Single-port video-assisted thoracic surgery for early lung cancer: initial experience in Japan
Introduction
The advent of video-assisted thoracic surgery (VATS), a minimally invasive surgery, has significantly altered respiratory surgical procedures (1,2). VATS has conferred remarkable benefits, such as reduced wound pain and a shorter hospital stay, on patients (3,4). However, since the most popular conventional VATS (c-VATS) using 3 or 4 ports is performed with cylindrical trocars placed in the intercostal spaces, it may damage the intercostal nerves at the sites of trocar placement. Though VATS is thought to be the minimally invasive surgery, the prevalence of persistent pain after VATS is almost same as that after thoracotomy (5).
Single-port VATS (SPVATS), which was recently introduced in Southern Europe, is a surgical technique used to treat diseases in the field of thoracic surgery and incorporates an intrathoracic approach through a single small incision. Dr. Migliore, an Italian surgeon, started to perform surgery with SPVATS for pleural biopsy in 2001 (6). Dr. Rocco reported the experiences of lung wedge resections in 2004 (7). Dr. Gonzalez-Rivas subsequently performed lung lobectomy for small-cell lung cancer in 2011 (8). Since then, lung segmentectomy and surgery for advanced lung cancer have been reported (9-11), and SPVATS has become widespread in Asia. Dr. Ng et al. in Hong Kong promptly introduced SPVATS for lung cancer (12), which is becoming more widespread in Taiwan, South Korea, China, and Singapore.
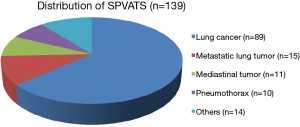
In Japan, our center has been actively performing SPVATS since 2012. We reviewed our experience of 139 patients who underwent SPVATS for anatomical lung resection for early-stage lung cancer, lung biopsy for advanced lung cancer, metastatic lung tumors, mediastinal tumors, spontaneous pneumothorax, and pleural biopsy (Figure 1). In the present study, we present the efficacy of this procedure in 84 patients who were definitively diagnosed with pathological stage I lung cancer and our operative procedures.
Patients and methods
A retrospective review of a prospectively maintained database identified 84 patients with pathological stage I non-small cell lung cancer who underwent curative thoracoscopic surgery at Nippon Medical Chiba Hokusoh Hospital between September 2012 and September 2015. SPVATS was performed on 84 patients by the same thoracic surgeon. Informed consent to use the data-use agreement was obtained from all patients before surgery.
Methods
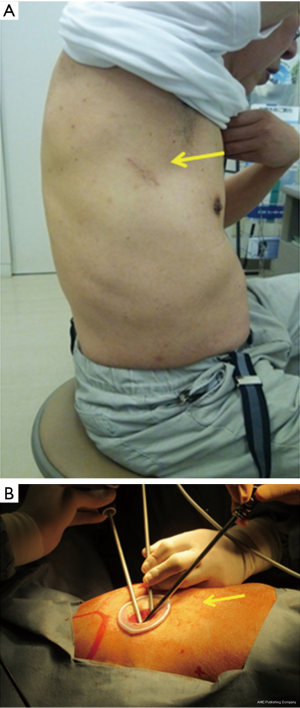
In SPVATS, an approximately 4-cm incision was made on the anterior (upper and lower lobectomy) or middle (lower lobectomy) axillary line (Figure 2A), followed by the attachment of a Wrap Protector mini (Hakko Inc., Japan). Protective procedures to the intercostal nerves were provided by the surgeon and assistants. The procedure was a traumatically performed as much as possible in order to prevent contact between the thoracoscope and forceps and intercostal nerves. The thoracoscope was mostly handled at an angle of more than 45 degrees from the chest wall (Figure 2B). Surgery was performed using a 10-mm or 5-mm 30-degree oblique-viewing thoracoscope and facing/inverted dual-monitors without any specific device. The Wrap Protector mini was used to open incisions, and a small rib retractor was not used. Trocars (Endopath, 12-mm Ethicon, USA) were used in the port holes. The energy device, HARMONIC scalpel (Ethicon, USA) was used for mediastinal lymph node dissection. Various surgical factors (operative outcomes), the incidence of complications, postoperative complications, and 30-day mortality were evaluated. Postoperative wound pain was monitored using the numeric rating scale (NRS). NRS was evaluated on postoperative day 7. Additionally, the frequency of allodynia related to neuropathic pain was evaluated by a detailed interview and a writing brush on postoperative day 30.
The maximum value of creatine phosphokinase (CPK) was measured as the assessment of chest muscle damage after surgery.
Statistical analysis
Quantitative variables are presented as medians (± standard deviation) or ranges where appropriate. Continuous variables were compared using the Mann-Whitney U test where appropriate. All statistical analyses were performed with the software SPSS version 17 for Windows (IBM Corp, Armonk, NY). P values of <0.05 were considered to indicate significance for all parameters.
Results
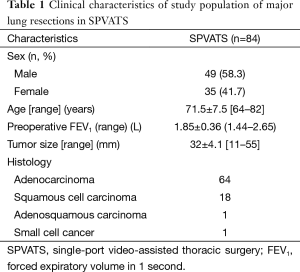
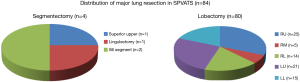
Eighty-four patients underwent anatomical lung resection for postoperative pathological stage I lung cancer. The mean preoperative wound length was 4.2 cm. Eighty-four patients underwent lobectomy and segmentectomy, respectively. Our patients, with a mean age of 71.5±7.5 years, consisted of 49 men (58.3%) and 35 women (41.7%). The mean preoperative forced expiratory volume in 1 second (FEV1) was 1.85±0.36. Histologically, 64, 18, 1, and 1 out of the 84 patients had adenocarcinoma, squamous cell carcinoma, adenosquamous carcinoma, and small-cell lung cancer, respectively (Table 1). Figure 3 shows the types of procedures performed. Four patients underwent segmentectomy, while 80 underwent lobectomy: 25 (31.3%) right upper lobectomy and 21 (26.3%) left upper lobectomy. The mean operative time was 175±21 min, operative blood loss 92±18 mL, CPKmax 342±42 IU/L, C-reactive protein max (CRPmax) 5.2±1.9 mg/dL, and duration of drain placement 1.9±0.6 days. The duration of the postoperative hospital stay was 7.1±1.7 days, NRS 1 week after surgery 2.8±0.6, and occurrence rate of allodynia 1 month after surgery 10.7% (Table 2). Figure 4 shows changes in the mean CPKmax level between 2012 and 2015. The postoperative CPKmax level (320±42) in 2015 was less than normal values in a large number of patients, and was significantly lower than that in 2012 (315±41 vs. 474±34, P<0.05). Although one patient developed multiple lung metastases and another adrenal metastasis, there was no evidence that local or lymph node recurrence was secondary to surgery. At present, the 2-year survival rate is 100%. No patients developed serious complications. Two patients (2.4%) were converted to open thoracotomy (two required antero-lateral thoracotomy).
Full table
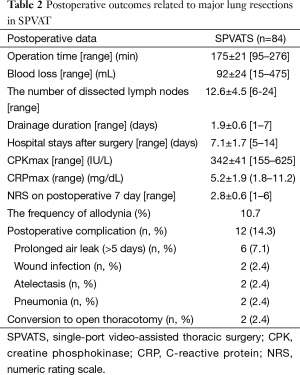
Full table

Discussion
As a recent topic in the thoracoscopic surgical treatment of lung cancer, several studies have reported the effectiveness and outcomes of SPVATS (13,14). SPVATS (including our technique) using an approach through the 4th or 5th intercostal space provides easy access to the interlobar space, and involves thoracotomy similar to that conventionally used. Therefore, vascular handling, the repair of injured lung, and lymph node dissection are not expected to significantly affect the quality of surgery if the surgeon is proficient in the use of forceps. Interlobar bleeding may be controlled by taping the central or peripheral side of the injured blood vessel. Moreover, even when bleeding cannot be handled through a single port, hemostasis may be safely performed by creating an additional hole or extending the original incision. Several studies have recently reported bronchoplasty or angioplasty for advanced lung cancer (9,11,15), suggesting that indications for SPVATS will be expanded.
Although the advantages of SPVATS, such as reduced postoperative wound pain, are readily expected to be greater for patients, this procedure has not yet become popular worldwide, possible because of its high degree of difficulty and complex operative techniques. Considering how laparoscopic surgery spread in the early 1990s, it is, of course, necessary to present the superiority of SPVATS over c-VATS in addition to the outcomes of treatments and demonstrate its curability and safety in order to raise its adoption rate.
We started to perform SPVATS for spontaneous pneumothorax, mediastinal tumors, pleural biopsy, and biopsy of benign lung lesions in 2011 and for early-stage lung cancer in 2012. In a comparison of SPVATS and c-VATS, we evaluated the advantages of SPVATS for patients in terms of the amelioration of various symptoms related to neuropathic pain in the postoperative wound (Figure 5) (16). During SPVATS, the surgeon and assistant take care not to touch or injure the intercostal neurovascular bundle. We think highly of such awareness on their part during operative procedure and it is assumed that this awareness will reduce the incidence of intercostal nerve injury. We also speculate that, during the manipulation of cylindrical trocars, muscles and nerves are less often crushed advertently in SPVATS than in c-VATS, leading to a decrease in the frequency of postoperative sedative use and in CPKmax. CPKmax, which appears to be affected by the learning curve for surgical procedures, gradually decreased in the later period (after SPVATS was started), and was within normal limits in many patients (Figure 4). At present, there is no evidence to show that local or lymph node recurrence is attributable to surgical procedures, and the rate of conversion to open thoracotomy in our department was 2.4%, which is similar to that in other centers, thereby supporting the validity and safety of this procedure.
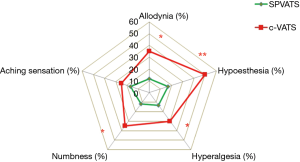
In Japan, from the viewpoint of lymph node dissection in lung cancer surgery, an emphasis is placed on peeling off the sheaths of pulmonary vessels as much as possible. In SPVATS, unlike c-VATS, vascular sheaths are sometimes challenging to peel off in terms of the viewing angle; therefore, difficulties may initially be associated with performing this technique after the introduction of SPVATS. The surgical field was successfully expanded to facilitate lymph node dissection, and SPVATS compared favorably with c-VATS in the number of lymph nodes dissected (17). Our experiences of SPVATS are shown in Figure 6. The knacks of SPVATS in our institute are indicated in Table 3.


Full table
In our experience the transition from c-VATS to SPVAT has proceeded smoothly. According to some recent reports (13,14,16), the operative factors of SPVATS showed the same as those of c-VATS. And as for postoperative wound pain we have recently demonstrated that SPVATS was more minimally invasive compared with c-VATS (16). With the increasing experiences of SPVATS for stage I lung cancer, the beneficial efficacy of this operative procedure for patients should be announced still more. Further investigations will be required to validate the advantage of SPVATS.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
- Hanna JM, Berry MF, D'Amico TA. Contraindications of video-assisted thoracoscopic surgical lobectomy and determinants of conversion to open. J Thorac Dis 2013;5 Suppl 3:S182-9. [PubMed]
- McKenna RJ Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg 2006;81:421-5; discussion 425-6. [PubMed]
- Swanson SJ, Herndon JE 2nd, D'Amico TA, et al. Video-assisted thoracic surgery lobectomy: report of CALGB 39802--a prospective, multi-institution feasibility study. J Clin Oncol 2007;25:4993-7. [PubMed]
- Cheng D, Downey RJ, Kernstine K, et al. Video-assisted thoracic surgery in lung cancer resection: a meta-analysis and systematic review of controlled trials. Innovations (Phila) 2007;2:261-92. [PubMed]
- Furrer M, Rechsteiner R, Eigenmann V, et al. Thoracotomy and thoracoscopy: postoperative pulmonary function, pain and chest wall complaints. Eur J Cardiothorac Surg 1997;12:82-7. [PubMed]
- Migliore M, Deodato G. A single-trocar technique for minimally-invasive surgery of the chest. Surg Endosc 2001;15:899-901. [PubMed]
- Rocco G, Martin-Ucar A, Passera E. Uniportal VATS wedge pulmonary resections. Ann Thorac Surg 2004;77:726-8. [PubMed]
- Gonzalez-Rivas D, de la Torre M, Fernandez R, et al. Single-port video-assisted thoracoscopic left upper lobectomy. Interact Cardiovasc Thorac Surg 2011;13:539-41. [PubMed]
- Gonzalez-Rivas D, Delgado M, Fieira E, et al. Left lower sleeve lobectomy by uniportal video-assisted thoracoscopic approach. Interact Cardiovasc Thorac Surg 2014;18:237-9. [PubMed]
- Gonzalez-Rivas D, Delgado M, Fieira E, et al. Uniportal video-assisted thoracoscopic pneumonectomy. J Thorac Dis 2013;5 Suppl 3:S246-52. [PubMed]
- Gonzalez-Rivas D, Fernandez R, Fieira E, et al. Uniportal video-assisted thoracoscopic bronchial sleeve lobectomy: first report. J Thorac Cardiovasc Surg 2013;145:1676-7. [PubMed]
- Ng CS. Uniportal VATS in Asia. J Thorac Dis 2013;5 Suppl 3:S221-5. [PubMed]
- Wang BY, Liu CY, Hsu PK, et al. Single-incision versus multiple-incision thoracoscopic lobectomy and segmentectomy: a propensity-matched analysis. Ann Surg 2015;261:793-9. [PubMed]
- Shen Y, Wang H, Feng M, et al. Single- versus multiple-port thoracoscopic lobectomy for lung cancer: a propensity-matched study. Eur J Cardiothorac Surg 2016;49 Suppl 1:i48-i53. [PubMed]
- Gonzalez-Rivas D, Delgado M, Fieira E, et al. Single-port video-assisted thoracoscopic lobectomy with pulmonary artery reconstruction. Interact Cardiovasc Thorac Surg 2013;17:889-91. [PubMed]
- Hirai K, Takeuchi S, Usuda J. Single-incision thoracoscopic surgery and conventional video-assisted thoracoscopic surgery: a retrospective comparative study of perioperative clinical outcomes. Eur J Cardiothorac Surg 2016;49 Suppl 1:i37-i41. [PubMed]
- Delgado Roel M, Fieira Costa EM, González-Rivas D, et al. Uniportal video-assisted thoracoscopic lymph node dissection. J Thorac Dis 2014;6:S665-8. [PubMed]
- Hirai K, Takeuchi S, Usuda J. The SPVATS techniques of the right upper lobectomy, right lower lobectomy, the vessel treatment during left upper lobectomy, and right middle lobectomy with partial resection of S3b. Asvide 2016;3:073. Available online: http://www.asvide.com/articles/826


