Date of posting of results summaries | 960 (56.1) | 160 (34.8) |
| URL hyperlink(s) related to results and publications | 537 (31.4) | 101 (22.0) | ||
| Baseline characteristics | 666 (38.9) | 35 (7.6) | ||
| Participant flow | 666 (38.9) | 35 (7.6) | ||
| Adverse events | 658 (38.5) | 32 (7.0) | ||
| Primary outcome measures | 662 (38.7) | 35 (7.6) | ||
| Primary outcome statistical analyses | 172 (10.1) | 7 (1.5) | ||
| Secondary outcome measures | 575 (33.6) | 29 (6.3) | ||
| Secondary outcome statistical analyses | 142 (8.3) | 4 (0.9) | ||
| Brief summary* | 5 (0.3) | 9 (2.0) | ||
| Total average | 504 (29.5) | 45 (9.7) | ||
| 21 | IPD sharing statement | Plan to share IPD (yes, no, undecided) | 109 (6.4) | 15 (3.3) |
| Available IPD/information type ↑ | 210 (12.3) | 18 (3.9) | ||
| Total average | 160 (9.3) | 17 (3.6) | ||
| 22 | Data monitoring committee | – | 752 (44.0) | 83 (18.0) |
*, brief summary: a short description of the clinical study, including a brief statement of the clinical study’s hypothesis, written in language intended for the lay public; ↑, available IPD (individual participant data)/information type: the type of data set or supporting information being shared, including individual participant data set, study protocol, statistical analysis plan, informed consent form, clinical study report, analytic code, and other types.
Regarding additional study information, the contact details of principal investigators (PI) were provided in 970 (56.69%) interventional and 234 (50.76%) observational studies. Most (>80%) studies provided information regarding the affiliation and title of the PI, while several (50–75%) studies mentioned the name and degree of the PI. Telephone numbers were more frequently provided for interventional (36.53%) than for observational studies (8.70%). E-mail addresses were the least frequently provided contact detail, only provided in 0.45% of interventional and 0.22% of observational studies.
The reporting rate of interventional design and study arms was as high as 95.08% in interventional studies. Most (>90%) studies provided basic information on the arm title, type, description, and further details of the interventions. In contrast, only 53.19% of observational studies reported the group and interventions completely. The group/cohort label, group/cohort description, and interventions reporting rates of observational studies were 57.61%, 57.39%, and 44.57%, respectively.
Almost all (>96%) studies mentioned information regarding the inclusion criteria of age, sex, and medical diagnosis of the participants, while 94.62% of interventional and 82.17% of observational studies reported the exclusion criteria. Moreover, observational studies mostly mentioned the study population description (93.91%) and sampling method (98.04%).
Study type (interventional or observational) was reported in all of the registered research. Information on the primary purpose, study phase, study model, and allocation was reported in the majority (>99%) of interventional studies. Complete information on the masking method was reported in 99.53% of studies, while a few (0.41%) studies provided the allocation concealment mechanism. Regarding the design of observational studies, most (>95%) reported the model and time perspective information (retrospective or prospective). However, information on whether biological samples were collected was only reported in 28.70% of studies. Furthermore, 6.74% of studies reported the target follow-up duration. In general, the registration of study design was more common in interventional (85.33%) than observational studies (59.60%).
Most (>90%) studies provided the primary outcome(s). For interventional studies, the measurement(s) and timepoint(s) reporting rates were 66.22% and 81.77%, respectively. The same information in observational studies was less frequently reported, with 49.13% and 70.87%, respectively. Fewer studies reported secondary outcome(s), with a reporting rate of 71.73% in interventional and 47.68% in observational studies.
Almost all (>99%) studies reported the sample size, both target and achieved. Only 6.37% of interventional studies and 3.26% of observational studies chose to share the individual participant data (IPD), and a few (<15%) provided the available information, such as study protocol, statistical analysis plan, or informed consent form.
The rate of reported summary results was higher in interventional studies (56.11%) than in observational studies (34.78%). Only 31.39% of interventional studies and 21.96% of observational studies linked the resulting publications to ClinicalTrials.gov. About 40% of interventional studies reported the baseline characteristics, participant flow, and adverse events, while the reporting rate of these aspects in observational studies was <8.00%. In interventional studies, the reporting rate of primary outcome measures and statistical analyses accounted for 38.69% and 10.05% of studies, while that of the secondary outcome measures and statistical analyses accounted for 33.61% and 8.30%, respectively. In observational studies, the registration rate of all outcome measures was <8.00%, and <2% for statistical analyses. Similarly, few (<2.00%) studies submitted a research summary. In general, the reporting rate of summaries in interventional studies (29.47%) was significantly higher than that in observational studies (9.72%).
Among the 2,171 studies, 987 (45.46%) were of high quality, and the rest were of low quality in terms of registration. Taking registration quality as the dependent variable, prospective registration studies were more likely to have high registration quality than retrospective registration studies [adjusted odds ratio (aOR), 2.18, 95% confidence interval (95% CI), 1.79–2.65] (Table 3). Multi-center studies were more likely to have high registration quality than single-center studies (aOR, 1.73; 95% CI, 1.39–2.16), and government-sponsored studies were more likely to have a high registration quality than industry-sponsored studies (aOR, 3.09; 95% CI, 1.48–6.42). Published studies were more likely to have a high registration quality than unpublished studies (aOR, 1.43; 95% CI, 1.15–1.78).
Table 3
| Variables | n (%) | Registration quality | |||
|---|---|---|---|---|---|
| Crude OR (95% CI) | P value | Adjusted OR (95% CI) | P value | ||
| Time of registration | |||||
| Retrospective registration | 862 (39.70) | 1 | 1 | ||
| Prospective registration | 1,309 (60.30) | 2.59 (2.17, 3.09) | <0.001* | 2.18 (1.79, 2.65) | <0.001* |
| Number of centers | |||||
| Single-center | 952 (43.85) | 1 | 1 | ||
| Multi-center | 1,098 (50.58) | 2.74 (2.29, 3.29) | <0.001* | 1.73 (1.39, 2.16) | <0.001* |
| Not reported | 121 (5.57) | 0.48 (0.30, 0.77) | 0.002* | 0.42 (0.26, 0.70) | 0.001* |
| Sponsor | |||||
| Industry | 747 (34.41) | 1 | 1 | ||
| Government | 46 (2.12) | 2.57 (1.29, 5.14) | 0.007* | 3.09 (1.48, 6.42) | 0.003* |
| Academic institutions | 881 (40.58) | 0.31 (0.25, 0.38) | <0.001* | 0.46 (0.36, 0.59) | <0.001* |
| Other+ | 497 (22.89) | 1.19 (0.95, 1.50) | 0.136 | 1.49 (1.15, 1.94) | 0.003* |
| Duration of the studies, years | |||||
| <2 | 586 (26.99) | 1 | 1 | ||
| 2–5 | 1,240 (57.12) | 2.04 (1.66, 2.50) | <0.001* | 1.86 (1.48, 2.32) | <0.001* |
| >5 | 301 (13.86) | 2.28 (1.71, 3.02) | <0.001* | 2.51 (1.82, 3.46) | <0.001* |
| Not reported | 44 (2.03) | 0.38 (0.17, 0.87) | 0.021* | 0.34 (0.14, 0.81) | 0.015* |
| Publication | |||||
| Unpublished | 579 (26.67) | 1 | 1 | ||
| Publish | 1,592 (73.33) | 1.57 (1.29, 1.91) | <0.001* | 1.43 (1.15, 1.78) | 0.001* |
Registration quality was assigned as the dependent variable and binary variable; scores >36 signified a high registration quality, which was assigned as 1. Adjustment included registration, location, sponsor, study duration, and publication. *, there were significant differences between groups; +, industry + government, industry + academic institutions, government + academic institutions, industry + government + academic institutions. OR, odds ratio; CI, confidence interval.
Discussion
Current situation of lung cancer clinical research registrations
In this study, we examined 6,448 studies on lung cancer registered on ClinicalTrials.gov from 1999 to 2021. The number of lung cancer research registrations has increased since 2003. In this study, we showed that most (57.34%) studies were registered retrospectively. The timing of clinical trial registration is important, and studies enrolling participants after 1 July 2005 must be registered on ClinicalTrials.gov prior to or upon beginning patient enrolment (23). However, even several years after these urgent calls for prospective research registration, studies still miss this target (24). The number of prospective registrations over the past 5 years has exceeded the number of retrospective registrations. It is suggested that researchers have gradually realized the importance of timely registration, which may be due to the member journals of the International Committee of Medical Journal Editors (ICMJE) rejecting retrospectively registered trials (25). In conclusion, prospective clinical research registration is an important step to increase research transparency, the visibility of unpublished studies, and the minimization of selective result publications. Moreover, several research organizations have called for prospective study registration (26-29).
Of the 6,448 studies eligible for analysis, 2,479 studies (38.45%) had a completed status. This could be attributed to the fact that most of the studies were retrospective registrations, which had been completed or nearly completed by the time of registration. Furthermore, only 15.80% of studies registered results data. Even among the completed or terminated studies, the result registration rate was 29.56%. The FDA requires the reporting of basic summary results to ClinicalTrials.gov by the sponsor within 1 year after the completion of data collection for the pre-specified primary outcome or within 1 year after the date of early termination (8,9). It is considered irresponsible not to make the results available to the public after trial registration (30,31), as the lack of result dissemination may affect clinical practice, research, and ultimately, patients (32,33). Sponsors and researchers must prioritize publishing the results, which may be positive, negative, or inconclusive.
The majority (81.89%) of the 6,448 research registrations were interventional studies. Among these, the most common designs were randomized, single group, and open-label. Most interventional studies were treatment-oriented, rather than diagnostic investigations. Most observational studies were prospective cohorts by design. The enrolled patients included both sexes aged between 18 and 75 years, with <100 participants. Almost half (45.87%) of the 6,448 studies were sponsored by academic institutions.
In the present study, the number of observational studies was significantly lower than that of intervention studies, which may be related to the fact that purely observational studies were exempt from registration (34). Randomized clinical trials (RCTs) are important scientific experiments in global health research (5), as they assess the efficacy of a treatment by minimizing the selection bias and creating groups with comparable prognostic factors (35,36). RCTs only accounted for 41.88% of the interventional studies on lung cancer. More efficient research designs are necessary to generate high-level evidence to inform medical decision-making. In contrast, our findings suggested that open-label was the most prevalent type of trial masking, accounting for 82.86%. In this study, the proportion of interventions, such as surgical, behavioral, and pharmaceutical, was 44.59%, which may have limited the implementation of blinding. It was reported that trials without blinding may exaggerate the intervention benefits by 14% (37). Therefore, researchers should pay more attention to the blinding design of drug-related interventional clinical trials.
The majority (60.44%) of studies had small sample sizes (<100 participants), which had limited power in establishing the effectiveness of treatments (38,39). Furthermore, rare adverse events were unlikely to be captured in these studies (38). The details of sample size calculations and rationale for the study size should be included in the study registration (6). Furthermore, lung cancer is a disease with a median age at diagnosis of 70 years (40); however, we found that the most frequent age category was 18–75 years.
Quality evaluation of lung cancer clinical research registrations
Our study is the first to evaluate the overall registration quality specific to clinical studies in lung cancer using the modified WHO TRDS (version 1.3.1). The modified TRDS includes 13 simple items and nine complex items, with a maximum score of 54. We reported deficiencies in registrations, especially in observational research, as requirements were less strict than for intervention studies.
Each clinical study was required to register a complete protocol, summary results, and links to resulting publications (41,42). We found that poor registration quality was accounted for by the following three categories. Firstly, there were several points of protocol that were not adequately reported, especially the contact for PI, collaborators, allocation concealment of randomized trials, method, and outcome time points. This may be attributable to the fact that the reporting of these data is optional (43). Furthermore, allocation concealment in an RCT ensures confidentiality to avoid selection bias (13), but only seven studies reported on this.
Secondly, registered clinical trials should promptly report a summary of results (≤500 words or a table), ideally within 1–2 years after completion (34,44,45). Yet, less than half (<45%) of the studies reported their results, and <2.00% of studies submitted a brief results summary. This indicated that investigators registered trials only to gain a registration identification number, but not to disseminate the results (46,47). Hartung et al. found that 55% of the trials had neither linked publications nor summary results reported (48). This common phenomenon is not conducive to clinical practice and research (33), and reporting of results must be improved (18).
Thirdly, there was a lack of IPD sharing, which refers to the sharing of clinical (and other) data collected from each clinical trial participant (49). This has only recently become a requirement; for publication in ICMJE journals, studies have been required to contain a data sharing statement from 1 July 2018, and clinical trials starting after 1 January 2019 must include a data sharing plan (29). Sharing IPD is the new standard in clinical trial transparency (36,38), and we encourage scientists to engage in this to support efficient clinical research and benefit patients (50).
We found that registration quality was independently associated with factors such as time of registration, number of centers, sponsor, study duration, and publication of the results. Prospective registrations were more likely to have a high registration quality than retrospective registrations, which could be due to several factors. Firstly, researchers with retrospective registrations could be less familiar with the process than those who plan their registrations ahead. Secondly, researchers with retrospective registrations may not register to avoid publishing negative results or to enable them to change the primary outcomes, and hence, could deliberately avoid registration until a journal requires it (51). This study also found that government-sponsored studies were more likely to have high registration quality than industry-sponsored studies, which was consistent with previous studies (52,53). Government-sponsored studies were often required to be published in journals as part of the funding conditions (54), and researchers would need to follow registration requirements (52). Furthermore, due to “trade secrets”, commercially-funded researchers were more resistant to register (53). Lastly, our study found that published research was more likely to be of high quality than unpublished studies. In 2005, the ICMJE announced that journals would decline any manuscripts describing unregistered research (55); then, from 2015, the journal would not publish any clinical research that was registered retrospectively (54). These steps could improve the registration awareness of researchers, resulting in a high registration quality.
Limitations
There were some limitations to this study that should be noted. Firstly, this study ended on 7 July 2021, which does not encompass all studies on lung cancer. Secondly, the ClinicalTrials.gov registry is influenced by evolving reporting incentives that influence which studies are registered on it as well as the amount of information submitted. Therefore, to mitigate the changes reported over time, the quality evaluation of this study only analyzed studies submitted after July 2007.
Conclusions
This study analyzed the characteristics of lung cancer studies registered on ClinicalTrials.gov, evaluated the quality of these based on the adjusted TRDS model, and assessed the independent factors influencing registration quality. We found that the proportion of prospectively registered studies had increased over the past two decades, but several studies provided incomplete or incorrect data during registration, especially in the reporting of results. Univariate and multivariate logistic regressions showed that prospective registration, multi-center studies, government-funded, and published studies were independent protective factors for high registration quality. Awareness of prospective registration should be further improved and government investment should be increased. At the same time, the quality of clinical registration and more efficient and extensive data sharing after completion of the studies should be actively promoted.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-975/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Learn about clinical studies: US National Library of Medicine; [cited 2019]. Available online: https://clinicaltrials.gov/ct2/about-studies/learn
- De Angelis C, Drazen JM, Frizelle FA, et al. Clinical trial registration: a statement from the International Committee of Medical Journal Editors. Lancet 2004;364:911-2. [Crossref] [PubMed]
- Dickersin K, Rennie D. Registering clinical trials. JAMA 2003;290:516-23. [Crossref] [PubMed]
- Simes RJ. Publication bias: the case for an international registry of clinical trials. J Clin Oncol 1986;4:1529-41. [Crossref] [PubMed]
- Guinn D, Wilhelm EE, Shoulson I. Reasons for Premature Conclusion of Late Phase Clinical Trials: An Analysis of ClinicalTrials.gov Registered Phase III Trials. Ther Innov Regul Sci 2020;54:232-9. [Crossref] [PubMed]
- Liang R, Long J, Zheng Q, et al. Current landscape of type 1 diabetes mellitus-related interventional clinical trials registered on ClinicalTrials.gov: a cross-sectional study. Acta Diabetol 2021;58:723-33. [Crossref] [PubMed]
- Zwierzyna M, Davies M, Hingorani AD, et al. Clinical trial design and dissemination: comprehensive analysis of clinicaltrials.gov and PubMed data since 2005. BMJ 2018;361:k2130. [Crossref] [PubMed]
- Anderson ML, Chiswell K, Peterson ED, et al. Compliance with results reporting at ClinicalTrials.gov. N Engl J Med 2015;372:1031-9. [Crossref] [PubMed]
- Zarin DA, Tse T, Williams RJ, et al. Trial Reporting in ClinicalTrials.gov - The Final Rule. N Engl J Med 2016;375:1998-2004. [Crossref] [PubMed]
- Zhang X, Tian R, Yang Z, et al. Quality assessment of clinical trial registration with traditional Chinese medicine in WHO registries. BMJ Open 2019;9:e025218. [Crossref] [PubMed]
- Chen R, Desai NR, Ross JS, et al. Publication and reporting of clinical trial results: cross sectional analysis across academic medical centers. BMJ 2016;352:i637. [Crossref] [PubMed]
- WHO Trial Registration Data Set (Version 1.1 - archived): World Health Organization. Available online: https://www.who.int/clinical-trials-registry-platform/network/who-data-set/archived/1.1-archived
- WHO Trial Registration Data Set (Version 1.3.1): World Health Organization. Available online: https://www.who.int/clinical-trials-registry-platform/network/who-data-set
- International standards for clinical trial registries: the registration of all interventional trials is a scientific, ethical and moral responsibility, version 3.0: World Health Organization; [cited 2018]. Available online: https://apps.who.int/iris/handle/ 10665/ 274994?show=full
- Zhang X, Lan L, Chan JCP, et al. WHO Trial Registration Data Set (TRDS) extension for traditional Chinese medicine 2020: recommendations, explanation, and elaboration. BMC Med Res Methodol 2020;20:192. [Crossref] [PubMed]
- ClinicalTrials.gov Protocol Registration Data Element Definitions for Interventional and Observational Studies: ClinicalTrials.gov; [cited 2020 10-01]. Available online: https://prsinfo.clinicaltrials.gov/definitions.html
- ClinicalTrials.gov Results Data Element Definitions for Interventional and Observational Studies: ClinicalTrials.gov; [cited 2021 02-01]. Available online: https://prsinfo.clinicaltrials.gov/results_definitions.html
- The Lancet Oncology. Clinical trial registry reporting: a transparent solution needed. Lancet Oncol 2019;20:741. [Crossref] [PubMed]
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Subramanian J, Regenbogen T, Nagaraj G, et al. Review of ongoing clinical trials in non-small-cell lung cancer: a status report for 2012 from the ClinicalTrials.gov Web site. J Thorac Oncol 2013;8:860-5. [Crossref] [PubMed]
- Al-Shbool G, Latif H, Farid S, et al. Publication Rate and Characteristics of Lung Cancer Clinical Trials. JAMA Netw Open 2019;2:e1914531. [Crossref] [PubMed]
- Williams RJ, Tse T, Harlan WR, et al. Registration of observational studies: is it time? CMAJ 2010;182:1638-42. [Crossref] [PubMed]
- DeAngelis CD, Drazen JM, Frizelle FA, et al. Clinical trial registration: a statement from the International Committee of Medical Journal Editors. JAMA 2004;292:1363-4. [Crossref] [PubMed]
- Trinquart L, Dunn AG, Bourgeois FT. Registration of published randomized trials: a systematic review and meta-analysis. BMC Med 2018;16:173. [Crossref] [PubMed]
- Viergever RF, Ghersi D. The quality of registration of clinical trials. PLoS One 2011;6:e14701. [Crossref] [PubMed]
- Chan AW, Song F, Vickers A, et al. Increasing value and reducing waste: addressing inaccessible research. Lancet 2014;383:257-66. [Crossref] [PubMed]
- Zarin DA, Tse T, Williams RJ, et al. Update on Trial Registration 11 Years after the ICMJE Policy Was Established. N Engl J Med 2017;376:383-91. [Crossref] [PubMed]
- McGauran N, Wieseler B, Kreis J, et al. Reporting bias in medical research - a narrative review. Trials 2010;11:37. [Crossref] [PubMed]
- Trofimova AV, Bluemke DA. Prospective Clinical Trial Registration: A Prerequisite for Publishing Your Results. Radiology 2022;302:1-2. [Crossref] [PubMed]
- Berlin JA, Wacholtz MC. Selective Reporting, Publication Bias and Clinical Trial Registry: An Industry Perspective. International Journal of Pharmaceutical Medicine 2005;19:277-84. [Crossref]
- Li YP, Wu TX, Li J, et al. Joint statement of establishing Chinese Clinical Trial Registration and Publishing system. Zhong Xi Yi Jie He Xue Bao 2006;4:331-2. [Crossref] [PubMed]
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1789-858. [Crossref] [PubMed]
- Chalmers I, Glasziou P. Avoidable waste in the production and reporting of research evidence. Lancet 2009;374:86-9. [Crossref] [PubMed]
- Laine C, Horton R, DeAngelis CD, et al. Clinical trial registration--looking back and moving ahead. N Engl J Med 2007;356:2734-6. [Crossref] [PubMed]
- Department of Economic and Social Affairs: United Nations; [cited 2019]. Available online: https://population.un.org/wpp/Publications/Files/WPP2019_ Highlights.pdf
- Chang AY, Skirbekk VF, Tyrovolas S, et al. Measuring population ageing: an analysis of the Global Burden of Disease Study 2017. Lancet Public Health 2019;4:e159-67. [Crossref] [PubMed]
- Moher D, Pham B, Jones A, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet 1998;352:609-13. [Crossref] [PubMed]
- Yusuf S, Collins R, Peto R. Why do we need some large, simple randomized trials? Stat Med 1984;3:409-22. [Crossref] [PubMed]
- Mocco J, O'Kelly C, Arthur A, et al. Randomized clinical trials: the double edged sword. J Neurointerv Surg 2013;5:387-90. [Crossref] [PubMed]
- Cancer Stat Facts: Lung and Bronchus Cancer: National Cancer Institute. Available online: https://seer.cancer.gov/statfacts/html/lungb.html
- Hudson KL, Collins FS. Sharing and reporting the results of clinical trials. JAMA 2015;313:355-6. [Crossref] [PubMed]
- Taichman DB, Backus J, Baethge C, et al. Sharing Clinical Trial Data: A Proposal From the International Committee of Medical Journal Editors. Ann Intern Med 2016;164:505-6. [Crossref] [PubMed]
- Chinese clinical trial registry. Guideline of registration. [cited 2021]. Available online: http://www.chictr.org.cn/registry.aspx
- Tan S, Chen Y, Dai L, et al. Characteristics and publication status of gastrointestinal endoscopy clinical trials registered in ClinicalTrials.gov. Surg Endosc 2021;35:3421-9. [Crossref] [PubMed]
- Clinical Trials. Available online: https://www.icmje.org/recommendations/browse/ publishing-and -editorial-issues/clinical-trial-registration.html#one
- Li ZJ, Liu ML, Wang JN, et al. Method and current situations on acupuncture clinical trial registration in the world. Zhen Ci Yan Jiu 2012;37:86. inside back cover. [PubMed]
- Li Y, Wu T, Shang H, et al. Strategies for promoting the development of evidence-based medicine in China. J Evid Based Med 2009;2:47-52. [Crossref] [PubMed]
- Hartung DM, Zarin DA, Guise JM, et al. Reporting discrepancies between the ClinicalTrials.gov results database and peer-reviewed publications. Ann Intern Med 2014;160:477-83. [Crossref] [PubMed]
- Xu Y, Dong M, Liu X. Characteristics and trends of clinical studies primarily sponsored by China in WHO primary registries between 2009 and 2018: a cross-sectional survey. BMJ Open 2020;10:e037262. [Crossref] [PubMed]
- Taichman DB, Sahni P, Pinborg A, et al. Data Sharing Statements for Clinical Trials: A Requirement of the International Committee of Medical Journal Editors. PLoS Med 2017;14:e1002315. [Crossref] [PubMed]
- Harriman SL, Patel J. When are clinical trials registered? An analysis of prospective versus retrospective registration. Trials 2016;17:187. [Crossref] [PubMed]
- Blümle A, Wollmann K, Bischoff K, et al. Investigator initiated trials versus industry sponsored trials - translation of randomized controlled trials into clinical practice (IMPACT). BMC Med Res Methodol 2021;21:182. [Crossref] [PubMed]
- Krleza-Jerić K. Clinical trial registration: the differing views of industry, the WHO, and the Ottawa Group. PLoS Med 2005;2:e378. [Crossref] [PubMed]
- Cook C, Jull G, Moore A. Registration of clinical trials for publication. Man Ther 2014;19:279-80. [Crossref] [PubMed]
- Zarin DA, Tse T, Ide NC. Trial Registration at ClinicalTrials.gov between May and October 2005. N Engl J Med 2005;353:2779-87. [Crossref] [PubMed]
(English Language Editor: A. Kassem)