
EGFR T790M testing through repeated liquid biopsy over time: a real-world multicentric retrospective experience
Introduction
Lung cancer is the leading cause of cancer-related death and non-small cell lung cancer (NSCLC) represents about 85% of lung cancers (1). About 15% of patients with advanced NSCLC harbor epidermal growth factor receptor (EGFR) mutations, the most common being exon 19 deletion and the exon 21 L858R substitution (2). EGFR mutation testing is recommended at diagnosis for patients with lung adenocarcinoma and for light smokers regardless of histology, considering the survival benefit shown by EGFR tyrosine kinase inhibitors (TKIs) over chemotherapy for EGFR-positive patients (3-6).
EGFR exon 20 T790M mutation constitutes the predominant acquired resistance mechanism to first and second generation TKIs (7-10). Osimertinib is a third generation TKI that targets activating mutations and the T790M resistance mutation. This compound irreversibly binds to the C797 residue in the EGFR mutant protein ATP-binding pocket, sparing wild-type EGFR (11). At disease progression after first or second generation TKIs treatment, the analysis of circulating tumor DNA (ctDNA) through liquid biopsy (LB) or tissue rebiopsy (TB), when feasible, is carried out to identify T790M mutation and, whenever present, switch to osimertinib (6,11).
Recently, osimertinib has become the standard of care first line treatment for EGFR-positive patients (12,13). Notably, resistance mechanisms to first line osimertinib do not include T790M mutation (14-16). Nonetheless, characterizing tumor genomics after progression to TKIs remains crucial, in order to find druggable alterations and personalize further treatments. After osimertinib failure, extensive ctDNA analysis through next generation sequencing (NGS) could be performed through liquid and tissue biopsy to evaluate resistance mechanisms (14-16). No targeted treatments are currently approved by European Medicines Agency for upfront osimertinib acquired resistance, although several clinical trials are ongoing, especially in the setting of MET-amplified patients (17-20).
Compared to tissue biopsy, LB is a minimally invasive technique that can better picture tumor heterogeneity, be safely repeated over time, and overcome feasibility issues (21,22). However, tissue biopsy remains the only technique providing histological information and the gold standard for re-assessing mutational status (23). Since LB sensitivity is 50–90%, a negative LB result does not exclude the presence of a molecular alteration, but might be due to the lack of ctDNA or to presence of mutant allele at lower frequency than the limit of detection (24-28). Extrathoracic disease and bone metastases have been correlated with a higher probability to detect EGFR T790M mutation on ctDNA (28,29), while detection rates of genomic alterations is lower in patients with exclusively intracranial disease (30).
To date, it is unknown whether a specific timing of testing or clinical features could enhance LB sensitivity, whether a resistance mutation could be found in ctDNA before radiological progression, and whether repeating LBs after a negative result would be clinically meaningful (31,32). Therefore, optimizing LB procedures and ctDNA workflow is still an unmet clinical need (33). The clinical data derived from the use of LB to detect T790M mutation can help fill these knowledge gaps and may be translated into other clinical scenarios in which the analytic methods rely on ctDNA, such as NGS after progression to first line osimertinib (14-20).
The primary aims of this study were the description of the prevalence of EGFR T790M mutation at LB and in repeated LBs over time in a real-life cohort of EGFR mutant advanced NSCLC patients progressing to upfront first or second generation EGFR TKIs. Secondary aims were to assess the correlation of EGFR activating mutations and T790M mutation at LB with clinical-pathological features and the prevalence of EGFR T790M mutation in TB. Finally, we described the decision-making process for LB request and for TB. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-745/rc).
Methods
We designed an observational retrospective study at two Italian Oncology Centers. The inclusion criteria were histologically confirmed diagnosis of advanced NSCLC between 2016 and 2019, the presence of EGFR activating mutation at diagnosis, upfront treatment with first or second generation EGFR TKIs (erlotinib, gefitinib, or afatinib) and the presence of at least two LBs after starting first or second generation EGFR TKI, with the first LB resulting as T790M negative. Patients with T790M mutation at diagnosis were excluded, as patients with only one LB test.
This study was conducted according to current rules of Good Clinical Practice and the principles of Helsinki Declaration (as revised in 2013). Informed consent form for diagnostic procedures and treatment was signed by all patients, according to clinical practice and to the Regulation EU 2016/679 of the European Parliament and of the Council on personal data protection. Patients’ data collected in the study were anonymized. Ethics Committee approval by our Institutions was not required because of the observational intent of the data collection from the diagnostic routine practice.
An electronic questionnaire was submitted to medical oncologists and biologists at each involved Oncology Center [Supplementary file (Appendix 1)]. The survey was distributed in November 2020 and was collected in January 2021. The following clinical data were collected for each LB: date and result of LB, reason for LB request, progression type, progression sites, subsequent systemic therapy after LB; tissue biopsy after LB, tissue biopsy site and result, and, if tissue biopsy was not done, the reason for not performing it.
The possible pre-set reasons for LB request included “at radiological progression”, “at clinical and radiological progression” or “arbitrarily”. Progression type was classified as “only radiological and not meeting Response Evaluation Criteria version 1.1 (RECIST 1.1) for progression”, “only radiological and meeting RECIST 1.1 criteria for progression”, and “both clinical and radiological progression”. Progression sites included central nervous system, intrathoracic, liver, adrenal gland, metastatic lymph nodes and bone. Subsequent therapies were classified as TKI, chemotherapy, immunotherapy, or best supportive care. The possible pre-set reasons for which tissue biopsy was not carried out included “T790M positive liquid biopsy outcome”, “tissue biopsy not feasible”, “patient’s decision”, “only radiological progression”, or “clinical deterioration”.
EGFR mutational analysis
At the time of histologic diagnosis, mutational analysis of EGFR gene exons 18 to 21 was performed according to standard clinical practice.
At disease progression, LB was performed to assess the EGFR T790M mutational status in ctDNA. In T790M negative patients at LB, a tissue biopsy was carried out, when feasible. If test was still T790M negative, new plasma samples were collected.
In case of LB, cell free-DNA was isolated from 2 mL of plasma using the cobas® cf-DNA Sample Preparation kit (Roche, Basel, Switzerland) or the Helix® circulating Nucleic Acid assay (Diatech Pharmacogenetics, Jesi, Italy), and the EGFR mutational analysis was performed by polymerase chain reaction (PCR)-based methods (cobas® EGFR Mutation Test v2, Roche, Basel, Switzerland, or Easy EGFR kit, Diatech Pharmacogenetics, Jesi, Italy).
In case of tissue biopsy, DNA was extracted from formalin-fixed paraffin-embedded tissue sections using the QIAamp DNA Mini kit (Qiagen, Hilden, Germany) and analyzed by mass spectrometry-based methods (Sequenom MassARRAY, Diatech Pharmacogenetics, Jesi, Italy).
Results of liquid and tissue biopsies were classified as: positive (when EGFR T790M mutation was present); negative (when neither T790M nor activating mutations were found); other (when only EGFR activating mutations but not T790M were reported).
Treatment and tumor assessment
First-line systemic treatment was administered according to national and international guidelines. Patients received a first (oral erlotinib 150 mg daily or gefitinib 250 mg daily) or second generation TKI (oral afatinib 40 mg daily) until radiological disease progression (PD), clinical treatment failure, unacceptable toxicity, physician and/or patient decision, or death.
Tumor assessment was performed through chest and abdomen computerized tomography (CT) scan; brain CT scan or nuclear magnetic resonance was performed as clinically indicated. Response to treatment was classified according to RECIST, version 1.1.
Statistical analysis
Statistical analysis was performed through R software (version 4.0.5; the R foundation, Vienna, Austria). Mean and standard deviation, median, first (Q1) and third (Q3) quartile were reported for quantitative variables. The Cochran-Mantel-Haenszel test for monotonic trend was used to evaluate statistically significative changes in the reasons for LB request or in the systemic therapy, over time or through repeated LBs, and changes in conditional probability of T790M mutation over time. Associations of EGFR T790M positivity by LB and any EGFR mutation positivity by LB with clinical factors were evaluated through logistic and mixed effects logistic models, respectively. Odds ratios (ORs) with 95% confidence intervals (95% CIs) were reported. Significance level was set at P<0.05.
Results
Study population and repeated LBs
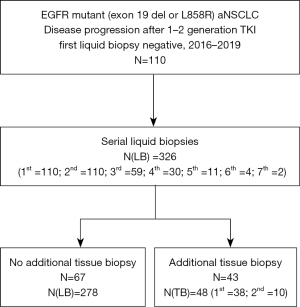
Between January 2016 and December 2019, 110 consecutive patients with progression to a first or second generation EGFR TKI and a first T790M negative LB were included in this study. A second LB was performed in all patients. Data cut-off was carried out on 5 January 2021 (Figure 1).
Twenty-four patients out of 110 (21.8%) were enrolled in 2016, 25 (22.7%) in 2017, 29 (26.4%) in 2018 and 32 (29.1%) in 2019.
Overall, 326 LBs were carried out (Figure 1). Twenty-nine (8.9%) out of 326 were collected in 2016, 74 (22.7%) in 2017, 72 (35.7%) in 2018, 110 (33.7%) in 2019 and 41 (12.6%) in 2020.
The mean number of LBs per patient was 3.0 (standard deviation: 1.2). The median number of LBs per patient was 3 (Q1: 2; Q3: 6). The median time between subsequent blood draws was 124 days (Q1: 70 days; Q3: 230 days). The median time between each subsequent LB is detailed in Table 1.
Table 1
| Number of repeated liquid biopsy per patient | First | Second | Third | Fourth | Fifth | Sixth | Seventh | Total |
|---|---|---|---|---|---|---|---|---|
| Number of liquid biopsies | 110 | 110 | 59 | 30 | 11 | 4 | 2 | 326 |
| Time from previous liquid biopsy (days) | ||||||||
| Median | – | 119 | 128 | 137 | 104 | 90 | 116 | 124 |
| Q1, Q3 | – | 70, 197 | 66, 291 | 82, 242 | 88, 152 | 61, 118 | 72, 159 | 70, 230 |
| Range | – | 3–784 | 14–892 | 21–423 | 30–386 | 28–146 | 28–203 | 3–892 |
Q1, first quartile; Q3, third quartile.
LBs results and prevalence of EGFR T790M mutation
Seventy-seven out of 110 patients (70.0%) showed any EGFR mutation at LB. The T790M mutation was detected through LB in 38 of 110 patients (34.5%); in 22 of 38 cases both T790M and baseline EGFR activating mutation were present, whereas 16 of 38 cases were T790M positive and baseline EGFR activating mutation negative. Thirty-nine of 110 patients (35.5%) displayed only the baseline EGFR activating mutations and were T790M negative. Finally, thirty-three patients out of 110 (30.0%) had a negative LB.
Reasons for LB testing over time and through LBs
We reported the reason for requesting each LB. A significant reduction in the “arbitrarily” reason, compared to “at clinical and radiological progression” and “at radiological progression”, was shown over time (P=0.0114) (Table 2). The “arbitrarily” motivation increased through subsequent biopsies, although not significantly (Table 3).
Table 2
| Year of liquid biopsy | 2016 | 2017 | 2018 | 2019 | 2020 | Total | P value (trend test) |
|---|---|---|---|---|---|---|---|
| Reason for liquid biopsy request, n (%) | 0.0114 | ||||||
| At clinical and radiological progression | 2 (6.9) | 13 (17.6) | 18 (25.0) | 20 (18.3) | 12 (30.8) | 65 (20.1) | |
| At radiological progression | 15 (51.7) | 47 (63.5) | 40 (55.6) | 73 (67.0) | 22 (56.4) | 197 (61.0) | |
| Arbitrarily | 12 (41.4) | 14 (18.9) | 14 (19.4) | 16 (14.7) | 5 (12.8) | 61 (18.9) | |
| Missing | 0 | 0 | 0 | 1 | 2 | 3 | |
| Totala | 29 | 74 | 72 | 110 | 41 | 326 | |
a, percent values were calculated excluding missing data.
Table 3
| Number of repeated liquid biopsy | First | Second | Third | Fourth | Fifth | Sixthb | Seventhb | Total | P value (trend test) |
|---|---|---|---|---|---|---|---|---|---|
| Reason for liquid biopsy request, n (%) | 0.0651 | ||||||||
| At clinical and radiological progression | 17 (15.5) | 23 (21.1) | 14 (23.7) | 6 (21.4) | 3 (27.3) | 1 (25.0) | 1 (50.0) | 65 (20.1) | |
| At radiological progression | 80 (72.7) | 60 (55.0) | 34 (57.6) | 16 (57.2) | 5 (45.4) | 2 (50.0) | 0 (0.0) | 197 (61.0) | |
| Arbitrarily | 13 (11.8) | 26 (23.9) | 11 (18.7) | 6 (21.4) | 3 (27.3) | 1 (25.0) | 1 (50.0) | 61 (18.9) | |
| Missing | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 3 | |
| Totala | 110 | 110 | 59 | 30 | 11 | 4 | 2 | 326 | |
a, percent values were calculated excluding missing data; b, sixth and seventh liquid biopsies were excluded from the trend evaluation because of limited numerosity.
We collected information about the treatment that patients received after each LB (Figure 2). Forty-four patients out of 110 (40%) changed systemic therapy, whereas 66 (60%) patients did not. A significant increase in the probability of administering chemotherapy after LB compared to other therapies was found throughout subsequent LBs (P<0.001) (Table S1).
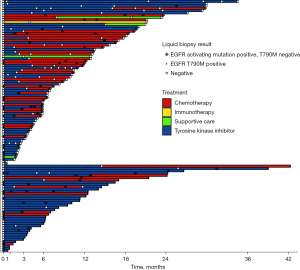
Prevalence of EGFR T790M mutation throughout LBs
We analyzed the prevalence of T790M mutation across each subsequent LB (Table 4). The probability of finding the T790M mutation for a patient across subsequent LBs did not significantly change (Table 4).
Table 4
| Number of repeated liquid biopsy | Firsta | Second | Third | Fourth | Fifth | Sixth | Seventha | Total | P value (trend test) |
|---|---|---|---|---|---|---|---|---|---|
| T790M, n (%) | 0.7164 | ||||||||
| Negative | 110 (100.0) | 89 (80.9) | 49 (83.1) | 26 (86.7) | 9 (81.8) | 3 (75.0) | 2 (100.0) | 288 (88.3) | |
| Positive | 0 (0.0) | 21 (19.1) | 10 (16.9) | 4 (13.3) | 2 (18.2) | 1 (25.0) | 0 (0.0) | 38 (11.7) | |
| Total | 110 | 110 | 59 | 30 | 11 | 4 | 2 | 326 |
a, first and seventh liquid biopsies were excluded from the trend evaluation. EGFR, epidermal growth factor receptor.
Features associated with EGFR T790M positivity and ctDNA positivity
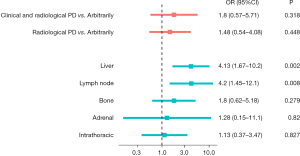
We evaluated the association between EGFR T790M positivity at LB and the reason for LB request. A trend was shown for association of T790M positivity when “clinical and radiological progression” motivation was present, compared to “radiological progression” and “arbitrarily” reason (Figure 3, Table S2). Conversely, liver and lymph node progression were significantly correlated to T790M positivity (OR 4.13, 95% CI: 1.67–10.2, P=0.002; and OR 4.2, 95% CI: 1.45–12.1, P=0.008, respectively) (Figure 3, Table S2).
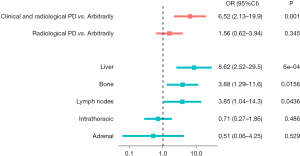
Moreover, the association between LB positivity for any EGFR mutation (i.e., T790M or other EGFR mutations) and the reason prompting LB were evaluated. Compared to “arbitrarily” reason, the reason “at clinical and radiological progression” was significantly associated to increased odds of finding any mutation (OR 6.52, 95% CI: 2.13–19.9, P=0.001) (Figure 4, Table S2). When the metastatic disease spread to the liver, bone or lymph nodes, the probability of detecting any mutation at LB was significantly higher (OR 8.62, 95% CI: 2.52–29.5, P=0.0006; OR 3.88, 95% CI: 1.29–11.6, P=0.0156, and OR 3.85, 95% CI: 1.04–14.3, P=0.0436, respectively) (Figure 4, Table S2).
TB as a salvage procedure
When LB was T790M negative and the procedure was feasible, TBs were performed in 43 out of 72 patients (59.7%) (Figure 1). Thirty-eight out of 43 (88.4%) patients had one tissue biopsy, while 5 (11.6%) patients had two tissue biopsies, for a total of 48 procedures. The site of tissue biopsy was intrathoracic in 40 out of 48 (83.3%) biopsies, liver in 4 cases (8.3%), lymph nodes in 3 cases (6.2%) and breast in 1 case (2.1%). Data on biopsy procedure was available for 47 cases: in 22 out of 47 cases (46.8%) the procedure was CT or ultrasound-guided, in 20 (42.5%) was in bronchoscopy, in 5 (10.7%) in thoracoscopy. Nine out of 48 tissue biopsies (18.8%) were both EGFR T790M and activating mutation positive, 16 (33.3%) were positive for EGFR activating mutation and T790M negative, and 23 (47.9%) were negative. The prevalence of T790M at tissue biopsy in our study was 20.9% (9 out of 43 patients).
Notably, in most patients (67 out of 110, 60.9%), tissue biopsy was not carried out. For two hundred seventy-three out of 278 LBs we recorded the reason for which tissue biopsy was not done: in 135 out of 273 blood draws (49.5%) there was no clinical progression, in 67 cases (24.6%) tissue biopsy was not feasible due to the biopsy site, in 38 cases (13.9%) LB was T790M positive, in 22 cases (8.0%) the patient’s conditions worsened and for patient refusal in 11 cases (4.0%).
The overall T790M prevalence in the study population by liquid and tissue biopsy was 47/110 (42.7%).
Discussion
This real-world, multicenter study evaluated the use of EGFR LB for detecting resistance mechanisms in a cohort of patients with EGFR positive advanced NSCLC, after progression to first or second generation EGFR TKI. The impact of LB on the diagnostic-therapeutic pathway was also described.
Randomized clinical trials and several real-world studies demonstrated osimertinib efficacy in second and in further lines in EGFR T790M mutation positive patients (11,34-39). Thus, international guidelines include EGFR mutation testing through LB at disease progression after first or second generation TKIs treatment to find T790M acquired mutation (6). When LB is T790M negative, tissue biopsy is recommended to identify T790M mutation and, if present, switch to osimertinib (6). However, TB is frequently not feasible due to technical issues or worsening conditions of the patient (40-42). In this case, a common clinical strategy is to repeat LB to seek for T790M mutation. Few real-world studies described this medical oncologist attitude (43-46) and, to the best of our knowledge, this is the first work to specifically focus on this topic in a large patient population, and to include only patients with repeated EGFR testing at LB and a first T790M negative LB.
Among the real-world experiences, a work by Spence et al. included 343 sequential patients with progression after first of second generation TKI, of which 82 patients were T790M positive at initial LB testing. Eighty-four patients received additional follow-up testing. Fifty-one patients had serial blood specimens (a total of 59 LBs), 34 patients TB (for a total of 35 procedures) and 16 patients cytology testing (18 specimens in total). Eight of 59 (13.6%) repeated LBs were T790M positive, 15 of 35 (42.9%) tissue biopsies and 7 of 18 (38.8%) cytology specimens (43).
The work by Reale and colleagues reported data on 308 consecutive patients with progression to upfront first or second generation TKI, of which 169 underwent a LB and 66 (39%) were T790M positive. Forty-eight patients underwent further testing with liquid or tissue biopsy, 24 (50%) of these patients were T790M positive (44).
A third study by Minari et al. included 120 consecutive patients with progression to upfront first or second generation TKI, 23 (19.2%) of which tested T790M positive at first LB. Forty patients underwent a second LB with 5 (12.5%) positive samples for T790M. Twelve patients underwent a third LB, with 3 (25.0%) patients positive for T790M and, finally, two patients underwent a fourth LB with no T790M positive samples. Fifty-four (60.7%) of 89 T790M negative patients underwent TB, with 28 (56%) patients positive for T790M (45).
Finally, Silveira and colleagues evaluated 111 consecutive patients, with 40 (36.0%) patients T790M positive at LB. Eighteen T790M negative patients received further testing: 6 patients had another LB, with the detection of T790M in 3 cases (50%), while 12 had tissue biopsy, with 2 T790M positive cases (16.7%) (46).
The prevalence of EGFR T790M mutation using LB in our cohort was 34.5%, consistently with other real-life studies where prevalence ranged from 24% to 52% (43-46). This value is less than the overall proportion of T790M mutation as a resistance mechanism (about 50%), as expected, due to the limited sensitivity of LB compared to tissue biopsy.
Furthermore, we described the decision-making process prompting LB request. The most reported reasons for testing were “at radiological progression” and “at radiological and clinical progression”, compared to “arbitrarily”. We showed a significantly reduction of the “arbitrarily” reason over time. This evolution over time can be explained by the improvement of clinicians’ learning curve about LB and increasing data showing better sensitivity for LB in patients with high disease burden (28,29,47).
Interestingly, we showed that the prevalence of EGFR T790M mutation across repeated LBs did not significantly change over time. This original finding and the observation that osimertinib is effective also beyond second line (34-38) suggest the utility of repeated LB in T790M negative patients at every disease progression event in order to detect T790M mutation.
Moreover, we explored the correlation of EGFR mutations and T790M mutation at LB with clinical and pathological features. The features associated with the presence of any EGFR mutation at LB, an estimate of ctDNA “shedding” status, was clinical and radiological progression, or, similarly, the presence of liver, bone or nodal metastases. These factors correlate to disease burden and spread. Conversely, only liver and lymph node progression were associated to T790M positivity. These results are coherent with other published works (28,29,43,47).
We finally collected data on TBs. TB was carried out in 59.7% of T790M negative patients. The most frequent reason accounting for no TB was technical feasibility, followed by the patient’s clinical deterioration. Other real-life experiences reported similar or lower proportions of patients undergoing TB and the same issues we found for not carrying out the procedure (40,41,45,46). In our study, the prevalence of EGFR T790M mutation in TB was 20.9%. This relatively low value could possibly be explained by tumor spatial heterogeneity, which could be overcome by LB.
Last, the overall prevalence of T790M mutation in our study, considering both liquid and tissue biopsy, was 42.7%, which is in line with previous studies (9,10).
The main limitations of our work are its retrospective nature and the relatively small number of patients with data on TBs.
Future research should aim to prospectively clarify the best timing, strategies and factors driving serial LB testing, in order to optimize the diagnostic yield and the clinical utility of this test. These findings could be translated to the management of patients receiving upfront osimertinib and experiencing acquired resistance, monitored through serial NGS, and, similarly, to patients receiving other targeted treatments.
Conclusions
Repeated EGFR LB mutational analysis can be used in a real-life scenario to detect EGFR T790M mutation acquisition.
Acknowledgments
Funding: This study was funded by Ricerca Corrente funding from the Italian Ministry of Health.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-745/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-745/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-745/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study complies with the Declaration of Helsinki (as revised in 2013). Ethics Committee approval by our Institutions was not required because of the observational intent of the data collection from the diagnostic routine practice. Informed consent form for diagnostic procedures and treatment was signed by all patients, according to clinical practice and to the Regulation EU 2016/679 of the European Parliament and of the Council on personal data protection.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Zhang YL, Yuan JQ, Wang KF, et al. The prevalence of EGFR mutation in patients with non-small cell lung cancer: a systematic review and meta-analysis. Oncotarget 2016;7:78985-93. [Crossref] [PubMed]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [Crossref] [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327-34. [Crossref] [PubMed]
- Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018;29:iv192-237. [Crossref]
- Yun CH, Mengwasser KE, Toms AV, et al. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc Natl Acad Sci U S A 2008;105:2070-5. [Crossref] [PubMed]
- Oxnard GR, Miller VA, Robson ME, et al. Screening for germline EGFR T790M mutations through lung cancer genotyping. J Thorac Oncol 2012;7:1049-52. [Crossref] [PubMed]
- Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res 2013;19:2240-7. [Crossref] [PubMed]
- Westover D, Zugazagoitia J, Cho BC, et al. Mechanisms of acquired resistance to first- and second-generation EGFR tyrosine kinase inhibitors. Ann Oncol 2018;29:i10-9. [Crossref] [PubMed]
- Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N Engl J Med 2017;376:629-40. [Crossref] [PubMed]
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:113-25. [Crossref] [PubMed]
- Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N Engl J Med 2020;382:41-50. [Crossref] [PubMed]
- Ramalingam SS, Cheng Y, Zhou C, et al. Mechanisms of acquired resistance to first-line osimertinib: Preliminary data from the phase III FLAURA study. Ann Oncol 2018;29:viii740. [Crossref]
- Schoenfeld AJ, Chan JM, Kubota D, et al. Tumor Analyses Reveal Squamous Transformation and Off-Target Alterations As Early Resistance Mechanisms to First-line Osimertinib in EGFR-Mutant Lung Cancer. Clin Cancer Res 2020;26:2654-63. [Crossref] [PubMed]
- Leonetti A, Sharma S, Minari R, et al. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br J Cancer 2019;121:725-37. [Crossref] [PubMed]
- Bauml J, Cho BC, Park K, et al. Amivantamab in combination with lazertinib for the treatment of osimertinib-relapsed, chemotherapy-naïve EGFR mutant (EGFRm) non-small cell lung cancer (NSCLC) and potential biomarkers for response. J Clin Oncol 2021;39:abstr 9006.
- Oxnard GR, Yang JC, Yu H, et al. TATTON: a multi-arm, phase Ib trial of osimertinib combined with selumetinib, savolitinib, or durvalumab in EGFR-mutant lung cancer. Ann Oncol 2020;31:507-16. [Crossref] [PubMed]
- Oxnard GR, Cantarini M, Frewer P, et al. SAVANNAH: A Phase II trial of osimertinib plus savolitinib for patients (pts) with EGFR-mutant, MET-driven (MET+), locally advanced or metastatic non-small cell lung cancer (NSCLC), following disease progression on osimertinib. J Clin Oncol 2019;37: abstr TPS9119.
- Yang JCH, Ellers-Lenz B, Straub J, et al. INSIGHT 2: Tepotinib plus osimertinib in patients with EGFR-mutant NSCLC having acquired resistance to EGFR TKIs due to MET-amplification: A phase II trial in progress study. Ann Oncol 2019;30:ix181. [Crossref]
- Siravegna G, Mussolin B, Venesio T, et al. How liquid biopsies can change clinical practice in oncology. Ann Oncol 2019;30:1580-90. [Crossref] [PubMed]
- Volckmar AL, Sültmann H, Riediger A, et al. A field guide for cancer diagnostics using cell-free DNA: From principles to practice and clinical applications. Genes Chromosomes Cancer 2018;57:123-39. [Crossref] [PubMed]
- Lindeman NI, Cagle PT, Aisner DL, et al. Updated Molecular Testing Guideline for the Selection of Lung Cancer Patients for Treatment With Targeted Tyrosine Kinase Inhibitors: Guideline From the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. Arch Pathol Lab Med 2018;142:321-46. [Crossref] [PubMed]
- Mok T, Wu YL, Lee JS, et al. Detection and Dynamic Changes of EGFR Mutations from Circulating Tumor DNA as a Predictor of Survival Outcomes in NSCLC Patients Treated with First-line Intercalated Erlotinib and Chemotherapy. Clin Cancer Res 2015;21:3196-203. [Crossref] [PubMed]
- Oxnard GR, Paweletz CP, Kuang Y, et al. Noninvasive detection of response and resistance in EGFR-mutant lung cancer using quantitative next-generation genotyping of cell-free plasma DNA. Clin Cancer Res 2014;20:1698-705. [Crossref] [PubMed]
- Li Z, Zhang Y, Bao W, et al. Insufficiency of peripheral blood as a substitute tissue for detecting EGFR mutations in lung cancer: a meta-analysis. Target Oncol 2014;9:381-8. [Crossref] [PubMed]
- Oxnard GR, Thress KS, Alden RS, et al. Association Between Plasma Genotyping and Outcomes of Treatment With Osimertinib (AZD9291) in Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2016;34:3375-82. [Crossref] [PubMed]
- Passiglia F, Rizzo S, Di Maio M, et al. The diagnostic accuracy of circulating tumor DNA for the detection of EGFR-T790M mutation in NSCLC: a systematic review and meta-analysis. Sci Rep 2018;8:13379. [Crossref] [PubMed]
- Dal Maso A, Lorenzi M, Roca E, et al. Clinical Features and Progression Pattern of Acquired T790M-positive Compared With T790M-negative EGFR Mutant Non-small-cell Lung Cancer: Catching Tumor and Clinical Heterogeneity Over Time Through Liquid Biopsy. Clin Lung Cancer 2020;21:1-14.e3. [Crossref] [PubMed]
- Aldea M, Hendriks L, Mezquita L, et al. Circulating Tumor DNA Analysis for Patients with Oncogene-Addicted NSCLC With Isolated Central Nervous System Progression. J Thorac Oncol 2020;15:383-91. [Crossref] [PubMed]
- Remon J, Menis J, Hasan B, et al. The APPLE Trial: Feasibility and Activity of AZD9291 (Osimertinib) Treatment on Positive PLasma T790M in EGFR-mutant NSCLC Patients. EORTC 1613. Clin Lung Cancer 2017;18:583-8. [Crossref] [PubMed]
- Iwama E, Sakai K, Hidaka N, et al. Longitudinal monitoring of somatic genetic alterations in circulating cell-free DNA during treatment with epidermal growth factor receptor-tyrosine kinase inhibitors. Cancer 2020;126:219-27. [Crossref] [PubMed]
- Cortiula F, Pasello G, Follador A, et al. A Multi-Center, Real-Life Experience on Liquid Biopsy Practice for EGFR Testing in Non-Small Cell Lung Cancer (NSCLC) Patients. Diagnostics (Basel) 2020;10:765. [Crossref] [PubMed]
- Ahn MJ, Tsai CM, Shepherd FA, et al. Osimertinib in patients with T790M mutation-positive, advanced non-small cell lung cancer: Long-term follow-up from a pooled analysis of 2 phase 2 studies. Cancer 2019;125:892-901. [Crossref] [PubMed]
- Mu Y, Xing P, Hao X, et al. Real-World Data Of Osimertinib In Patients With Pretreated Non-Small Cell Lung Cancer: A Retrospective Study. Cancer Manag Res 2019;11:9243-51. [Crossref] [PubMed]
- Auliac JB, Pérol M, Planchard D, et al. Real-life efficacy of osimertinib in pretreated patients with advanced non-small cell lung cancer harboring EGFR T790M mutation. Lung Cancer 2019;127:96-102. [Crossref] [PubMed]
- Auliac JB, Saboundji K, Andre M, et al. Real-Life Efficacy of Osimertinib in Pretreated Octogenarian Patients with T790M-Mutated Advanced Non-small Cell Lung Cancer. Target Oncol 2019;14:307-14. [Crossref] [PubMed]
- Dal Maso A, Lorenzi M, Ferro A, et al. Real-world data on treatment outcomes in EGFR-mutant non-small-cell lung cancer patients receiving osimertinib in second or further lines. Future Oncol 2021;17:2513-27. [Crossref] [PubMed]
- Jin Y, Lin C, Shi X, et al. Impact of clinical and molecular features on efficacy and outcome of patients with non-small cell lung cancer receiving second-line osimertinib. BMC Cancer 2022;22:586. [Crossref] [PubMed]
- Kawamura T, Kenmotsu H, Taira T, et al. Rebiopsy for patients with non-small-cell lung cancer after epidermal growth factor receptor-tyrosine kinase inhibitor failure. Cancer Sci 2016;107:1001-5. [Crossref] [PubMed]
- Kim TO, Oh IJ, Kho BG, et al. Feasibility of re-biopsy and EGFR mutation analysis in patients with non-small cell lung cancer. Thorac Cancer 2018;9:856-64. [Crossref] [PubMed]
- Nieva J, Reckamp KL, Potter D, et al. Retrospective Analysis of Real-World Management of EGFR-Mutated Advanced NSCLC, After First-Line EGFR-TKI Treatment: US Treatment Patterns, Attrition, and Survival Data. Drugs Real World Outcomes 2022;9:333-45. [Crossref] [PubMed]
- Spence T, Perera S, Weiss J, et al. Clinical implementation of circulating tumour DNA testing for EGFR T790M for detection of treatment resistance in non-small cell lung cancer. J Clin Pathol 2021;74:91-7. [Crossref] [PubMed]
- Reale ML, Chiari R, Tiseo M, et al. Be-TeaM: An Italian real-world observational study on second-line therapy for EGFR-mutated NSCLC patients. Lung Cancer 2020;140:71-9. [Crossref] [PubMed]
- Minari R, Mazzaschi G, Bordi P, et al. Detection of EGFR-Activating and T790M Mutations Using Liquid Biopsy in Patients With EGFR-Mutated Non-Small-Cell Lung Cancer Whose Disease Has Progressed During Treatment With First- and Second-Generation Tyrosine Kinase Inhibitors: A Multicenter Real-Life Retrospective Study. Clin Lung Cancer 2020;21:e464-73. [Crossref] [PubMed]
- Silveira C, Sousa AC, Janeiro A, et al. Detection and quantification of EGFR T790M mutation in liquid biopsies by droplet digital PCR. Transl Lung Cancer Res 2021;10:1200-8. [Crossref] [PubMed]
- Passiglia F, Rizzo S, Rolfo C, et al. Metastatic Site Location Influences the Diagnostic Accuracy of ctDNA EGFR- Mutation Testing in NSCLC Patients: a Pooled Analysis. Curr Cancer Drug Targets 2018;18:697-705. [Crossref] [PubMed]


