
The clinical application of lateral chest flap transfer combined with relay skin flap transfer to repair soft tissue defects of the chest wall after tumor resection
Introduction
Resection of malignant tumors originating in the chest wall and breast tissue often leads to large-scale soft tissue defects in the chest wall, accompanied by defects in rib and rib cartilage tissue. Such wounds are accompanied by exposed bone tissue, so wound repair cannot be performed with free skin grafts. In addition, due to the large area of soft tissue defects in the chest wall, it is impossible to repair directly with local flaps, so the common repair method is mainly to use the latissimus dorsi muscle flap for flap transfer repair. However, because malignant tumors originating in the chest wall or breast are prone to recurrence, when the latissimus dorsi muscle flap repair defect, once the tumor recurs again, the available local flap sources are extremely limited. Therefore, soft tissue defects after thoracic tumor resection require concurrent surgical repair, but the latissimus dorsi muscle flap needs to be retained as a backup for possible secondary repair or breast reconstruction.
The study found that the lateral pectoral flap is a thoracic flap located under the axillary, and the maximum incision range of the flap is 80 mm × 100 mm. The upper boundary of the flap reaches the edge of the axillary hairs, the lower boundary reaches the 7th to 8th rib, the posterior boundary reaches between the posterior axillary line and the scapula line, and the anterior boundary reaches the anterior axillary wall. Partial soft tissue defects in the chest wall can be repaired by rotating the flap during surgery, and if the defect is large, combined with the rotational propulsion of the local flap next to the chest wall defect, multiple flaps are combined to repair (1). Therefore, in order to preserve the latissimus dorsi muscle flap as much as possible as a backup flap for re-repair, during the same period of tumor resection, we used the advantages of local rotational propulsion flap combined with the lateral chest flap to repair the soft tissue defect of the chest wall after tumor surgery, and the results of the study are described as follows. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1067/rc).
Methods
Clinical data
A total of 26 patients, who suffered from soft tissue defects of the chest wall and rib exposure and sought medical attention at the Burn and Plastic Surgery Department, Zhongda Hospital from January 2017 to October 2021 were enrolled in this study. None of the patients required breast reconstructions. The patients’ ages ranged from 34 to 56 years. Of the patients, 21 were females suffering from non-healing radiation ulcers after breast lumpectomies. The remaining 5 patients were male with dermatofibrosarcoma protuberans (DFSP) relapse. Patients with contraindications were excluded before the surgery. Of the 25 patients, 1 was a hepatitis B virus carrier, while the rest of the patients denied having any infectious or autoimmune diseases.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of Zhongda Hospital (No. 2017ZDKYSB093) and individual consent for this retrospective analysis was waived.
Preoperative design
The lateral chest flap is located on the axillary part of the thoracic cage. It is bounded superiorly by a border of axillary hair and inferiorly by the 7th or 8th rib. The posterior border of the lateral chest flap is between the posterior axillary line and scapular line, and its anterior border is the anterior wall of the axilla. If the recipient site requires hair, the area of the lateral chest flap can be extended to the skin with axillary hair. Depending on the anatomy, the maximum area of the flap we can obtain is 150 mm × 80 mm. We designed relay skin flap transfers, and then rotated and advanced them according to contralateral defect tissue.
Surgery procedure
The deep fascia of the lateral chest was dissected to expose the pectoralis major, serratus anterior, latissimus dorsi, lateral thoracic artery, brachial-thoracic cutaneous artery, long thoracic nerve, thoracoepigastric veins, and anterior and posterior branches of the cutaneous branch of the intercostal nerves. The thoracoepigastric veins empty superiorly into the axillary veins, inferiorly into the superficial epigastric veins, and anteriorly into the perforators of the internal thoracic veins. The incision should reach the inferior surface of the deep fascia. The inferior border of the flap was elevated, and the vessel pedicle was then searched retrogradely the point of rotation of the flap. The obvious cutaneous arteries and veins that entered the flap were dissected, or the lateral thoracic artery was dissected. The vessel pedicle was lengthened, and we tried to cover the wound. The wound of the donor site was closed directly by suture. The deep surface of the subcutaneous tissue was dissected. The flap near the wound was used to repair the adjacent side of the wound. The distal part of the flap was advanced to repair the secondary wound after the distal part had been thoroughly dissected. A drain was placed in the cavity of the wound and the wound was dressed with compression dressing (or Negative Pressure Wound Therapy was used as the sealing treatment, and the window for the blood supply of the skin flap was observed). After the surgery, the blood supply of the flap was observed, and the volume was expanded by rehydration to prevent vasospasm and infection. Once the wound had healed, radiotherapy or comprehensive therapy was performed depending on each patient’s condition.
Postoperative observation and follow-up
After surgery, we collated clinical data containing basic information about the patient, age, sex, tumor nature, size of chest wall defects after tumor resection, size of the selected side chest flap, assessment of the healing status of chest wall wounds, recurrence and chest wall scars. Scar hyperplasia is assessed using the Vancouver Scar Scale. The observational indicators to evaluate postoperative healing mainly include whether the wound heals in the first stage, whether there are manifestations of red and swollen infection, and whether the complications of infection are improved after treatment. The follow-up period mainly focuses on chest wall wound healing, whether the tumor has recurred, and scarring.
Statistical analysis
Postoperative follow-up data were analyzed by Excel 2020. Mean was calculated for continuous variables in normal distribution. For variables in skewed distribution, we calculated their medians. Percentages were calculated for ranked variables.
Results
In this research, 25 of the 26 patients’ incisions achieved primary healing, but 1 patient’s incision only healed after suture removal and 10 days of dressing changing due to malnutrition. The incisions of all patients healed well without complications such as skin necrosis and infection. All the patients were followed-up for 6–18 months (with an average of 12 months). Among the patients, 2 males suffered from a recurrence of dermatofibrosarcoma carina 6 and 10 months after the operation, respectively. After extensive resection, ipsilateral latissimus dorsi myocutaneous flap transfers were used to repair the defects, and the postoperative incision heals well Vancouver scar scale was used to evaluate the scar hyperplasia of all the incisions 6 months after the surgery. The average score was 5.92±1.6. The patients’ clinical data are summarized in Table 1.
Table 1
| Patient No. | Age, year | Sex (M, F) | Etiology | Defect region | Defect size, cm | Flap size, cm | Late complication | VSS score |
|---|---|---|---|---|---|---|---|---|
| 1 | 54 | F | RU | Right chest | 8×6 | 10×5 | None | 8 |
| 2 | 35 | M | DFSP | Right chest | 15×12 | 15×7 | None | 8 |
| 3 | 36 | F | RU | Right chest | 6×5 | 8×5 | None | 5 |
| 4 | 54 | F | RU | Right chest | 6×5 | 12×6 | None | 7 |
| 5 | 53 | F | RU | Left chest | 8×5 | 10×5 | None | 6 |
| 6 | 46 | F | RU | Left chest | 5×4 | 12×5 | None | 3 |
| 7 | 47 | F | RU | Left chest | 7×5 | 10×5 | None | 5 |
| 8 | 51 | F | RU | Left chest | 5×4 | 12×5 | None | 7 |
| 9 | 34 | M | DFSP | Right chest | 10×6 | 12×6 | None | 9 |
| 10 | 55 | F | RU | Left chest | 6×5 | 10×5 | None | 8 |
| 11 | 47 | F | RU | Right chest | 6×4 | 12×5 | None | 6 |
| 12 | 45 | F | RU | Right chest | 5×4 | 10×5 | None | 4 |
| 13 | 47 | F | RU | Right chest | 4×4 | 10×5 | None | 4 |
| 14 | 42 | M | DFSP | Right chest | 12×8 | 14×7 | Recurrence | 6 |
| 15 | 52 | F | RU | Left chest | 7×6 | 10×5 | None | 7 |
| 16 | 37 | F | RU | Left chest | 5×4 | 8×4 | None | 5 |
| 17 | 42 | F | RU | Left chest | 7×5 | 9×5 | None | 4 |
| 18 | 45 | M | DFSP | Left chest | 10×8 | 12×6 | None | 6 |
| 19 | 39 | F | RU | Left chest | 7×6 | 10×5 | None | 4 |
| 20 | 41 | F | RU | Right chest | 5×5 | 10×6 | None | 7 |
| 21 | 56 | F | RU | Right chest | 6×5 | 10×5 | None | 6 |
| 22 | 51 | M | DFSP | Left chest | 12×7 | 14×6 | Recurrence | 5 |
| 23 | 39 | F | RU | Right chest | 8×6 | 10×5 | None | 8 |
| 24 | 46 | F | RU | Right chest | 5×4 | 6×4 | None | 4 |
| 25 | 53 | F | RU | Left chest | 4×4 | 12×5 | None | 7 |
| 26 | 51 | F | RU | Left chest | 6×4 | 10×5 | None | 5 |
VSS, Vancouver scar scale; RU, radiation ulcer after lumpectomy of breast; DFSP, dermatofibrosarcoma protuberans.
Case 1
A 35-year-old male was hospitalized, who had undergone DFSP dissection on his right chest 7 years ago. The patient found a 3 cm subcutaneous lump on his right chest wall 8 months before presentation, and he felt that the incision broadened after the resection at a local hospital. The post-resection pathology was DFSP. The tumor relapsed in the spring of 2017, and it enlarged gradually. A special examination revealed a circle tumor of 10 cm on the right side of the chest wall, and engorgement was observed on the skin. The texture of the tumor was firm and barely movable (see Figure 1A). The chest computed tomography scan showed that there were multiple soft tissue masses with reticular enhancement and skin thickening. The largest cross-section was 5.12 mm × 9.07 mm. After completing the preoperative examination, a lumpectomy of the chest wall was performed under general anesthesia as well as a lateral chest flap transposition combined with a relay skin flap transfer.
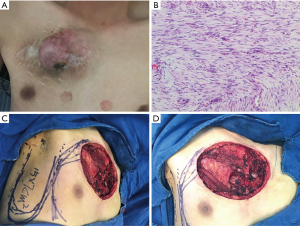
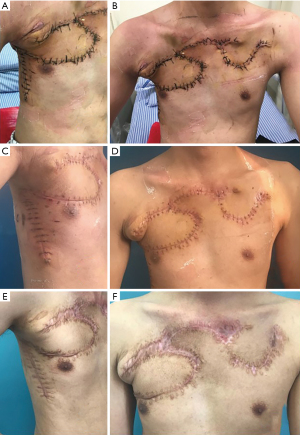
Pathological section results showed that the tumor had invaded the dermis and surrounding striated muscle (see Figure 1B). During the operation, we cut the skin and subcutaneous tissue 3 cm away from the border of the tumor. As the tumor had invaded the joint between the 3rd rib and the sternum, we removed a 4-cm section of the 3rd rib, which was near the sternum. We designed a right lateral chest flap (approximately 15 cm × 7 cm) (see Figure 1C,1D). We rotated the flap to cover the right side of the chest wound. We also designed a dolabriform flap on the left chest. We rotated and advanced the flap to cover the left side of the chest wound and secondary wound separately. We placed a drain and closed the wound by suturing. The postoperative pathology results were as follows: DFSP; fibrosarcoma type. The longest diameter was 9.9 cm. The immunohistochemistry results were as follows: cluster of differentiation (CD)34(+), Bcl-2(–), CD99(+), SMA(–), S-100(-), Sox10(–), Ki67(about 10% was +), NF(–), Des(–), and CD10(partly +). After the incision had healed, the patient underwent radiotherapy at our hospital’s oncology department. The patient was followed-up for 6 months after therapy, and there was no recurrence (see Figure 2).
Discussion
Currently, radical surgeries are often performed on varieties of malignant tumors on or near the chest wall. Large quantities of the chest wall soft tissue and even parts of the ribs and costal cartilage have to be removed during the surgery. The exposure of these bony tissues retrains the application of free skin grafts. Many surgeons choose pedicled myocutaneous flap transfers, such as latissimus dorsi myocutaneous flap transfers, and rectus abdominis myocutaneous flap transfers, to repair the defects of the chest wall, and free skin grafts, such as anterolateral thigh flap transfers. These skin flaps, whose structure is like the soft tissue of the chest wall, are the best source for not only chest wall defect repair, but also female breast reconstruction after mastectomy (2,3). However, the possibility of relapse remains, and myocutaneous flap transfer is the ultimate solution for repair.
Additionally, radiation therapy are given to some patients after breast cancer surgery in women, most of these radiation wounds are chronic and refractory bone ulcers, and the wound and the soft tissue around the wound are obviously scarred (4). These non-healing wounds can severely harm patients physically and mentally. Thus, we choose local flap transfers based on patients’ anatomy to repair the defects of the chest wall. Relay skin flap transfers can also be used when necessary. No skin grafts are required after the surgery, and the wounds can be sutured directly. Additionally, there is no dissipation of functional skin flaps or myocutaneous flaps, which provides more treatment options for patients.
To repair a defect of the chest wall, we prioritized the lateral chest flap, which is in the axilla. Generally, depending on the anatomical evidence, the maximum area of the flap we can obtain is 150 mm × 80 mm. The blood supply originates from the axillary-thoracic cutaneous artery of the axillary artery (15%, external diameter 1.40 mm), the brachial-thoracic cutaneous artery of the brachial artery (37%, external diameter 1.55 mm), the thoracodorsal artery (47%, external diameter 1.10 mm), and the cutaneous artery of the lateral thoracic artery (77%, external diameter 0.60 mm). Clinically, lateral chest arteries and thoracodorsal arteries are often used as the vessel pedicle of the lateral chest. The distribution of blood supply to the brachial-thoracic cutaneous artery is relatively large, and it anastomoses anteriorly with the perforating branches of the internal thoracic artery, and posteriorly with the lateral branches of the posterior intercostal artery, emptying into the thoracoepigastric vein and accompanying the veins of the cutaneous artery (5). Some surgeons use lateral chest flaps to repair chronic radiation ulcers on the chest, but others prefer the neighboring myocutaneous flaps (6). To protect functional myocutaneous flaps and avoid grafting on secondary wounds, we chose to reserve the length of the flaps along the vessel axis. We also reduced the flap widths appropriately in accordance with the size of the chest wall defects and the elasticity of the lateral chest wall skin. In this case, we determined that the size of the flap would be 70 mm × 150 mm; thus, we were able to extend the flap with the help of a Doppler ultrasonic blood stream detector. If only a lateral chest flap is used, wounds of the chest wall defect cannot be repaired thoroughly, especially those ulcers that are near the sternum. The exposure of bones often occurs on patients whose chest skin is tight. Thus, we combined lateral chest flap transfers with relay skin flap transfers to repair the wounds.
Currently, the relay skin flap transfer approach has no precise definition. It is generally agreed that this kind of flap transfer is used to repair a primary wound with the flap of 1 donor site and a secondary wound with the flap of the other donor site, using the tension difference of the 2 adjacent donor sites (7,8). This approach can lead to the direct suture of donor sites and avoids other disadvantages, such as grafting on wounds and donor site enlargement (9). Thus, we chose the relay skin flap method combined with the lateral chest flap method to repair defects of the chest wall soft tissue, using the different tension of the skins near the sternum.
In relation to the flaps in our research, we designed these individually based on the different tensions between the skin at different locations. In our research, 25 of the 26 patients’ incisions achieved primary healing, and 1 patient’s incision healed after suture removal and 10 days of dressing changing. This 1 patient underwent radiotherapy after mastectomy and suffered from resultant malnutrition. Her wound healed after 10 days of dressing changing and nutritional support. These cases remind us that patients suffering from tumors require individualized and multimodal treatment, not just operations. The 26 patients in our study all healed well after surgery and did not have complications such as skin necrosis and infection. All the patients were followed-up for 6 to 18 months, (with an average of 12 months). No recurrence was observed
Additionally, in relation to the DFSP in the case, it was a localized low-grade fibrous histiocytoma that stemmed from the interstitium, which was considered representative of classic intermediate fibrous tissue tumors. DFSP is prone to relapse, as occurred in the case above (10). The principle of treatment is to perform an extended local resection; otherwise, the disease is likely to relapse and the lesion will then deteriorate. The change may present as fibrosarcomatoid degeneration, which is also called fibrosarcomatoid DFSP in the literature (11-13). For such patients, we resect as much surrounding tissue as possible and conduct a postoperative frozen section analysis to determine the range of resection, followed by radiotherapy. For patients who do not require breast reconstruction, in choosing the surgery approach, we repair defects with local flaps, which addresses the issue of their being a lack of donor sites in case of tumor relapse. During the operation, bilateral nipples should be located on the same horizon line based on aesthetic principle. In this study, 2 male patients suffered from a recurrence of dermatofibrosarcoma carina 6 months and 10 months after surgery, respectively, and the ipsilateral latissimus dorsi myocutaneous flap was used to repair the defect wounds after extended resection. Thus, the lateral thoracic flap and local flap were used to repair the recurrent tumors after resection to provide more local treatment methods for subsequent wound soft tissue defects.
To summarize, in our view, patients with chest wall soft tissue defects caused by malignant tumors and rib exposure and costal cartilage should be treated with lateral chest flap transfers combined with relay skin flap transfers, as this repair method avoid wounds caused by harvesting and grafting and provides more options for subsequent treatments of malignant tumors.
Acknowledgments
Funding: The study was supported by the Natural Science Foundation of Jiangsu Province (No. BK20170705).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1067/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1067/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1067/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of Zhongda Hospital (No. 2017ZDKYSB093) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chang S, Tang X, Wei Z, et al. Repair of skin and soft tissue defects of auricle and donor site with relay flap. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2019;33:66-9. [PubMed]
- Smolanka II, Bagmut IY, Sheremet MI, et al. Delayed breast reconstruction with tram-flap and various modifications after radical mastectomy. J Med Life 2021;14:847-52. [PubMed]
- Li YJ, Tang XN, Li XQ. Effect of modified radical mastectomy combined with latissimus dorsi musculocutaneous flap breast reconstruction on patients' psychology and quality of life. Am J Transl Res 2021;13:11548-55. [PubMed]
- Porosnicu AL, Ghiurco I, Atanasescu VP, et al. The Impact of Post-Mastectomy Radiotherapy on Delayed Alloplastic Breast Reconstruction - Experience of One Center. Chirurgia (Bucur) 2021;116:224-31. [Crossref] [PubMed]
- Zhu H, Gao Z, Tremp M, et al. Application of Tissue Expansion with Perforator Flaps for Reconstruction of Challenging Skin Lesions. J Reconstr Microsurg 2018;34:13-20. [Crossref] [PubMed]
- Ma X, Jin Z, Li G, et al. Classification of chronic radiation-induced ulcers in the chest wall after surgery in breast cancers. Radiat Oncol 2017;12:135. [Crossref] [PubMed]
- Lee S, Jung Y, Bae Y. Dermoglandular rotation flap with subaxillary advancement flap as an oncoplastic technique for breast cancer. Breast J 2020;26:420-6. [Crossref] [PubMed]
- Neiman R, Finkemeier CG, Swentik AG. Distally Based Peroneus Brevis Rotation Flap. J Orthop Trauma 2020;34:S44-5. [Crossref] [PubMed]
- Lee S, Lee J, Jung Y, et al. Oncoplastic surgery for inner quadrant breast cancer: fish-hook incision rotation flap. ANZ J Surg 2017;87:E129-33. [Crossref] [PubMed]
- Brooks J, Ramsey ML. Dermatofibrosarcoma Protuberans. Treasure Island (FL): StatPearls Publishing; 2022.
- Choong P, Lindsay D, Khoo M, et al. Dermatofibrosarcoma protuberans: the diagnosis of high-grade fibrosarcomatous transformation. Skeletal Radiol 2021;50:789-99. [Crossref] [PubMed]
- Li Y, Liang J, Xu X, et al. Clinicopathological features of fibrosarcomatous dermatofibrosarcoma protuberans and the construction of a back-propagation neural network recognition model. Orphanet J Rare Dis 2021;16:48. [Crossref] [PubMed]
- Sharma D, Singh G, Kakkar N, et al. Reply to comment on: Orbital dermatofibrosarcoma protuberans with frontal and ethmoid sinus involvement: A case report and brief review of literature. Indian J Ophthalmol 2018;66:351. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)


