Impact of previous upper gastrointestinal cancer surgery on complications after lobectomy for lung cancer
Introduction
The incidence of patients diagnosed with primary lung cancer after recovering from other forms of cancer is increasing because of the improved modalities of cancer diagnosis, improvement in cancer therapy, and accuracy of postoperative follow-up surveillance (1). Because the development of lung cancer substantially and adversely impacts the long-term survival of patients after recovering from a previous cancer (2), its management is important. The incidence risk of primary lung cancer is higher in patients with a cancer history (3), especially in patients with smoking-related malignancies and in patients with a history of esophageal, gastric, or head and neck cancer, and subsequent primary lung cancer is diagnosed during follow-up for other cancers (4-6). Comparatively, patients with esophageal cancer who had received definitive treatment had a significantly increased risk of lung cancer (standardized incidence rate =1.67) (7). In gastric cancer patients, primary lung cancer is one of the most common types of cancer that occurs after surgery (8), and the development of lung cancer is associated with higher fatality rates (1). In these upper gastrointestinal cancers (UGIC), surgery is the mainstay of treatment (9), and the surgical procedure may affect the safety of surgery for subsequent lung cancer. The anatomical alternation and functional state of the digestive tract (stomach and small intestine) following surgery for UGIC may lead to nutritional deficiencies (10); nutritional status worsens as patients often eat less and lose weight (11). In lung cancer patients, good nutrition appears beneficial in decreasing complications (12). Although some studies have evaluated long-term prognosis of patients who have undergone lung cancer surgery, there are only a few studies on the short-term outcomes of surgery for lung cancer arising after UGIC surgery (1).
In this study, we aimed to assess the impact of a previous UGIC surgery on postoperative complications after thoracoscopic lobectomy for lung cancer. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-798/rc).
Methods
In this retrospective, observational study, we examined the records of all patients with primary lung cancer who underwent video-assisted and robot-assisted thoracoscopic curative lobectomy at our hospital between 2011 and 2021. There was no missing data. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Institutional Review Board for Clinical Research of Cancer Institute Hospital of Japanese Foundation for Cancer Research approved this study on 29th June 2021 (No. 2021-1086), and informed consent was obtained from all individuals included in the study.
We collected information on the history of cancer surgeries performed at our hospital or other hospitals. Those who underwent open or laparoscopic surgeries under general anesthesia were included, while those who underwent endoscopic treatment or radiotherapy were excluded. The group with a history of other cancer surgery was further subdivided into three groups: UGIC surgery that included esophagectomy and gastrectomy; lower gastrointestinal cancer surgery that included the liver, pancreas, and colorectal cancer; and other cancer surgery classified as non-gastrointestinal cancer surgery. The prevalence of postoperative pulmonary complications for each subgroup was compared. Moreover, serum levels of preoperative albumin and prealbumin were evaluated as nutritional indicators.
Postoperative complications that occurred during hospitalization or within 3 months after surgery were extracted. We defined postoperative complications as those included in Clavien–Dindo grade ≥ II complications (13,14). We included the following postoperative pulmonary complications, as in our previous study: pneumonia; pneumonitis such as atelectasis and aspiration pneumonia; bronchopleural fistula; empyema; and respiratory failure including asthma attack, acute exacerbation of idiopathic pulmonary fibrosis, pleural effusion due to pleurisy, pulmonary thromboembolism, and chylothorax (15). All clinical data and the information of complications were obtained from our institutional database. We compared and evaluated the risk of postoperative pulmonary complications according to cancer surgery history, including UGIC surgery.
Statistical analysis
Dichotomous data are presented as numbers with percentages, and continuous data as means ± standard deviations. We used univariable and multivariable analyses to identify the factors associated with postoperative pulmonary complications by logistic regression models. A backward stepwise selection method was used to build logistic regression models. We checked the multicollinearity of these factors in logistic regression models by Hosmer-Lemeshow test. The rate of postoperative pulmonary complications according to the type of previous cancer was also evaluated. All tests were two-sided, and P values <0.05 were considered statistically significant. We used the IBM SPSS statistical software package (version 27.0; DDR3 RDIMM, SPSS Inc., Chicago, IL, USA) for all statistical analyses.
Results
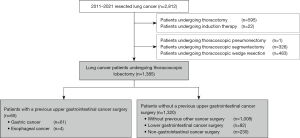
Over the 10-year period, 1,385 patients underwent curative thoracoscopic lobectomy, of whom 377 had a history of other cancer surgery, including 65 (4.7%) with a history of UGIC surgery (Figure 1). Of these 65 patients, 10 underwent perioperative chemotherapy for UGIC and no patient received radiation therapy before and after UGIC surgery. There were four patients with esophageal cancer. Postoperative pulmonary complications were observed in 155 (11.2%) patients, including 39 with a history of UGIC surgery. Three patients (0.2%) died within 90 days after surgery due to postoperative pulmonary complications. Figure 2 shows the prevalence of postoperative pulmonary complications. Accounting for some overlap, 7.7% (5 in 65 patients in the UGIC group) of patients with a history of UGIC surgery developed pneumonia and bronchopleural fistula, and 6.1% (4 in 65 patients in the UGIC group) developed empyema (Figure 2). Patient characteristics according to a history of UGIC surgery are shown in Table 1. Patients with a history of UGIC tended to be older, heavier smokers, and had lower lung function in terms of vital capacity. Although the frequency of postoperative pulmonary complications was significantly higher in patients with a history of UGIC surgery, there were no significant differences in all postoperative complications. The preoperative serum albumin and prealbumin levels were significantly lower in patients with a history of UGIC surgery than in those without a history of UGIC surgery (Table 1).
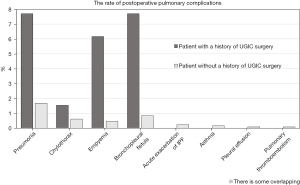
Table 1
| Variables | Patients with a history of UGIC (n=65) | Patients without a history of UGIC (n=1,320) | P value |
|---|---|---|---|
| Age, years | 75 ± 7.7 | 68 ± 10.2 | <0.001 |
| Sex, male | 51 (78.4) | 658 (49.8) | <0.001 |
| Smoking index, pack-year | 20±35.7 | 3.5±27.3 | <0.001 |
| FEV1.0%, % | 74.5±8.9 | 75±8.6 | 0.345 |
| %VC, % | 115.2±15.1 | 107.2±15.4 | 0.008 |
| Cardiopulmonary comorbidity, yes | 18 (27.7) | 237 (18.0) | 0.092 |
| Diabetes, yes | 10 (15.4) | 178 (13.5) | 0.663 |
| Serum level of albumin, g/dL | 4.0±0.4 | 4.2±0.3 | <0.001 |
| Serum level of prealbumin, g/dL | 23.0±5.9 | 28.9±6.2 | <0.001 |
| Histology, Ad | 50 (76.9) | 1140 (86.4) | 0.082 |
| Tumor size, mm | 23.0±10.6 | 22.0±10.6 | 0.257 |
| Duration of surgery, min | 183±46.1 | 189±52.4 | 0.287 |
| Bleeding, mL | 20±79.0 | 20±65.7 | 0.632 |
| Pathological stage (8th) | 0.268 | ||
| 0 | 6 (9.2) | 84 (6.4) | |
| I | 51 (78.5) | 1039 (78.7) | |
| II | 6 (9.2) | 115 (8.7) | |
| III | 2 (3.1) | 82 (6.2) | |
| Overall complications, yes | 11 (16.9) | 144 (10.9) | 0.137 |
| Pulmonary complications, yes | 6 (9.2) | 33 (2.5) | 0.003 |
| Hospital stay, days | 6.0±4.3 | 5.0±4.3 | 0.695 |
| 90-day mortality, cases | 0 (0) | 3 (0.2) | 0.753 |
Values are mean ± standard deviation or n (%). UGIC, upper gastrointestinal cancer; FEV1.0%, forced expiratory volume in one second percent; %VC, percent vital capacity; Ad, adenocarcinoma.
Table 2 shows the results of univariable and multivariable analyses performed to identify factors associated with the prevalence of postoperative pulmonary complications after thoracoscopic lobectomy for lung cancer. History of UGIC surgery, age, male sex, smoking index, forced expiratory volume 1.0%, the presence of cardiopulmonary comorbidities, and tumor size were risk factors for postoperative pulmonary complications in the univariable analysis. However, no significant association was observed between the prevalence of postoperative pulmonary complications and the serum levels of albumin and prealbumin. Multivariable analysis revealed that the history of UGIC surgery was an independent factor associated with postoperative pulmonary complications [odds ratio (OR) =2.92, P=0.016], as were smoking index (OR =1.01, P<0.001) and tumor size (OR =1.03, P=0.038).
Table 2
| Variables | Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|---|
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | ||
| Age, years | 1.05 (1.01–1.09) | 0.010 | |||
| Sex, male | 3.82 (1.74–8.37) | 0.001 | |||
| Smoking index, pack-year | 1.01 (1.01–1.02) | <0.001 | 1.01 (1.01–1.02) | <0.001 | |
| FEV1.0%* | 1.05 (1.02–1.09) | 0.001 | |||
| %VC* | 1.02 (0.99–1.04) | 0.063 | |||
| Cardiopulmonary comorbidities | 2.28 (1.16–4.50) | 0.017 | |||
| Diabetes | 1.96 (0.91–4.19) | 0.084 | |||
| UGIC surgery, yes | 3.97 (1.60–9.84) | 0.003 | 2.92 (1.12–7.65) | 0.016 | |
| Tumor size, mm | 1.03 (1.01–1.06) | 0.008 | 1.03 (1.01–1.05) | 0.038 | |
| Serum levels of albumin, g/dL | 0.70 (0.24–2.04) | 0.516 | |||
| Serum levels of prealbumin, g/dL | 0.93 (0.68–1.19) | 0.420 | |||
*, odds ratio for 1% increment of FEV1.0% and %VC. CI, confidence interval; FEV1.0%, forced expiratory volume one second percent; UGIC, upper gastrointestinal cancer; %VC, percent vital capacity.
We also compared the risk of postoperative pulmonary complications according to the type of previous cancer (Table 3). Of the 1,385 patients, 377 (27.2%) had a history of other cancer surgery. Among these patients, 65 had a history of UGIC surgery, 82 had a history of lower gastrointestinal cancer surgery, and the remaining 230 had a history of non-gastrointestinal cancer surgery. The prevalence of postoperative pulmonary complications in patients with a history of UGIC surgery was significantly higher than that of patients without a history of other cancer surgery (OR =4.00, P=0.003). However, there was no difference in the prevalence of pulmonary complications between patients with previous lower gastrointestinal cancer surgery or non-gastrointestinal cancer surgery and those without previous cancer surgery.
Table 3
| Variables | N | The prevalence of pulmonary complications | Odds ratio (95% CI)* | P value |
|---|---|---|---|---|
| All patients | 1,385 | 39 (2.8%) | ||
| Patients without previous other cancer surgery | 1,008 | 25 (2.5%) | 1 | – |
| Patients with previous other cancer surgery | 377 | 14 (3.7%) | 1.52 (0.78−2.96) | 0.220 |
| Upper gastrointestinal cancer surgery | 65 | 6 (9.2%) | 4.00 (1.58–10.12) | 0.003 |
| Lower gastrointestinal cancer surgery | 82 | 1 (1.2%) | 0.49 (0.07–3.63) | 0.481 |
| Non-gastrointestinal cancer surgery | 230 | 7 (3.0%) | 1.23 (0.53–2.89) | 0.628 |
*, odds ratio was calculated in comparison with patients without a history of cancer surgery. CI, confidence interval.
Discussion
The results of this study confirmed that the frequency of pulmonary complications after thoracoscopic lobectomy for lung cancer was significantly higher in patients with a previous UGIC surgery, with the previous UGIC surgery being an independent associated factor for postoperative pulmonary complications.
The use of minimally invasive surgery like a thoracoscopic surgery has contributed to the reduction of postoperative complications. Several reports showed that postoperative pulmonary complications developed in approximately 3–5% of patients, and of the patients who had undergone thoracoscopic lobectomy, 0.6% died within 30 days post-surgery (15-17). Additionally, the rate of postoperative pulmonary complications after thoracoscopic lobectomy was found to be 2.9% and the 90-day mortality rate was 0.2% (16,17). In recent years, surgeries like robotic and laparoscopic surgeries for management of extrapulmonary cancers have become less invasive with a decreased incidence rate of postoperative complications (2). Since the number of patients with good general health and favorable prognosis after extrapulmonary surgery is increasing, the opportunities to consider surgery for primary lung cancer management have also been on the rise, even if the patients had undergone previous surgeries for the management of other malignancies. In our study, approximately 30% of all lung cancer patients who underwent thoracoscopic lobectomy had a history of cancer surgery.
Several reports have shown that a history of treatment for gastric cancer had a low impact on long-term outcome after resection of lung cancer (1). Pulmonary resection for primary lung cancers following esophagectomy for esophageal carcinoma is acceptable and has low impact on prognosis (18). Nevertheless, the effect of UGIC surgeries on short-term outcomes has not been fully investigated. Generally, surgery for UGIC can have a profound effect on postoperative nutritional status, thus increasing the risk of postoperative complications. The deterioration in nutritional status 6 months post-esophagectomy could affect the complications following lung cancer surgery (19). We evaluated preoperative nutritional status using serum albumin and prealbumin levels and found that they were significantly lower in patients with a history of UGIC surgery than in those without a history of UGIC surgery. However, although nutritional status may have some influence on the risk of morbidity, serum albumin and prealbumin levels were not risk factors for postoperative pulmonary complications in our study.
Following UGIC surgery, there are many anatomical changes in the structures around the chest, such as the location of the intestinal tract and lymphatic systems. The lymphatic flow around the lower paraoesophageal and supradiaphragmatic lymph nodes changes after gastric cancer surgery (20). In the current study, bronchopleural fistulas were nine times more frequent in patients with a history of UGIC surgery than in those without, and all bronchopleural fistulas developed after right lower lobectomy. We performed lymph node dissection in the paraoesophageal region at the time of lower lobectomy (21), which may have influenced this result. Moreover, the regurgitation of gastrointestinal contents following UGIC surgery may also affect the outcome after lung cancer surgery. Fifty percent of postoperative pulmonary aspiration occurs during gastroesophageal procedures (22). Therefore, focus should be given to gastroesophageal reflux in patients after UGIC surgery.
Smoking index was also an independent risk factor for pulmonary complications in this study. Smoking itself affects lung respiratory function and causes airway inflammation, thereby increasing the vulnerability to postoperative pulmonary complications (15). Our previous study reported that smoking is a predictor for postoperative pulmonary complications after thoracoscopic lobectomy and segmentectomy (15). In addition, smoking significantly increases the risk of developing UGIC and lung cancer. Smoking was considered a confounding factor; however, multivariable analysis showed that previous UGIC surgery was an independent factor associated with postoperative pulmonary complications.
This study had several limitations. First, this retrospective study was conducted at a single institution; thus, more data accumulation is needed to validate our findings. Considering that this was a small cohort study and the number of events was also small, the use of a large multi-institutional database may enhance the statistical power of this analysis. Second, owing to the ambiguous definition of non-treatment complications, we only included Clavien–Dindo grade ≥ II complications. The results of this study may change if grade I complications are included.
Conclusions
Postoperative pulmonary complications after thoracoscopic lobectomy for primary lung cancer are three times more likely to develop in patients with a history of UGIC surgery than in those with a history of other surgeries for cancer or in those with no history of cancer surgery. Thus, efforts including preoperative respiratory rehabilitation and smoking cessation should be made to prevent postoperative pulmonary complications in patients with a history of UGIC surgery.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-798/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-798/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-798/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-798/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tsubokawa N, Mimae T, Aokage K, et al. Surgical outcomes of non-small-cell lung carcinoma in patients previously treated for gastric cancer. Eur J Cardiothorac Surg 2015;47:648-52. [Crossref] [PubMed]
- Ko KH, Huang HK, Chen YI, et al. Surgical outcomes of second primary lung cancer after the extrapulmonary malignancy. J Cancer Res Clin Oncol 2020;146:3323-32. [Crossref] [PubMed]
- Wu GX, Nelson RA, Kim JY, et al. Non-Small Cell Lung Cancer as a Second Primary Among Patients With Previous Malignancy: Who Is at Risk? Clin Lung Cancer 2017;18:543-550.e3. [Crossref] [PubMed]
- Tamjid B, Phan P, John T, et al. Outcomes for patients with synchronous and metachronous primary lung cancer after diagnosis of head and neck cancer. Head Neck 2017;39:1544-9. [Crossref] [PubMed]
- Takemura M, Sakurai K, Takii M, et al. Metachronous pulmonary metastasis after radical esophagectomy for esophageal cancer: prognosis and outcome. J Cardiothorac Surg 2012;7:103. [Crossref] [PubMed]
- Kim JY, Jang WY, Heo MH, et al. Metachronous double primary cancer after diagnosis of gastric cancer. Cancer Res Treat 2012;44:173-8. [Crossref] [PubMed]
- Mitani S, Kadowaki S, Oze I, et al. Risk of second primary malignancies after definitive treatment for esophageal cancer: A competing risk analysis. Cancer Med 2020;9:394-400. [Crossref] [PubMed]
- Yamamoto M, Yamanaka T, Baba H, et al. The postoperative recurrence and the occurrence of second primary carcinomas in patients with early gastric carcinoma. J Surg Oncol 2008;97:231-5. [Crossref] [PubMed]
- Huang ZD, Gu HY, Zhu J, et al. The application of enhanced recovery after surgery for upper gastrointestinal surgery: Meta-analysis. BMC Surg 2020;20:3. [Crossref] [PubMed]
- Fujiya K, Kawamura T, Omae K, et al. Impact of Malnutrition After Gastrectomy for Gastric Cancer on Long-Term Survival. Ann Surg Oncol 2018;25:974-83. [Crossref] [PubMed]
- Shim H, Cheong JH, Lee KY, et al. Perioperative nutritional status changes in gastrointestinal cancer patients. Yonsei Med J 2013;54:1370-6. [Crossref] [PubMed]
- Ji X, Ding H. The efficacy of enteral nutrition combined with accelerated rehabilitation in non-small cell lung cancer surgery: A randomized controlled trial protocol. Medicine (Baltimore) 2020;99:e23382. [Crossref] [PubMed]
- Clavien PA, Sanabria JR, Strasberg SM. Proposed classification of complications of surgery with examples of utility in cholecystectomy. Surgery 1992;111:518-26. [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Yamamichi T, Ichinose J, Iwamoto N, et al. Correlation Between Smoking Status and Short-term Outcome of Thoracoscopic Surgery for Lung Cancer. Ann Thorac Surg 2022;113:459-65. [Crossref] [PubMed]
- Agostini PJ, Lugg ST, Adams K, et al. Risk factors and short-term outcomes of postoperative pulmonary complications after VATS lobectomy. J Cardiothorac Surg 2018;13:28. [Crossref] [PubMed]
- Ichinose J, Kohno T, Fujimori S, et al. Locoregional control of thoracoscopic lobectomy with selective lymphadenectomy for lung cancer. Ann Thorac Surg 2010;90:235-9. [Crossref] [PubMed]
- Komatsu H, Izumi N, Tsukioka T, et al. Surgical outcomes of primary lung cancers following esophagectomy for primary esophageal carcinoma. Jpn J Clin Oncol 2021;51:786-92. [Crossref] [PubMed]
- Baker M, Halliday V, Williams RN, et al. A systematic review of the nutritional consequences of esophagectomy. Clin Nutr 2016;35:987-94. [Crossref] [PubMed]
- Kunisaki C, Shimada H, Nomura M, et al. Lymph node dissection in surgical treatment for remnant stomach cancer. Hepatogastroenterology 2002;49:580-4. [PubMed]
- Mun M, Nakao M, Matsuura Y, et al. Oncological outcomes after lobe-specific mediastinal lymph node dissection via multiport video-assisted thoracoscopic surgery. Eur J Cardiothorac Surg 2020;58:i92-9. [Crossref] [PubMed]
- Sakai T, Planinsic RM, Quinlan JJ, et al. The incidence and outcome of perioperative pulmonary aspiration in a university hospital: a 4-year retrospective analysis. Anesth Analg 2006;103:941-7. [Crossref] [PubMed]