Veno-venous extracorporeal membrane oxygenation support during lung volume reduction surgery for a severe respiratory failure patient with emphysema
Introduction
Veno-venous extracorporeal membrane oxygenation (ECMO) provides support for severe respiratory failure where the body blood flow is maintained entirely by cardiac function. Venous blood outflow from the patient is accessed from the large central veins, and inflow is returned near the right atrium after oxygenation. Normally veno-venous ECMO is, however, combined with mechanical ventilation. This article reports the case of the clinical use of veno-venous ECMO in a severe respiratory failure patient who required lung volume reduction surgery (LVRS) because of emphysema, but could not afford single-lung ventilation during anaesthesia.
Case presentation
A 60-year-old man had undergone chronic cough with sputum and short of breath for eight years, with a past history of hypertension for ten years and inguinal hernia repair surgery for two years. Two years before the admission, his lung function test showed a forced expiratory volume in 1 s (FEV1) of 37.1% predicted and the ratio of FEV1/FVC (a forced vital capacity) was 59.18%. The lung computed tomography (CT) scan confirmed panlobuia emphysema in the lung with bulla formation (Figure 1).
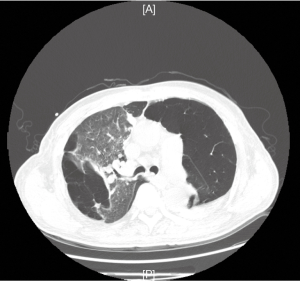
He subsequently presented himself as an emergency with an acute exacerbation of dyspnea. Severe pulmonary emphysema was diagnosed on clinical. He then developed impending respiratory failure due to the emphysema aggravation and atelectasis of the remaining lung, when was intubated in our intensive care unit. The blood gas analysis showed pH 7.257, pO2 75.1 mmHg, pCO2 98.9 mmHg, SO2 92%; with mechanical ventilation [Mode: volume assist/control (VA/C), parameters: tidal volume 400 mL (weight 51 Kg), PEEP 6 cmH2O, frequency 18 per minute, FiO2 1.0]. Laboratory examinations showed the count of white blood cells was 10.12×109/L and the percentage of neutrophilic granulocyte was 91.1%, C-reactive protein and procalcitonin were both normal.
LVRS was considered the only option to control the life-threatening hypercapnia. Due to his poor respiratory reserve, the conventional anaesthesia with single lung ventilation was inappropriate. Veno-venous ECMO was used to allow operation on a non-ventilated lung.
Under ultrasound guidance, a 17-Fr armoured catheter was placed into the right internal jugular vein. A 23-Fr venous cannula was inserted into the right femoral vein. Then the cannulas were connected to the ECMO system (Maquet PLS) via heparin-coated tubing primed with physiological sodium chloride solution, and the pump was started. Blood flow through the device was increased to 3.6−4 L and 3,300−3,800 revolutions per minute (rpms).
During the cannulation, patient was given a bolus intravenous (iv) dose of unfractionated heparin (UFH) 2,500 IU (51 kg) to avoid thrombosis phenomena after placement of the guide wire at 1:44 pm. Activated partial thromboplastin time (aPTT) was common used to monitor anticoagulation management in our department, because it had an acceptable degree of correlation with heparin concentration and was therefore to be considered superior to the activated clotting time (ACT) (1). Thromboelastography (TEG) test was not available in our center at that time. After cannulation and onset of ECMO, no heparinization was undertaken and aPTT was 77.8 s at 2:15 pm. Then the patient was sent to the operating room and anaesthetized, a bilateral bulla resection was performed by video assisted thoracic surgery (VATS) from 2:45 pm to 7:20 pm.
The lung was deflated and up to 5 L/min of ECMO blood flow maintained the SpO2 above 85%. Bullectomy was then performed using the linear stapler. Blood pressure was 93−154/36−59 mmHg, heart rate was 68−90 bpm without vasoactive agent during the operation. Total input fluid was 1,500 mL without blood transfusion, urine output was 350 mL and blood loss was 20 mL. Echocardiography showed left ventricular ejection fraction was 74% and 76% before and after the operation, no right ventricular hypertrophy and pulmonary hypertension. It was our limitation that we did not monitor the right ventricular function by the pulmonary artery catheterization (PAC) with the Swan-Ganz catheter. Although echocardiography was the best tool to evaluate the cardiac function, PAC was still considered the gold standard of haemodynamic monitoring (2).
ECMO worked very well and the blood gas analysis showed pH 7.49, pO2 131 mmHg, pCO2 46 mmHg, SO2 99% without mechanical ventilation (ECMO mode: blood flow 5 L/min and oxygen flow 5.5 L/min).
Given the residual effects of surgery, no anticoagulation was taken for the first 12 h to avoid massive postoperative bleeding. UFH infusion was started (10−20 IU/kg/h) on the second postoperative day and aPTT was controlled between 50 to 60 s. PaO2 increased and PaCO2 decreased remarkably and the CT scan showed the remaining lung reflated well (Figure 2).
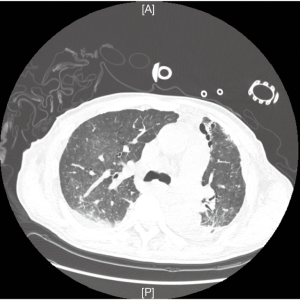
In the course of the first four postoperative days, ECMO was used to assist recovery; blood flow velocity was 2.51 to 4.28 L per minute with 2,430 to 3,955 rpms, and oxygen flow was 1.0 to 3.5 L per minute. The ventilator mode was pressure support ventilation, pressure support (PS) 10 to 18 cmH2O, PEEP 5 to 6 cmH2O, and FiO2 0.25 to 0.6. Patient’s SaO2 was 85% to 100%. Four days later, weaning from ECMO was accomplished by gradually reduced blood and oxygen flow. After the oxygen tube was clamped to stop gas flow for 12 hours, the tidal volume was 280 to 360 mL and respiratory rates were 13 to 18 times per minute. The blood gas analysis showed pH 7.387, pO2 92.5 mmHg, pCO2 60.6 mmHg, SO2 96% with mechanical ventilation (PS 15 cmH2O, PEEP 6 cmH2O, and FiO2 0.6). Then the ECMO cannulas were removed. UFH infusion was continued to maintain aPTT about 50 s for 6 hours after ECMO was stopped, and Nadroparin calcium 0.4 mL subcutaneous injection once a day was sequential. Then he was extubated until the fourteenth day after operation due to the weakness of respiratory muscles. He was finally discharged from the ICU twenty days after surgery, and went home ten days later.
Discussion
Surgical resection of giant occupying space emphysematous bullae allows the compress functioning lung to expand, permits a better ventilation and perfusion, decrease both dead space and residual volume and improves the chest mechanics with remodelling of the diaphragm and the chest wall (3). Surgical bullectomy is indicated for patients with dyspnoea having compressive space-occupying non-functioning bullae developed on near normal or emphysematous lung (4). In high-risk patients with emphysematous bulla, the best surgical circumstance in which to perform bullectomy is to isolate the affected lung. Therefore, lung isolation allows a nonventilated, nonfunctioning lung and optimal conditions for safely performing a pulmonary resection.
In view of emphysema in our patient’s remaining lung, ECMO was selected as the method of operating on a non-ventilated lung whilst maintaining gas exchange. And ECMO has, over the last 2 decades, become a valuable tool in the management of pediatric and increasingly, adult patients (5,6).
As demonstrated in this case, it is a useful method when other modes of pulmonary support are inadequate. Our familiarity with this disease process suggested that standard ventilatory management would not afford sufficient intraoperative support due to moderate ventilatory support, low PaO2/FiO2 ratio, and the operative requirement or lung isolation.
Our team also discussed cardiopulmonary bypass (CPB) as possible options in this instance. We decided not to use CPB as it usually requires full heparinisation and anticoagulation, which may have worsened bleeding and altered surgical operating conditions (7). Given its size and complexity, a CPB circuit would also not have permitted ongoing postoperative support. The Novalung® interventional lung assist (ILA®) maybe an alternative for this patient, which requires the femorofemoral implantation of a specific low-resistance artificial lung, is the easiest way to provide an extracorporeal respiratory support in an awake patient and had been used as a bridge to lung transplantation in patients with good cardiac function (8). But The Novalung® is not for sale in the market in Mainland China. Although ECMO is not without risks, in the setting of a carefully planned and executed elective procedure, it is well-tolerated and provides optimal pulmonary support. We routinely use percutaneous VV (veno-venous)-ECMO cannulation through the right femoral and internal jugular veins, heparin-bonded circuit, and centrifugal pump without systemic anticoagulation. Although VA (veno-arterial)-ECMO provides both gas exchange and cardiac support, but reduces pulmonary artery flow. Lung ischemia produced by inadequate pulmonary artery circulation can cause acute lung injury if followed by reperfusion with oxygenated blood (9). Due to the bleeding risk or complications after Bullectomy operation, we have a possibility of weaning from VV-ECMO without anticoagulation by the gas flow gradually reduced and the blood flow maintained on high rpms (10). This strategy is not recommended for VA-ECMO, because its weaning increases the risk of thrombotic events without anticoagulation.
Conclusions
In summary, we believe ECMO in this setting of complex surgery requiring deflation of the remaining lung provided excellent intra-operative support and stability that was successfully extended into the postoperative period. We recommend that ECMO be considered as a feasible and beneficial technique to facilitate thoracoscopic surgery in cases with multiple or more complex comorbidities.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
- Hirsh J, Raschke R, Warkentin TE, et al. Heparin: mechanism of action, pharmacokinetics, dosing considerations, monitoring, efficacy, and safety. Chest 1995;108:258S-275S. [Crossref] [PubMed]
- Porhomayon J, El-Solh A, Papadakos P, et al. Cardiac output monitoring devices: an analytic review. Intern Emerg Med 2012;7:163-71. [Crossref] [PubMed]
- Nakahara K, Nakaoka K, Ohno K, et al. Functional indications for bullectomy of giant bulla. Ann Thorac Surg 1983;35:480-7. [Crossref] [PubMed]
- De Giacomo T, Venuta F, Rendina EA, et al. Video-assisted thoracoscopic treatment of giant bullae associated with emphysema. Eur J Cardiothorac Surg 1999;15:753-6; discussion 756-7. [Crossref] [PubMed]
- Brodie D, Bacchetta M. Extracorporeal membrane oxygenation for ARDS in adults. N Engl J Med 2011;365:1905-14. [Crossref] [PubMed]
- Javidfar J, Brodie D, Iribarne A, et al. Extracorporeal membrane oxygenation as a bridge to lung transplantation and recovery. J Thorac Cardiovasc Surg 2012;144:716-21. [Crossref] [PubMed]
- Øvrum E, Tangen G, Tølløfsrud S, et al. Heparinized cardiopulmonary bypass circuits and low systemic anticoagulation: an analysis of nearly 6000 patients undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg 2011;141:1145-9. [Crossref] [PubMed]
- Fischer S, Hoeper MM, Bein T, et al. Interventional lung assist: a new concept of protective ventilation in bridge to lung transplantation. ASAIO J 2008;54:3-10. [Crossref] [PubMed]
- Baudouin SV. Lung injury after thoracotomy. Br J Anaesth 2003;91:132-42. [Crossref] [PubMed]
- Lappa A, Donfrancesco S, Contento C, et al. Weaning from venovenous extracorporeal membrane oxygenation without anticoagulation: is it possible? Ann Thorac Surg 2012;94:e1-3. [Crossref] [PubMed]


