Non-invasive ventilation in immunocompromised patients with acute hypoxemic respiratory failure
Introduction
The survival rate of immunocompromised patients, such as those with hematological malignancies, solid organ transplant, acquired immunodeficiency syndrome, and those receiving corticosteroid or cytotoxic therapy for a non-malignant disease, has progressively improved due to the remarkable advances in diagnostic and therapeutic options (1). Simultaneously, there has been an increase in the number of immunocompromised patients with life threatening complications (2-4), with recent studies showing that 15% of patients with acute leukemia and 20% of bone marrow transplantation recipients require intensive care unit (ICU) admission (5). The main reason for ICU admission in these patient populations is acute hypoxemic respiratory failure (5,6), which is associated with a high mortality rate, particularly when invasive mechanical ventilation is required (2,7). This raises the interest on non-invasive ventilation (NIV), a technique that provides ventilator assistance without the use of endotracheal tube. NIV carries the advantages of lower ventilator-associated pneumonia and sedation requirements when compared to invasive mechanical ventilation. Furthermore, although side effects of NIV have been described, including facial skin lesions, gastric distension and patient discomfort related to noise, claustrophobia, nasal or oral dryness and nasal congestion, their incidence is low and largely preventable with proper management of the technique (8). Therefore, applying NIV, and thus avoiding endotracheal intubation and invasive mechanical ventilation with its side effects (9,10), may potentially decrease the mortality rate in immunocompromised patients (5,11-13).
This perspective reviews the findings from a recent randomized controlled trial (RCT) assessing whether early intermittent respiratory support with NIV has a role in reducing the mortality rate of immunocompromised patients with non-hypercapnic hypoxemic respiratory failure in the context of the current critical care landscape, and in light of recent results from other trials focused on the early management of acute hypoxemic respiratory failure.
Current evidence and recommendations
Several small single center RCTs have demonstrated positive patient outcomes with the early use of NIV.
Hilbert et al. investigated this hypothesis in a seminal study published in 2001 (12). In this single center RCT, 52 immunocompromised patients (with immunosuppression from several different etiologies) were enrolled if they had pulmonary infiltrates, fever, and hypoxemic acute respiratory failure, defined by the presence of dyspnea at rest, respiratory rate greater than 30 breaths per minute and a partial pressure of arterial oxygen to fraction of inspired oxygen ratio (PaO2:FiO2) of less than 200 mmHg while breathing oxygen. These patients were randomly allocated to receive either standard oxygen treatment via facemask or intermittent NIV. Compared to standard oxygen therapy, the group treated with NIV had lower rates of endotracheal intubation (12/26 vs. 20/26 patients, P=0.03), and in-hospital mortality (50% vs. 81%, P=0.02).
Antonelli et al. reported the efficacy of NIV in reducing the need of endotracheal intubation and invasive mechanical ventilation in immunocompromised patients after solid organ transplantation with hypoxemic respiratory failure (14). In approximately 2 years, 40 patients with a PaO2:FiO2 of less than 200 mmHg while breathing oxygen and active use of accessory respiratory muscles were randomized to receive either NIV or treatment with supplemental oxygen via Venturi Mask. The group treated with NIV demonstrated significantly lower rates of endotracheal intubation (20% vs. 70%, P=0.002) and ICU mortality (20% vs. 50%, P=0.05). However, no significant difference was found in the in-hospital mortality rate.
Moreover, a more recent RCT investigated the potential role of early use of continuous positive airway pressure (CPAP) in patients with hematological malignancies (15). Forty patients on the ward with bilateral infiltrates, respiratory rate greater than 25 breaths/min and an oxygen saturation of less than 90% while breathing on room air, were randomized to receive oxygen (FiO2 =50%) either by facemask or helmet CPAP at 10 cmH2O. Overall, significantly fewer patients treated with CPAP required NIV or invasive mechanical ventilation (4 vs. 16 patients; P=0.0002).
Based on these data, NIV is currently considered in many centers as first line treatment for hypoxemic respiratory failure in patients with various causes of immunosuppression (16). Moreover, the 2011 Canadian guidelines for the use of NIV in critical care settings suggested the use of NIV in immunocompromised patients with a Grade 2B recommendation (17).
However, these very encouraging results have not been confirmed in subsequent observational (18) and randomized clinical (19) studies. In particular, a recent randomized trial investigated the role of early application of NIV in 86 patients with hypoxemic respiratory failure after allogeneic hematopoietic stem cell transplantation (19). In this study, early treatment with NIV did not affect the rate of endotracheal intubation, ICU admission, or patient survival. However, these results may be significantly affected by the high crossover rate given that 16 out of 44 patients in the group allocated to the treatment with conventional oxygen alone received NIV for failure to achieve the oxygenation target.
Therefore, the beneficial effect of NIV in immunocompromised patients has recently been questioned (20). Most of the studies showing a beneficial effect of NIV did not stratify patients for the cause of immunosuppression or timing (early vs. late) of NIV application. Moreover, the expected mortality rate of immunocompromised patients with acute respiratory failure, although still high, has progressively decreased from 50–80% in the year 2001 (12) to current 20–60% (21-23). This is likely due to the advancement in the management of critically ill patients, with particular regards to invasive mechanical ventilation, with a consequent potential lower clinical impact provided by treatment with NIV (24).
These observations directed the French group led by Lemiale and Azoulay to a new equipoise on the efficacy of NIV in immunocompromised patients with acute hypoxemic non hypercapnic respiratory failure.
The new trial: can early non-invasive ventilation (NIV) reduce mortality in immunocompromised patients with acute hypoxemic respiratory failure?
A multicenter RCT was conducted (25) to assess the potential benefit of early NIV in reducing the mortality rate among immunocompromised patients who developed non-hypercapnic hypoxemic respiratory failure in less than 72 h. In 28 intensive care units from France and Belgium with established experience in delivering NIV, 374 immunocompromised patients with PaO2 less than 60 mmHg on room air, or respiratory rate greater than 30/min, or signs of respiratory distress, were randomized to receive NIV or conventional oxygen therapy. Of note, patients were stratified according to the cause of immune deficiency in two groups, one with hematologic malignancy or solid cancer, and one with solid organ transplant or long-term/high-dose immunosuppressive treatment. No difference was found between groups with regards to the primary endpoint, the mortality rate at 28 days after randomization (NIV 24.1% vs. oxygen 27.3%; 95% CI, −12.1 to 5.6; P=0.47). Secondary outcomes were also similar between the two groups: proportion of patients requiring endotracheal intubation (NIV 38.2% vs. oxygen 44.8%; 95% CI, −16.6 to 3.4; P=0.20), time to intubation, ICU-acquired infections, duration of mechanical ventilation, and length of stay in ICU and hospital. Also the analysis of the two pre-specified subgroups did not result in any significant difference. The conclusion of the investigators was that among immunocompromised patients admitted to the ICU with hypoxemic acute respiratory failure, early NIV compared with oxygen therapy alone did not reduce 28-day mortality.
Table 1 highlights design and results of this trial in comparison to the previous RCTs from Antonelli et al. and Hilbert et al.
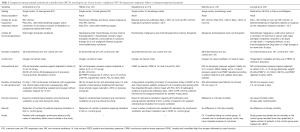
Full table
This was a large and well conducted RCT assessing the early use of NIV. There was a high protocol adherence among institutions with expertise in delivering NIV and caring for immunocompromised patients. This trial also powered the primary outcome to reducing patient mortality in comparison to the trials performed by Antonelli and Hilbert et al. who focused on reducing the need for intubation. However, this RCT carries a few limitations, acknowledged by the investigators. In particular, the lower mortality rate than expected in the control group reduced the power of the study to find a significant difference in the primary outcome. Indeed, the trial was designed anticipating a mortality of 35% in the oxygen treated group, whereas the observed mortality rate was 27.3%. As a result, the possibility of drawing definitive conclusions and a clinically meaningful effect based on the study findings is limited.
The reasons of this low mortality rate may be given by a few considerations related to the management of immunocompromised patients. Practices have changed and the prognosis has improved over recent years. Furthermore, the centers involved in the study carry high level of expertise in the field of immunocompromised ICU patients and in NIV. The relationship between case volume and outcomes has been evidenced in this specific field (2). Importantly, the authors speculated that the low mortality rate was potentially due to the higher number of patients in the control group that were treated with heated and humidified high flow oxygen delivered by nasal cannula (HFNC) system compared to the NIV group (44% vs. 31%, P=0.01, respectively). The support provided by HFNC in the control group could have remarkably reduced the need of invasive mechanical ventilation, thus masking the potential efficacious effect of NIV in this patient population. HFNC, which has gained increasing clinical and scientific interest (26-47), can deliver up to 100% of heated and humidified fraction of inspired oxygen at a maximum flow rate of 60 L/min. This flow rate is significantly higher than the one delivered via nasal prongs or facemask, which is able to provide a maximum flow of 15 L/min. This limited flow rate is important given that patients with severe respiratory distress often require inspiratory flow rates ranging between 30 and 120 L/min. The consequence of this difference in required inspiratory airflow and provided flow rate is the dilution of the oxygen therapy with room air, so that the delivered FiO2 is lower than the set FiO2 (48). The high flow rates delivered by HFNC may partially overcome this issue. In addition, the high airflow delivered directly to the nasopharynx, improves carbon dioxide clearance and reduces dead space, thereby improving alveolar ventilation (29,41,48), and may also induce generation of positive end expiratory pressure (PEEP) (27,30,36,37). In healthy volunteers treated with HFNC with closed mouth and a flow rate of 60 L/min the measured PEEP was as high as 7.4 cmH2O (30). Furthermore, the heated and humidified airflow delivered with HFNC may provide more comfort to patients requiring oxygen therapy (28,29,48). These potential benefits of HFNC should be studied in a systematic trial and compared to NIV, helmet CPAP and conventional oxygen therapy.
Some other limitations of the study may be related to the actual dose of NIV provided. First, the median durations of treatment were 8 h during the first 24 h, 6 h on day 2 and 5 h on day 3. At present we do not know whether longer durations of NIV would provide different outcomes. Previous studies in immunocompromised patients such as the one from Hilbert et al. (12) reported similar although slightly higher mean durations of treatment, with 9 h of NIV in the first day, and 7 h in the subsequent days. Second, the level of PEEP may play a significant role. As evidenced by the Editorial from Patel and Kress accompanying the study of Lemiale et al. (49), the physiologic goals of NIV in the treatment of acute hypoxemic respiratory failure rely on lung recruitment with proper use of PEEP and respiratory muscles unloading with addition of pressure support ventilation. Physiologic studies examining use of NIV in acute lung injury have suggested that a PEEP of at least 10 cmH2O is required to significantly improve PaO2:FiO2 ratio with therapy (50). The protocol of Lemiale et al. allowed an initial PEEP between 2 and 10 cmH2O, and then adjusted (together with FiO2) in order to maintain the peripheral capillary oxygen saturation at 92% or greater. Even if at present it is unclear and difficult to estimate the optimal clinical PEEP setting during NIV, either too low or too high PEEP values could potentially have deleterious consequences. Furthermore, interface-related problems such as facemask leaks or poor patient tolerance may limit accurate titration of PEEP and pressure support ventilation, thus decreasing the efficacy of NIV delivered via facemask (49). Third, excessive NIV support may cause alveolar overdistension or alveolar recruitment and derecruitment, the two main mechanisms of ventilator-induced lung injury (VILI), which may exacerbate the already established injury in patients with acute respiratory failure (48). The possible role of NIV in contributing to VILI may hence provide another explanation for the lack of efficacy of NIV in immunocompromised patients. Interestingly, Carteaux et al. assessed expired tidal volume in patients undergoing NIV for de novo acute hypoxemic respiratory failure in a recent prospective observational study involving 62 patients in a single institution university medical ICU, showing that delivered tidal volumes are higher than expected (49). In particular, the median (interquartile range) tidal volume was 9.8 mL/kg predicted body weight (8.1–11.1 mL/kg), although the targeted tidal volume was 6–8 mL/kg predicted body weight. In this study, high tidal volume was independently associated with NIV failure, which occurred in 51% of the cases. In the sub-group of patients with PaO2:FiO2 of less than 200 mmHg a tidal volume of 9.5 ml/kg accurately predicted NIV failure with a sensitivity of 82% and a specificity of 87%. These data are remarkable with regards of the potential contributing role of high tidal volume during NIV to VILI. In Lemiale et al.’s investigation, the median expiratory tidal volumes were 8.8 mL/kg of ideal body weight on day 1, 9.1 on day 2 and 9.5 on day 3, respectively. Although there were no significant differences in tidal volumes according to NIV success vs. failure or between survivors and non-survivors, the study may have not been adequately powered to make these distinctions based on tidal volume, and the role of excessively high tidal volumes achieved during NIV may have been underestimated.
Also differences in patient populations may be one of the reasons for the different findings in Lemiale et al.’s trial with respect to previous studies. Their patients showed lower degrees of tachypnea compared to Antonelli et al. and Hilbert et al.’s studies (respiratory rate of 25–27/min vs. 35–38/min) suggesting a difference in severity of the acute condition.
Limitations of current knowledge and future directions
Where do the recent findings from Lemiale et al.’s study leave the clinician at the bedside caring for immunocompromised patients in the ICU? Several questions remain open:
(I) The role of HFNC alone or in combination with NIV (using HFNC in between NIV sessions) in this patient population will need further investigation. As mentioned above, the higher number of patients in the control group that were treated with HFNC system compared to the NIV group in Lemiale et al.’s study may have partially explained the lower-than-predicted mortality observed. The data from the recent FLORALI study report that in a post hoc adjusted analysis that included the 238 patients with severe initial hypoxemia (PaO2:FiO2 ≤200 mmHg), the intubation rate was significantly lower among patients who received high-flow oxygen than among patients in the other two groups (P=0.009) (46). A multicenter parallel RCT in four intensive care units assessing the role of HFNC vs. Venturi mask oxygen in immunocompromised patients with acute hypoxemic respiratory failure was published by the group of Lemiale and Azoulay. Patients were randomized to 2 h of HFNC or Venturi mask oxygen (51). The primary endpoint was a need for invasive mechanical ventilation or NIV during the 2-h oxygen therapy period. They found no significant difference between the two groups (15% with HFNC and 8% with the Venturi mask, P=0.36). None of the secondary end-points, which included comfort, dyspnea and thirst, differed significantly between the two groups. The authors concluded that in immunocompromised patients with acute hypoxemic respiratory failure, a 2-h trial with HFNC did not improve mechanical ventilatory assistance or patient comfort compared with oxygen delivered via a simple Venturi mask. However, this study was underpowered given the low event rate and use of a one-sided hypothesis only. Furthermore, this trial focused only upon the initial 2 h after ICU admission and thus the role of HFNC for longer periods of time remains to be assessed.
(II) With improving technology in the near future, NIV might be delivered with interfaces that minimize facemask leaks thus improving the efficacy of treatment and leading to better patient outcomes. Furthermore, our capability to control tidal volumes more accurately may increase, helping us avoid propagation of injury through VILI.
(III) The concern around the potential detrimental effects of delaying intubation in patients who receive NIV remains open. A recent secondary analysis of a prospective observational cohort study published by Kangelaris et al. analyzed data on 457 patients with acute respiratory distress syndrome. Of them 106 (23%) were not intubated at the time of meeting all other acute respiratory distress syndrome criteria. Non-intubated patients had lower morbidity and severity of illness than intubated patients; however, mortality at 60 days was the same (36%) in both groups (P=0.91). Of the 106 non-intubated patients, 36 (34%) required intubation within the subsequent 3 days of follow-up, and this late-intubation subgroup had significantly higher 60-day mortality (56%) when compared with both early intubation group (36%, P<0.03) and patients never requiring intubation (26%; P=0.002). The increased mortality in the late intubation group persisted at 2-year follow-up (52). However, the authors reported that there was no evidence that NIV modified the association between intubation and mortality, i.e., delaying endotracheal intubation through the use of NIV did not account for increased mortality.
Conclusions
NIV remains an attractive modality when caring for immunocompromised patient with acute hypoxemic respiratory failure, in light of its potential to avoid the complications of invasive mechanical ventilation. Further adequately powered trials will help us understand which patient subpopulations will benefit the most from each technique (HFNC, NIV or invasive mechanical ventilation), and to identify the most appropriate timing of application of these techniques.
The ongoing efforts towards optimizing the management of acute hypoxemic respiratory failure in immunocompromised patients keep us hopeful that the mortality of these frail patients will continue to decrease in the coming years.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Brenner H, Gondos A, Arndt V. Recent major progress in long-term cancer patient survival disclosed by modeled period analysis. J Clin Oncol 2007;25:3274-80. [Crossref] [PubMed]
- Lecuyer L, Chevret S, Guidet B, et al. Case volume and mortality in haematological patients with acute respiratory failure. Eur Respir J 2008;32:748-54. [Crossref] [PubMed]
- Lecuyer L, Chevret S, Thiery G, et al. The ICU trial: a new admission policy for cancer patients requiring mechanical ventilation. Crit Care Med 2007;35:808-14. [Crossref] [PubMed]
- Khassawneh BY, White P Jr, Anaissie EJ, et al. Outcome from mechanical ventilation after autologous peripheral blood stem cell transplantation. Chest 2002;121:185-8. [Crossref] [PubMed]
- Molina R, Bernal T, Borges M, et al. Ventilatory support in critically ill hematology patients with respiratory failure. Crit Care 2012;16:R133. [Crossref] [PubMed]
- Gordon AC, Oakervee HE, Kaya B, et al. Incidence and outcome of critical illness amongst hospitalised patients with haematological malignancy: a prospective observational study of ward and intensive care unit based care. Anaesthesia 2005;60:340-7. [Crossref] [PubMed]
- Pène F, Aubron C, Azoulay E, et al. Outcome of critically ill allogeneic hematopoietic stem-cell transplantation recipients: a reappraisal of indications for organ failure supports. J Clin Oncol 2006;24:643-9. [Crossref] [PubMed]
- Carron M, Freo U. Complications of non-invasive ventilation techniques: a comprehensive qualitative review of randomized trials. Br J Anaesth 2013;110:896-914. [Crossref] [PubMed]
- Schnell D, Timsit JF, Darmon M, et al. Noninvasive mechanical ventilation in acute respiratory failure: trends in use and outcomes. Intensive Care Med 2014;40:582-91. [Crossref] [PubMed]
- Demoule A, Girou E, Richard JC, et al. Increased use of noninvasive ventilation in French intensive care units. Intensive Care Med 2006;32:1747-55. [Crossref] [PubMed]
- Rocco M, Dell'Utri D, Morelli A, et al. Noninvasive ventilation by helmet or face mask in immunocompromised patients: a case-control study. Chest 2004;126:1508-15. [Crossref] [PubMed]
- Hilbert G, Gruson D, Vargas F, et al. Noninvasive ventilation in immunosuppressed patients with pulmonary infiltrates, fever, and acute respiratory failure. N Engl J Med 2001;344:481-7. [Crossref] [PubMed]
- Principi T, Pantanetti S, Catani F, et al. Noninvasive continuous positive airway pressure delivered by helmet in hematological malignancy patients with hypoxemic acute respiratory failure. Intensive Care Med 2004;30:147-50. [Crossref] [PubMed]
- Antonelli M, Conti G, Bufi M, et al. Noninvasive ventilation for treatment of acute respiratory failure in patients undergoing solid organ transplantation: a randomized trial. JAMA 2000;283:235-41. [Crossref] [PubMed]
- Squadrone V, Massaia M, Bruno B, et al. Early CPAP prevents evolution of acute lung injury in patients with hematologic malignancy. Intensive Care Med 2010;36:1666-74. [Crossref] [PubMed]
- Demoule A, Chevret S, Carlucci A, et al. Changing use of noninvasive ventilation in critically ill patients: trends over 15 years in francophone countries. Intensive Care Med 2016;42:82-92. [Crossref] [PubMed]
- Keenan SP, Sinuff T, Burns KE, et al. Clinical practice guidelines for the use of noninvasive positive-pressure ventilation and noninvasive continuous positive airway pressure in the acute care setting. CMAJ 2011;183:E195-214. [Crossref] [PubMed]
- Azoulay E, Thiéry G, Chevret S, et al. The prognosis of acute respiratory failure in critically ill cancer patients. Medicine (Baltimore) 2004;83:360-70. [Crossref] [PubMed]
- Wermke M, Schiemanck S, Höffken G, et al. Respiratory failure in patients undergoing allogeneic hematopoietic SCT--a randomized trial on early non-invasive ventilation based on standard care hematology wards. Bone Marrow Transplant 2012;47:574-80. [Crossref] [PubMed]
- Azoulay E, Lemiale V. Non-invasive mechanical ventilation in hematology patients with hypoxemic acute respiratory failure: a false belief? Bone Marrow Transplant 2012;47:469-72. [Crossref] [PubMed]
- Azoulay E, Mokart D, Pène F, et al. Outcomes of critically ill patients with hematologic malignancies: prospective multicenter data from France and Belgium--a groupe de recherche respiratoire en réanimation onco-hématologique study. J Clin Oncol 2013;31:2810-8. [Crossref] [PubMed]
- Canet E, Osman D, Lambert J, et al. Acute respiratory failure in kidney transplant recipients: a multicenter study. Crit Care 2011;15:R91. [Crossref] [PubMed]
- Faguer S, Ciroldi M, Mariotte E, et al. Prognostic contributions of the underlying inflammatory disease and acute organ dysfunction in critically ill patients with systemic rheumatic diseases. Eur J Intern Med 2013;24:e40-4. [Crossref] [PubMed]
- Adda M, Coquet I, Darmon M, et al. Predictors of noninvasive ventilation failure in patients with hematologic malignancy and acute respiratory failure. Crit Care Med 2008;36:2766-72. [Crossref] [PubMed]
- Lemiale V, Mokart D, Resche-Rigon M, et al. Effect of noninvasive ventilation vs oxygen therapy on mortality among immunocompromised patients with acute respiratory failure: a randomized clinical trial. JAMA 2015;314:1711-9. [Crossref] [PubMed]
- Carratalá Perales JM, Llorens P, Brouzet B, et al. High-Flow therapy via nasal cannula in acute heart failure. Rev Esp Cardiol 2011;64:723-5. [PubMed]
- Corley A, Caruana LR, Barnett AG, et al. Oxygen delivery through high-flow nasal cannulae increase end-expiratory lung volume and reduce respiratory rate in post-cardiac surgical patients. Br J Anaesth 2011;107:998-1004. [Crossref] [PubMed]
- Cuquemelle E, Pham T, Papon JF, et al. Heated and humidified high-flow oxygen therapy reduces discomfort during hypoxemic respiratory failure. Respir Care 2012;57:1571-7. [Crossref] [PubMed]
- Dysart K, Miller TL, Wolfson MR, et al. Research in high flow therapy: mechanisms of action. Respir Med 2009;103:1400-5. [Crossref] [PubMed]
- Groves N, Tobin A. High flow nasal oxygen generates positive airway pressure in adult volunteers. Aust Crit Care 2007;20:126-31. [Crossref] [PubMed]
- Kernick J, Magarey J. What is the evidence for the use of high flow nasal cannula oxygen in adult patients admitted to critical care units? A systematic review. Aust Crit Care 2010;23:53-70. [Crossref] [PubMed]
- Lee JH, Rehder KJ, Williford L, et al. Use of high flow nasal cannula in critically ill infants, children, and adults: a critical review of the literature. Intensive Care Med 2013;39:247-57. [Crossref] [PubMed]
- Lenglet H, Sztrymf B, Leroy C, et al. Humidified high flow nasal oxygen during respiratory failure in the emergency department: feasibility and efficacy. Respir Care 2012;57:1873-8. [Crossref] [PubMed]
- Maggiore SM, Idone FA, Vaschetto R, et al. Nasal high-flow versus Venturi mask oxygen therapy after extubation. Effects on oxygenation, comfort, and clinical outcome. Am J Respir Crit Care Med 2014;190:282-8. [Crossref] [PubMed]
- Murphy M, Scanlon A. A randomised controlled study suggesting equivalence between full face vs. nasal high flow oxygen delivery on health outcomes. Aust Crit Care 2011;24:139-41. [Crossref] [PubMed]
- Parke R, McGuinness S, Eccleston M. Nasal high-flow therapy delivers low level positive airway pressure. Br J Anaesth 2009;103:886-90. [Crossref] [PubMed]
- Parke RL, Eccleston ML, McGuinness SP. The effects of flow on airway pressure during nasal high-flow oxygen therapy. Respir Care 2011;56:1151-5. [Crossref] [PubMed]
- Rello J, Pérez M, Roca O, et al. High-flow nasal therapy in adults with severe acute respiratory infection: a cohort study in patients with 2009 influenza A/H1N1v. J Crit Care 2012;27:434-9. [Crossref] [PubMed]
- Riera J, Pérez P, Cortés J, et al. Effect of high-flow nasal cannula and body position on end-expiratory lung volume: a cohort study using electrical impedance tomography. Respir Care 2013;58:589-96. [Crossref] [PubMed]
- Roca O, de Acilu MG, Caralt B, et al. Humidified high flow nasal cannula supportive therapy improves outcomes in lung transplant recipients readmitted to the intensive care unit because of acute respiratory failure. Transplantation 2015;99:1092-8. [Crossref] [PubMed]
- Roca O, Riera J, Torres F, et al. High-flow oxygen therapy in acute respiratory failure. Respir Care 2010;55:408-13. [PubMed]
- Schwabbauer N, Berg B, Blumenstock G, et al. Nasal high-flow oxygen therapy in patients with hypoxic respiratory failure: effect on functional and subjective respiratory parameters compared to conventional oxygen therapy and non-invasive ventilation (NIV). BMC Anesthesiol 2014;14:66. [Crossref] [PubMed]
- Sztrymf B, Messika J, Bertrand F, et al. Beneficial effects of humidified high flow nasal oxygen in critical care patients: a prospective pilot study. Intensive Care Med 2011;37:1780-6. [Crossref] [PubMed]
- Sztrymf B, Messika J, Mayot T, et al. Impact of high-flow nasal cannula oxygen therapy on intensive care unit patients with acute respiratory failure: a prospective observational study. J Crit Care 2012;27:324.e9-13.
- Parke RL, McGuinness SP, Eccleston ML, et al. A preliminary randomized controlled trial to assess effectiveness of nasal high-flow oxygen in intensive care patients. Respir Care 2011;56:265-70. [Crossref] [PubMed]
- Frat JP, Thille AW, Mercat A, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med 2015;372:2185-96. [Crossref] [PubMed]
- Vargas F, Saint-Leger M, Boyer A, et al. Physiologic effects of high-flow nasal cannula oxygen in critical care subjects. Respir Care 2015;60:1369-76. [Crossref] [PubMed]
- Ricard JD. High flow nasal oxygen in acute respiratory failure. Minerva Anestesiol 2012;78:836-41. [PubMed]
- Patel BK, Kress JP. The changing landscape of noninvasive ventilation in the intensive care unit. JAMA 2015;314:1697-9. [Crossref] [PubMed]
- L'Her E, Deye N, Lellouche F, et al. Physiologic effects of noninvasive ventilation during acute lung injury. Am J Respir Crit Care Med 2005;172:1112-8. [Crossref] [PubMed]
- Lemiale V, Mokart D, Mayaux J, et al. The effects of a 2-h trial of high-flow oxygen by nasal cannula versus Venturi mask in immunocompromised patients with hypoxemic acute respiratory failure: a multicenter randomized trial. Crit Care 2015;19:380. [Crossref] [PubMed]
- Kangelaris KN, Ware LB, Wang CY, et al. Timing of intubation and clinical outcomes in adults with acute respiratory distress syndrome. Crit Care Med 2016;44:120-9. [Crossref] [PubMed]


