Impact of attenuated plaques on TIMI grade flow and clinical outcomes of coronary artery disease patients: a systematic review and meta analysis
Introduction
Cardiovascular disease is the first and foremost cause of preventable death globally (1), The National Health and Nutrition Examination Survey (NHANES) estimated that 17,600 000 Americans >20 years of age had coronary artery disease (CAD) in 2003 to 2006 (2). Percutaneous coronary intervention (PCI) and pharmacologic therapies have improved the prognosis of CAD patients (2,3) and utilization of intravascular ultrasound (IVUS) has further contributed to reducing the incidence of stent thrombosis and management of stent underexpansion (4). These improvements led 15% decrease in cardiac deaths of CAD patients from 2000 to 2006 (2). Despite the remarkable progress, the picture still remains grim as 3.6% of US adults suffered from myocardial infarction (MI) from 2003 to 2006 (2).
Plaque rupture and subsequent thrombogenesis induce acute coronary syndrome (ACS) (3,5). Unfortunately, the underlying mechanisms and predictors of plaque rupture are unclear. Pathological studies have shown that the most common cause of MI and cardiac death is thrombotic coronary occlusion after rupture of a lipid-rich atheroma with only a thin fibrous layer of intimal tissue covering the necrotic core, the so-called thickening, thin-cap fibroatheromas (TCFA) (5,6). The PROSPECT study (7) demonstrated that major adverse cardiovascular events (MACEs) are attributed to TCFA with a large plaque burden and/or a small luminal area on gray-scale intravascular ultrasonography (IVUS). On IVUS, attenuated plaques manifest as echo attenuation behind lesion sites in the absence of dense calcification in ACS patients (Figure 1). When they are found during coronary intervention, patients are more likely to experience induced coronary microembolism and slow/no reflow phenomenon (8). Several studies have reported that attenuated plaques impact on thrombolysis in myocardial infarction (TIMI) grade flow and portend an adverse prognosis (9-19).
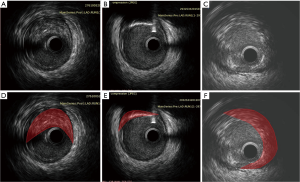
The incidence rate of slow/no reflow (TIMI flow grade 0–2) during PCI was reported to be between 15% and 26% (16,20). Tanaka et al. (9) confirmed that coronary microembolization as a result of PCI-induced plaque rupture caused no reflow phenomenon and the entry of the lipid pool into the final microcirculation. According to the modified American Heart Association (AHA) histological classification based on a large series of post-mortem human coronary specimens, IVUS attenuated plaque represents either a fibroatheroma containing a large necrotic core or pathological intimal thickening with a large lipid pool, which conforms to the morphology of vulnerable plaques that would induce slower TIMI grade flow.
Despite the significant implications of plaque rupture and subsequent thrombogenesis for ACS development, there has been no systemic review of the impact of attenuated plaques on TIMI grade and clinical outcomes of CAD patients. In the present study, we performed a meta-analysis and systematic review of the relationship between attenuated plaques detected by IVUS and the incidence of slow/no reflows during PCI and the clinical outcomes of CAD or ACS patients from pooled data of published eligible cohort studies.
Materials and methods
Sources and search strategy
We searched literature published from January, 2000 to June, 2015 on PubMed, Ovid, EMBASE, the Cochrane Library, CNKI and WanFang databases. The following keywords were used for the search: Attenuated Plaque, Attenuation Plaque, Attenuated, Attenuation, Acute Coronary Syndrome, Angina, Stable Angina, Coronary Disease, Coronary Artery Disease, IVUS, and Intravascular Ultrasound. We further searched the reference lists of all eligible studies, relevant review articles and previous meta-analyses. This meta analysis was carried out in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta Analyses) guidelines (10).
Eligibility criteria
The literature was exclusively limited to cohort studies on CAD patients who received IVUS during PCI. Any of the following efficacy outcomes was required: (I) the incidence of slow/no reflow during PCI of CAD patients with positive or negative attenuated plaques as detected by IVUS; and (II) major adverse cardiac events (MACE), including all-cause death, MI, and TVR. Fully duplicate studies were eliminated using Notexpress software and manual confirmation. Studies that failed to present original data or only provide confounding comparisons were also eliminated. Two investigators (Ruofei Jia and Lianmei Pu) performed the search independently and without language restriction. One investigator (Ruofei Jia) determined whether studies met the inclusion criteria.
Study selection
Titles and abstracts obtained from the above databases were independently evaluated by two reviewers (Ruofei Jia and Lianmei Pu) in order to assess the contents of potentially eligible studies. All articles satisfying the criteria were included. Disagreements were discussed by the two reviewers to reach a consensus was; if disagreement was still unresolved, a third reviewer (Zening Jin) was consulted. Un-weighted κ statistics was applied to assess agreement between the two reviewers. The quality of studies was evaluated using the cohort Newcastle-Ottawa Scale, which calculates the total scores and assess the bias risk based on the selection, comparability, and outcome items.
Data extraction
Two investigators (Ruofei Jia and Lianmei Pu) independently extracted the data and transferred it into Microsoft Excel 2007 without interposing each other until both of their tasks were completed. Information on participant characteristics such as age and gender, study design, and primary efficacy outcomes was extracted. After independent data extraction, the two investigators consulted each other to identify disagreement and to reach consensus by discussion. Any disagreement still existing was verified by Zening Jin. Missing data was supplemented by contacting the authors or from the data of previous meta analysis.
Statistical analysis
The original data from the eligible studies was imported from Microsoft Excel 2007 into the Stata system and Winbugs system for subsequent analysis. Traditional meta analysis was done with the aid of Stata software. The primary outcomes measured were relative risk (RR) and 95% confidence interval (95% CI) of developing TIMI in patients who had attenuated plaque. For each study, we calculated their RR and the corresponding 95% CI. The pooled RR with 95% CI was summarized to represent the total effect. To measure the outcome, we used Inverse-Variance random effect model (REM). Heterogeneity between studies, which was analyzed by two techniques: the Q statistic, which gave a qualitative indicator and was statistically significant for heterogeneity with P value less than 0.1, and the I2 statistic, which gave a quantitative measurement, with I2<50% indicating insignificant heterogeneity. Publication bias was addressed using Egger’s method, which shows good sensitivity when the number of references is small. Results synthesized from multiple studies and Forest plots were obtained with RevMan ver. 5.3 (The Cochrane Collaboration) and the Egger’s test was conducted by Stata ver. 12.0.
Results
Search results
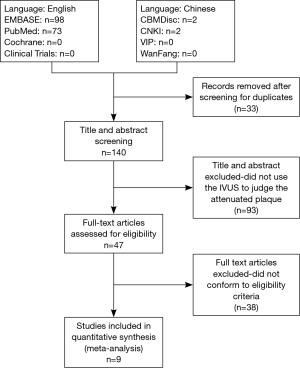
One hundred forty publications were identified for full text assessment with the search strategy (including 73 articles from PubMed, 98 articles from Embase and 2 from CNKI). Ninety-three articles were excluded because the titles and/or abstracts indicated that they were review articles [n=28, case reports (n=9) and others that did not qualify (n=56)]. Further screening by reading through the articles by two reviews excluded 35 articles that did not use the IVUS to determine attenuated plaques. Three articles were further excluded due to no record of slow/no reflow phenomenon or clinical outcomes (13,15,21). Five observational studies, which described the association between attenuated plaques and incidence of slow/no reflow during PCI (11,14,16,22) and five studies that presented MACEs were included in the final meta analysis (Figure 2) (12,14,18,19,23).
Qualitative study analysis
Table 1 summarizes the main charateristics of the included studies. A total of 3,833 patients (2,028 patients in the attenuated plague group and 1,805 patients in the non-attenuated plague group) were enrolled in the nine studies. Five studies described the relationship between attenuated plaques and incidence of slow/no reflow during PCI (11,14,16,17,22). The sample size was 97 to 687 for these studies. Two studies involved unstable and stable angina patients (17,22). Two studies involved ACS patients (13,14) and one study involved all CAD patients (11). Five other studies reported the MACEs (12,14,18,19,23). The sample size was 110 to 2,072 for these studies. Two studies involved ACS patients (14,18), two studies involved CAD patients (12,21) and one study involved patients with ST segment elevation myocardial infarction (STEMI) (23). The attenuated and non-attenuated plaque groups were comparable in age, gender and other demographic and baseline characteristics.
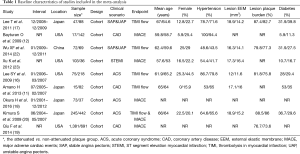
Full table
There was excellent agreement between investigators for full text screening (κ=0.93). The mean total cohort Newcastle-Ottawa Scale score was 7.7 (Table 2). All observational studies had intermediate to low intermediate bias risk according to the Newcastle-Ottawa Scale.
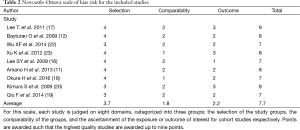
Full table
Meta analysis of attenuated plaques and the slow/no reflow phenomenon
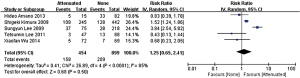
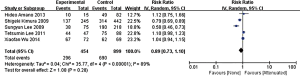
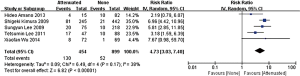
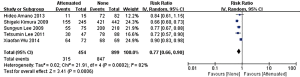
In the stage of angiography before PCI was performed, no statistically significant difference was observed between the attenuated and non-attenuated plaque group in the risk of TIMI grade 0–2 flow (RR =1.25; 95% CI: 0.65 to 2.41) (Figure 3) and TIMI 3 (RR =0.89; 95% CI: 0.73 to 1.10) (Figure 4). By contrast, after balloon dilation and stent implantation, the attenuated group showed a more than 4 fold increase in the risk of TIMI grade 0–2 flow compared to the non-attenuated group (RR =4.73; 95% CI: 3.03 to 7.40; P<0.001) (Figure 5). Among the attenuated plaque group, 130 of 454 plaques (28.6%) were associated with TIMI 0-2 flow after PCI, while only 52 of 899 non attenuated plaque (5.8%) were associated with TIMI 0-2 flow. On the other hand, the attenuated group exhibited a noticeable reduction in the incidence of TIMI grade 3 compared to the non-attenuated group (RR =0.77; 95% CI: 0.67 to 0.90; P=0.02) (Figure 6). These findings suggested that the presence of attenuated plaques was associated with increased risk for the slow/no reflow phenomenon after balloon dilation and stent implantation.
Attenuated plaques and clinical outcomes
Five studies analyzed the association between attenuated plaques and clinical outcomes. Xu et al. (23) reported that at the 1-year follow-up, only four MACEs occurred in the attenuated plaque group and no significant difference was observed between the two groups (P=0.21). At 3 years, 16 (12%) MACEs occurred in the attenuated plaque group and 9 (17.5%) in the non-attenuated plaque group (P=0.38). Kimura et al. (14) compared ACS patients with attenuated and non-attenuated plaques, 9 (7.6%) MACEs occurred in the attenuated plaque group and 7 (4.4%) in the non-attenuated plaque group (P=0.39). In addition, the two groups exhibited no statistically significant difference in other endpoints such as re-infarction and ischemic target vessel revascularization (TVR). Okura H et al. conducted a long-term study (18) with a median follow-up duration of 6.2 years. The authors found no statistically significant difference in the incidence of all cause death, cardiac death, non-cardiac death, congestive heart failure, and ACS between the two groups. The ADAPT-DES IVUS sub-study enrolled 2,072 ACS patients (16) and found no statistically significant difference in the 2-year rates of MACEs (P=0.41) or clinically driven TVR (P=0.46). Bayturan O et al. reported (12) that during the 2-year follow-up, one patient in the non-attenuated plaque group developed myocardial infarction (MI) and one patient had stroke, and no patients with attenuated plaques developed MI, death, or stroke. In conclusion, all the studies did not reveal different MACE rates between the two groups. Xu K et al. found that PES implant into attenuated plaques was particularly associated with late acquired stent malapposition (36.8% vs. 15.4% in non-attenuated plaques treated with PES, P=0.03) (23).
Publication bias
Due to the limited article number, we chose Egger’s test to estimate the publication bias without funnel plot. The results showed TIMI 0~2 grade (P=0.94; 95% CI: −15.24 to 14.42) and TIMI 3 grade (P=0.57; 95% CI: −12.29 to 8.15) in the stage of angiography, TIMI 0~2 grade (P=0.51; 95% CI: −26.36 to 16.39) and TIMI 3 grade (P=0.79; 95% CI: −7.99 to 6.62) after the balloon dilation and stent implantation without publication bias.
Discussion
Attenuated plaques are associated with ST-segment elevation MI and no reflow in patients with CAD who undergo PCI (24). However, there has been no systemic review of the impact of attenuated plaques on TIMI grade and clinical outcomes of CAD patients and attenuated plaques are not described in the current IVUS guidelines from the American College of Cardiology or the European Society of Cardiology. We systemically reviewed nine cohort studies that investigated the association between attenuated plaques and incidence of slow/no reflow during PCI (11,14,16,17,22) or the relation between attenuated plaques and MACEs (12,14,16,19,23). We found that the presence of attenuated plaques was associated with more than 4 fold increase in the risk of TIMI grade 0–2 flow after balloon dilation and stent implantation though this was not observed before PCI was performed, suggesting that the presence of attenuated plaques portends an adverse prognosis for CAD patients because of increased post procedural risk of no reflow. To the best of our knowledge, this is the first systemic review and meta analysis of the association of IVUS-detected attenuated plaques and the risk of TIMI grade no reflow, demonstrating that attenuated plaque has a high risk of no-reflow phenomenon after balloon dilation and stent implantation.
Attenuated plaque is common in ACS patients and Shiono Y. et al. have recently shown that IVUS-detected attenuated plaque is associated with MRI-derived microvascular obstruction (25), which is known to portend an adverse clinical outcome in acute MI patients (26). However, our analysis of five studies that investigated the association of attenuated plaques and MACEs (12,14,18,19,23) failed to reveal a significant correlation between IVUS-detected attenuated plaques and MACEs at 1 to 3 years of follow-up. It remains to be seen whether IVUS-detected attenuated plaques is associated with only transient deterioration in coronary flow during PCI (18) or an adverse long term clinical outcome.
The predictor of microvascular obstruction after PCI has not yet been fully elucidated and whether IVUS-detected attenuated plaque may serve as such a predictor still remains debatable. A histopathologic analyses of a small number of specimens showed that echo attenuation has been variously related to microcalcification, hyalinized fibrous tissue, cholesterol crystals, or organized thrombus (8,20,27). Kimura S et al. examined 30 atherectomy specimens with attenuated plaques and found advanced atherosclerosis consisting predominantly of cholesterol clefts, macrophage infiltration, and microcalcification (20). Plaque rupture occurs more commonly in patients with attenuated plaques having a larger size of lipid/necrotic core (28-30). Davies MJ et al. found that atheroma is at high risk for rupture when more than 40% of the plaque consists of lipid/NC (31). The meta-analysis by Ding S et al. revealed that compared with patients with normal flow, significantly higher absolute necrotic core volume and dense calcium were found in ACS patients with distal embolization (32,33). The HORIZONS-AMI Trial showed that the larger the attenuated plaque is, the greater the likelihood of no-reflow is (24). However, Stone GW et al. (7) found that, among 51 non-culprit-lesion related recurrent events occurring in the imaged segments, only 26 (51%) occurred at sites with thin-cap fibroatheromas while others were the most common thick-cap fibroatheromas. These findings indicated that predicting clinical outcomes based on ruptured plaque characteristics is still highly debatable and requires more careful studies in the future.
Study limitations
Several limitations of our study should be taken into account. First, though nine studies were included in the current analysis, half of these studies investigated attenuated plaque and TIMI grade flow, and the remaining described MACE rates without data on TIMI grade flow. Only one study provided data on both MACE rate and TIMI grade flow. Therefore, we were unable to obtain the incidence of distal embolization in the studies on MACEs without TIMI grade flow data. Secondly, there was considerable heterogeneity in TIMI 0~3 grade of angiography and TIMI 3 grade after balloon dilation and stent implantation. Thirdly, because of the limited article number, we chose Egger’s test instead of funnel plot to estimate publication bias.
Conclusions
Our study presents the evidence that plaque with ultrasound signal attenuation would induce slow/no reflow phenomenon and distal embolization during PCI, but this appearance has no impact on MACE rates within three years.
Acknowledgements
The authors of this study thank Yang Ruan for her help with data abstraction. The staff of center of clinical epidemiology in Beijing Children’s hospital had full access to the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding: This research was supported by a grant from the Development of Capital Health Scientific Fund, China (No.2014-2-2062).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Santulli G. Epidemiology of Cardiovascular Disease in the 21st Century: Updated Numbers and Updated Facts. J Cardiovasc Dis 2013;1:1-2.
- Writing group members, Lloyd-Jones D, Adams RJ, et al. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation 2010;121:e46-e215. [Crossref] [PubMed]
- Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non ST-Elevation Myocardial Infarction): developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons: endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. Circulation 2007;116:e148-304. [Crossref] [PubMed]
- Hibi K, Kimura K, Umemura S. Clinical utility and significance of intravascular ultrasound and optical coherence tomography in guiding percutaneous coronary interventions. Circ J 2015;79:24-33. [Crossref] [PubMed]
- Virmani R, Burke AP, Farb A, et al. Pathology of the vulnerable plaque. J Am Coll Cardiol 2006;47:C13-8. [Crossref] [PubMed]
- Naghavi M, Libby P, Falk E, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part I. Circulation 2003;108:1664-72. [Crossref] [PubMed]
- Stone GW, Maehara A, Lansky AJ, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med 2011;364:226-35. [Crossref] [PubMed]
- Tsunoda T, Hara H, Kunimasa T, et al. The histopathological validation of coronary athrosclerotic lesions using non-calcific plaque ultrasound attenuation images. Jpn J Interv Cardiol 2005;20:309-17.
- Tanaka A, Kawarabayashi T, Nishibori Y, et al. No-reflow phenomenon and lesion morphology in patients with acute myocardial infarction. Circulation 2002;105:2148-52. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [Crossref] [PubMed]
- Amano H, Wagatsuma K, Yamazaki J, et al. Virtual histology intravascular ultrasound analysis of attenuated plaque and ulcerated plaque detected by gray scale intravascular ultrasound and the relation between the plaque composition and slow flow/no reflow phenomenon during percutaneous coronary intervention. J Interv Cardiol 2013;26:295-301. [Crossref] [PubMed]
- Bayturan O, Tuzcu EM, Nicholls SJ, et al. Attenuated plaque at nonculprit lesions in patients enrolled in intravascular ultrasound atherosclerosis progression trials. JACC Cardiovasc Interv 2009;2:672-8. [Crossref] [PubMed]
- Kang SJ, Ahn JM, Han S, et al. Multimodality imaging of attenuated plaque using grayscale and virtual histology intravascular ultrasound and optical coherent tomography. Catheter Cardiovasc Interv 2014. [Epub ahead of print]. [Crossref] [PubMed]
- Kimura S, Yamakami Y, Kojima K, et al. Clinical significance of echo-attenuated plaque on intravascular ultrasound in patients with stable angina pectoris. Circulation 2014.130.
- Kubo T, Matsuo Y, Ino Y, et al. Optical coherence tomography analysis of attenuated plaques detected by intravascular ultrasound in patients with acute coronary syndromes. Cardiol Res Pract 2011;2011:687515.
- Lee SY, Mintz GS, Kim SY, et al. Attenuated plaque detected by intravascular ultrasound: clinical, angiographic, and morphologic features and post-percutaneous coronary intervention complications in patients with acute coronary syndromes. JACC Cardiovasc Interv 2009;2:65-72. [Crossref] [PubMed]
- Lee T, Kakuta T, Yonetsu T, et al. Assessment of echo-attenuated plaque by optical coherence tomography and its impact on post-procedural creatine kinase-myocardial band elevation in elective stent implantation. JACC Cardiovasc Interv 2011;4:483-91. [Crossref] [PubMed]
- Okura H, Kataoka T, Yoshiyama M, et al. Long-term prognostic impact of the attenuated plaque in patients with acute coronary syndrome. Heart Vessels 2016;31:23-8. [Crossref] [PubMed]
- Qiu F, Mintz G, Witzenbichler B, et al. Clinical impact of attenuated plaque after stent implantation: An adapt-desivus substudy. J Am Coll Cardiol 2014;63:A1763. [Crossref]
- Kimura S, Kakuta T, Yonetsu T, et al. Clinical significance of echo signal attenuation on intravascular ultrasound in patients with coronary artery disease. Circ Cardiovasc Interv 2009;2:444-54. [Crossref] [PubMed]
- Wu X, Maehara A, Mintz GS, et al. Virtual histology intravascular ultrasound analysis of non-culprit attenuated plaques detected by grayscale intravascular ultrasound in patients with acute coronary syndromes. Am J Cardiol 2010;105:48-53. [Crossref] [PubMed]
- Wu XF, Guo CJ, Chi YP, et al. Attenuated plaque is associated with plaque prolapse accompanied by cardiac enzyme elevation after drug-eluting stent implantation. Coron Artery Dis 2014;25:4-9. [Crossref] [PubMed]
- Xu K, Mintz GS, Kubo T, et al. Long-term follow-up of attenuated plaques in patients with acute myocardial infarction: an intravascular ultrasound substudy of the HORIZONS-AMI trial. Circ Cardiovasc Interv 2012;5:185-92. [Crossref] [PubMed]
- Wu X, Mintz GS, Xu K, et al. The relationship between attenuated plaque identified by intravascular ultrasound and no-reflow after stenting in acute myocardial infarction: the HORIZONS-AMI (Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction) trial. JACC Cardiovasc Interv 2011;4:495-502. [Crossref] [PubMed]
- Shiono Y, Kubo T, Tanaka A, et al. Impact of attenuated plaque as detected by intravascular ultrasound on the occurrence of microvascular obstruction after percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction. JACC Cardiovasc Interv 2013;6:847-53. [Crossref] [PubMed]
- de Waha S, Desch S, Eitel I, et al. Impact of early vs. late microvascular obstruction assessed by magnetic resonance imaging on long-term outcome after ST-elevation myocardial infarction: a comparison with traditional prognostic markers. Eur Heart J 2010;31:2660-8. [Crossref] [PubMed]
- Hara H, Tsunoda T, Moroi M, et al. Ultrasound attenuation behind coronary atheroma without calcification: mechanism revealed by autopsy. Acute Card Care 2006;8:110-2. [Crossref] [PubMed]
- Akyildiz AC, Speelman L, van Brummelen H, et al. Effects of intima stiffness and plaque morphology on peak cap stress. Biomed Eng Online 2011;10:25. [Crossref] [PubMed]
- Kawaguchi R, Oshima S, Jingu M, et al. Usefulness of virtual histology intravascular ultrasound to predict distal embolization for ST-segment elevation myocardial infarction. J Am Coll Cardiol 2007;50:1641-6. [Crossref] [PubMed]
- Hong YJ, Jeong MH, Choi YH, et al. Impact of plaque components on no-reflow phenomenon after stent deployment in patients with acute coronary syndrome: a virtual histology-intravascular ultrasound analysis. Eur Heart J 2011;32:2059-66. [Crossref] [PubMed]
- Davies MJ, Richardson PD, Woolf N, et al. Risk of thrombosis in human atherosclerotic plaques: role of extracellular lipid, macrophage, and smooth muscle cell content. Br Heart J 1993;69:377-81. [Crossref] [PubMed]
- Ding S, Xu L, Yang F, et al. Association between tissue characteristics of coronary plaque and distal embolization after coronary intervention in acute coronary syndrome patients: insights from a meta-analysis of virtual histology-intravascular ultrasound studies. PLoS One 2014;9:e106583. [Crossref] [PubMed]
- De Luca G, Gibson CM, Bellandi F, et al. Impact of distal embolization on myocardial perfusion and survival among patients undergoing primary angioplasty with glycoprotein IIb-IIIa inhibitors: insights from the EGYPT cooperation. J Thromb Thrombolysis 2010;30:23-8. [Crossref] [PubMed]