
Characteristics of sublingual microcirculatory changes during the early postoperative period following cardiopulmonary bypass-assisted cardiac surgery—a prospective cohort study
Introduction
Microcirculation refers to the blood circulation between arterioles and venules and is the prime site for the exchange of oxygen, nutrients, and metabolites and the most important regulator of oxygen delivery in tissue. Serious medical conditions such as sepsis, high-risk surgery, cardiac arrest, and respiratory failure can lead to microcirculatory dysfunction. Persistent microcirculatory dysfunction is the main mechanism of tissue oxygenation failure and early organ dysfunction, which have a significant impact on the prognosis of patients. Studies have reported that extensive microcirculatory changes occur after cardiac surgery (1-4). The changes in microcirculation after cardiac surgery are related to potential heart disease or cardiogenic shock, anesthesia, and surgical procedures, and the impact of cardiopulmonary bypass (CPB) on microcirculation should not be overlooked. During CPB, the systemic blood circulation is converted to extracorporeal circulation by a heart-lung machine, and blood cells and plasma proteins are activated due to exposure to polymers that are not biocompatible, thereby triggering inflammation and micro thrombosis. Hemodilution, hypothermia, cardiac arrest, and the conversion of pulsatile and nonpulsatile blood flow during CPB increase the adverse effects on microcirculation. Studies have shown that acute changes in microcirculatory blood flow occur after CPB-assisted cardiac surgery, including increased microcirculatory heterogeneity, decreased functional capillary density, and increased venular blood flow velocity (5-8). Microcirculatory changes can lead to organ dysfunction, prolonged hospitalization, and other adverse consequences (9-12), but microcirculation monitoring has not been widely used due to research in this filed are still limited and which parameter is most sensitive remains to be determined. Moreover, little is known about the pattern of these changes and recovery after CPB-assisted cardiac surgery (13,14).
During the progression of acute circulatory failure caused by hypovolemic shock, sepsis, and pericardial tamponade, both microcirculatory perfusion parameters and macroscopic hemodynamic indicators deteriorate simultaneously, suggesting that the changes of the two coincide. However, there is usually no consistency between microcirculatory perfusion changes and macrocirculation changes during resuscitation following circulatory failure (15,16). Knowing the relationship between microcirculation and macrocirculation is the key to guide interventions and provide personalized treatments in the perioperative period. Current research on the consistency between microcirculation and macrocirculation has been mainly focused on patients with sepsis and shock, and there is no research on whether there is a loss of consistency between microcirculation and macrocirculation after adult cardiac surgery. This study aimed to analyze changes in microcirculatory parameters after CPB-assisted cardiac surgery and their correlation with macroscopic hemodynamic indicators to explore the characteristics of microcirculatory changes after CPB-assisted cardiac surgery and guide postoperative cardiac resuscitation. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1159/rc).
Methods
Study design
In this single-centered prospective cohort study, patients who received CPB-assisted cardiac surgery and were admitted to the intensive care unit (ICU) after surgery at the Guangdong Provincial People’s Hospital, Department of Cardiac Surgery between December 1 2018 and January 31 2019 were recruited, to compare measurements of microcirculation at 4-time intervals within 24 hours postoperatively, and analyze correlation between microcirculatory parameters and macroscopic hemodynamic indicators at each time-point. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the ethics committee of Guangdong Provincial People’s Hospital (No. 2018-584H-1). The ethics committee of Guangdong Provincial People’s Hospital waived the need for patient’s written informed consent.
The inclusion criteria were patients ≥18 years of age with indwelling pulmonary artery catheter and with sedative and analgesic ventilator-assisted ventilation or spontaneous breathing but conscious and able to cooperate. The exclusion criteria were: patients with emergency surgery; pregnant women; patients with heart transplantation, intra-aortic balloon pump (IABP) or extracorporeal membrane oxygenation (ECMO); patients with oral disease, difficulty opening their mouth, or with massive oral bleeding that would affect the quality of image acquisition; patients with noninvasive ventilation; and those participating in other studies during the same period or unwilling to participate in this study. The elimination criteria were those who survived less than 24 hours after inclusion or withdrew halfway and those with poor image quality.
Data collection and processing
Patients who met the inclusion criteria were admitted to the ICU after cardiac surgery. Peripheral arterial and pulmonary arterial blood samples for blood gas analysis were collected at 0, 6, 16, and 24 hours after admission to determine arterial lactic acid, arterial and mixed venous oxygen saturation, arterial and mixed venous carbon dioxide partial pressure, arterial oxygen partial pressure, arterial oxygen saturation (SaO2), and hemoglobin (Hb) concentration. Cardiac index (CI) and systemic vascular resistance index were measured through a pulmonary artery catheter, and systolic blood pressure, mean arterial pressure (MAP), central venous pressure, and central body temperature (rectal temperature) were recorded at each time interval. Perfusion pressure, arterial oxygen content (CaO2), oxygen delivery index (DO2I), and venous-arterial carbon dioxide partial pressure difference (Pv-aCO2 or PCO2gap) were calculated at each time interval according to the formulae presented in Table 1.
Table 1
| Perfusion pressure = MAP − central venous pressure |
| CaO2 = (1.34 × SaO2 × Hb) + (0.003 × PaO2) |
| DO2I = 10 × CI × CaO2 |
| Pv-aCO2 = PvCO2 − PaCO2 |
MAP, mean arterial pressure; CaO2, arterial oxygen content; SaO2, arterial oxygen saturation; Hb, hemoglobin; PaO2, arterial oxygen pressure; DO2I, oxygen delivery index; CI, cardiac index; Pv-aCO2, venous-arterial carbon dioxide partial pressure difference; PvCO2, partial pressures of the dissolved CO2 in the mixed venous blood; PaCO2, partial pressures of the dissolved CO2 in the mixed arterial blood.
The video images of sublingual microcirculation were collected using a MicroSeeV100 handheld video microscope (Guangzhou Yiruan Intelligent Technology Co., Ltd., Guangzhou, China; software version 2.84) at 0, 6, 16, and 24 hours of ICU admission. A trained operator gently wiped off oral secretions, especially those under the tongue, with a 37 ℃ normal saline cotton swab. The tracheal intubation tube was moved to 1 side 2 minutes later. A microscopic video probe was used during image acquisition to avoid the influence of pressure on microcirculation, and the focal length was adjusted to obtain stable and clear video images. A total of 5 video images lasting 30 seconds were captured at 2 locations on each side of the lingual frenulum and at another optional location. All images were confirmed independently by two professional physicians. Five microcirculatory parameters, including total vascular density (TVD), perfused small vessel density (PSVD), proportion of perfused small vessels (PPV), microvascular flow index (MFI), and flow heterogeneity index (HI) could be obtained through the collected images.
The small vessels were traced by a computer, and supplementary traces were made artificially, depending on the situation. TVD in mm/mm2 is the ratio of the sum of microvascular length to the visual field area. PSVD in mm/mm2 is the ratio of the sum of the length of perfused small vessels to the visual field area. PPV in % is the ratio of the length of perfused small vessels to the length of all small vessels, reflecting the perfusion quality of microcirculation. A higher perfusion ratio represented better microcirculatory perfusion.
The MFI was calculated as follows. The obtained visual field was equally divided into 4 quadrants, and scores were based on the flow of small vessels in each quadrant: no blood flow (0 point), intermittent blood flow (1 point), slow blood flow (2 points), and normal flow (3 points). The average of the 4 scores represented the MFI and reflected microcirculatory blood flow. The difference between the maximum and minimum of the 4 scores divided by MFI was the HI, which was used to evaluate differences in the adjacent blood flow of microcirculation, reflecting the simultaneous occurrence of flowing capillaries and stasis or clotting capillaries after microcirculatory dysfunction.
Statistical analysis
R 3.5.5 software was used for statistical analysis. All continuous data are expressed as mean ± standard deviation (SD). Analysis of variance was used for the comparison of measurement data within groups of 4-time intervals (0, 6, 16, 24 hours). Pearson correlation analysis was used for normally distributed data, while Spearman correlation analysis was used for nonnormally distributed data. P≤0.05 was considered statistically significant.
Results
Among the 24 patients who met the inclusion criteria, there were 10 patients undergoing valve replacement surgery, 5 patients undergoing re-do cardiac surgery, 3 patients with hypertrophic obstructive cardiomyopathy, 2 patients undergoing coronary artery bypass graft plus valve surgery, 2 patients undergoing coronary artery bypass surgery, 1 patient undergoing major vascular surgery, and 1 patient with congenital heart disease. Due to various reasons, including the removal of pulmonary artery catheter, monitoring failure, and the noncompliance of patients, the number of data sets collected for each time interval were different, including 24 sets of data at 0 hour, 24 sets of data at 6 hours, 19 sets of data at 16 hours, and 16 sets of data at 24 hours (Tables 2,3).
Table 2
| Indicators | 0 hour (n=24) | 6 hours (n=24) | 16 hours(n=19) | 24 hours (n=16) |
|---|---|---|---|---|
| Perfusion pressure (mmHg) | 71.25±2.52 | 67.21±2.49 | 69.79±2.05 | 71.38±3.25 |
| Systolic pressure (mmHg) | 119.46±3.28 | 118.00±3.22 | 113.42±6.48 | 124.38±4.96 |
| MAP (mmHg) | 82.25±2.13 | 78.79±2.25 | 81.32±1.90 | 81.63±2.59 |
| Central body temperature (rectal temperature) (℃) | 36.62±0.18 | 37.97±0.13 | 37.85±0.19 | 37.66±0.10 |
| CI (L/min/m2) | 2.37±0.12 | 2.64±0.17 | 2.47±0.17 | 2.81±0.19 |
| Systemic vascular resistance index (dyn·sec/cm5/m2) | 2,716.25±177.65 | 2,370.38±173.45 | 2,421.00±178.55 | 2,000.25±175.91 |
| Arterial lactic acid (mmol/L) | 3.89±0.41 | 5.49±0.61 | 2.49±0.61 | 1.71±0.25 |
| Mixed venous oxygen saturation (%) | 0.68±0.03 | 0.65±0.02 | 0.64±0.02 | 0.67±0.03 |
| DO2I (mL/min·m2) | 373.73±22.23 | 405.50±25.77 | 373.40±24.91 | 424.31±28.97 |
| PCO2gap (mmHg) | 10.50±0.86 | 10.17±0.74 | 9.68±0.82 | 9.13±0.76 |
Continuous data is presented as mean ± SD. MAP, mean arterial pressure; CI, cardiac index; DO2I, oxygen delivery index; PCO2gap, carbon dioxide partial pressure difference; SD, standard deviation.
Table 3
| Parameters | 0 hour (n=24) | 6 hours (n=24) | 16 hours (n=19) | 24 hours (n=16) | P value |
|---|---|---|---|---|---|
| TVD (mm/mm2) | 29.69±1.96 | 31.38±1.55 | 32.97±1.57 | 34.18±1.20 | 0.012 |
| PSVD (mm/mm2) | 25.10±1.76 | 27.66±1.69 | 30.34±1.63 | 30.72±1.69 | 0.005 |
| PPV (%) | 84.41±2.46 | 86.54±2.57 | 91.51±1.28 | 88.13±3.04 | 0.314 |
| MFI | 2.41±0.09 | 2.59±0.09 | 2.63±0.07 | 2.57±0.09 | 0.162 |
| HI | 0.61±0.11 | 0.35±0.08 | 0.43±0.08 | 0.40±0.10 | 0.167 |
Continuous data is presented as mean ± SD. TVD, total vascular density; PSVD, perfused small vessel density; PPV, proportion of perfused small vessels; MFI, microvascular flow index; HI, flow heterogeneity index; SD, standard deviation.
There were statistical differences in the changes of TVD (P=0.012) and PSVD (P=0.005) during the 24-hour postoperative window following CPB-assisted cardiac surgery. There was no significant difference in PPV, MFI, and HI (Table 3, Figure 1).
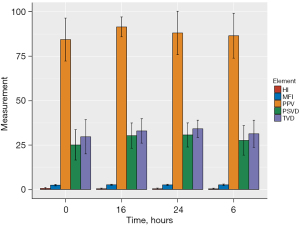
TVD and PSVD did not correlate with perfusion pressure, systolic pressure, MAP, rectal temperature, CI, systemic vascular resistance index, arterial lactic acid, mixed venous oxygen saturation, and PCO2gap at the 4-time intervals but were correlated with DO2I (TVD: r=−0.5059, P=0.0456; PSVD: r=−0.5499, P=0.0273) at 24 hours (Tables S1,S2).
PPV did not correlate with perfusion pressure, systolic pressure, MAP, rectal temperature, CI, systemic vascular resistance index, arterial lactic acid, mixed venous oxygen saturation, and DO2I at the 4-time intervals, while there was a moderate correlation with PCO2gap (r=0.4370, P=0.0327) at 0 hour (Table S3).
MFI did not correlate with perfusion pressure, systolic blood pressure, MAP, rectal temperature, CI, systemic vascular resistance index, arterial lactic acid, and mixed venous oxygen saturation at the 4-time intervals. On the other hand, MFI was strongly correlated with PCO2gap (r=0.6496, P=0.0006) at 0 hour (Table S4).
HI showed no correlation with perfusion pressure, systolic pressure, MAP, rectal temperature, CI, systemic vascular resistance index, arterial lactic acid, and mixed venous oxygen saturation at the 4-time intervals but was moderately correlated with PCO2gap (r=−0.5350, P=0.0071) at 0 hour (Table S5).
Discussion
Previous studies (17-19) have shown that changes in microcirculation such as decreased perfusion, functional shunting, and heterogeneous perfusion occur after CPB-assisted cardiac surgery, but there are few studies on the changes of microcirculatory parameters during the early postoperative period. A prospective observational study on microcirculatory changes in children after cardiac surgery by Scolletta et al. (20) concluded that microcirculation had different trends during and after cardiac surgery in children with cyanotic heart disease and noncyanotic heart surgery. TVD, PPV, and PSVD increased gradually over time, while MFI and HI did not change significantly in children with cyanotic heart disease. For children with noncyanotic heart disease, no parameters changed significantly. Our results suggested that there were significant differences in the changes of TVD and PSVD in adult patients within 24 hours of cardiac surgery, while there were no significant changes in the other parameters. The effect of CPB-assisted cardiac surgery on adult microcirculation is different from children with noncyanotic heart disease and resembles more that of children with cyanotic heart disease. The study by Scolletta et al. (20) looked at changes from the beginning of anesthesia to 12 hours after admission to the ICU. It is speculated that CPB-assisted cardiac surgery has little effect on the microcirculation of children with noncyanotic heart disease, while with the intraoperative correction of cardiac malformations, changes in the concentration of deoxyhemoglobin and oxygenated Hb result in the improvement of microcirculatory parameters in the children with cyanotic heart disease. In our study, CPB may have had a significant impact on the microvascular density parameters of TVD and PSVD in adult patients undergoing cardiac surgery, thus microcirculation changed significantly during postoperative cardiac resuscitation. A study by den Os et al. (7) suggested that CPB during cardiac surgery could reduce sublingual microcirculation perfusion and that the main manifestation of microcirculatory perfusion malfunction after CPB-assisted cardiac surgery was the decrease of perfused capillary, which resulted in decreased PSVD and PPV but had no effect on TVD.
Interestingly, no significant change in MFI after CPB was observed in this study. There are conflicting reports concerning the influence of CPB on MFI. Some studies have reported that MFI increased compared with the baseline after the start of CPB during surgery (18,21), while some did not observe this effect during cardiac surgery (20,22,23), and others reported that MFI significantly decreased compared with the baseline during ICU follow-up (24,25). It is speculated that the current measurement method of MFI only reflects the abnormal flow of slow microcirculation and does not consider the abnormal flow of hyperdynamic microcirculation. However, hyperdynamic circulation after CPB is common in clinical practice, making it difficult to identify the effect of CPB on MFI. The measurement method of HI is similar to that of MFI, so the limitation is also similar. In addition, HI is used to evaluate differences in the adjacent blood flow of microcirculation, which is common in patients with sepsis. Therefore, HI is mostly used for microcirculatory studies in sepsis (26-28) but rarely for microcirculatory studies after cardiac surgery (20).
Many studies have shown that microcirculatory perfusion parameters and macroscopic hemodynamic indicators deteriorate simultaneously during the progression of acute circulatory failure caused by hypovolemic shock, sepsis, and pericardial tamponade, indicating that the changes of the two coincide. A study of a porcine model of hemorrhagic shock by van Iterson et al. (29) indicated that changes in oxygen partial pressure of cardiac and intestinal microcirculation were closely related to changes in macroscopic hemodynamic indicators (CI, MAP, and DO2I) during the progression of hemorrhagic shock. In another study (30) on animal models of obstructive and endotoxin shock, the regional perfusion in sublingual, intestinal, and muscular vascular beds was measured by microcirculatory assessment, measurement of tissue oxygenation, and assessment of surrounding skin perfusion at different time intervals during the progression of circulatory failure, and the results showed that regional perfusion in these areas coincided with the changes in the systemic circulation [cardiac output (CO)]. However, changes in microcirculatory perfusion were not necessarily consistent with systemic circulation during resuscitation from circulatory failure. van Genderen et al. (30) reported that in a porcine model of cardiac tamponade with obstructive shock, the microcirculatory perfusion of sublingual, intestinal, and muscular vascular beds recovered to baseline level when CO was restored to baseline level using fluid resuscitation, suggesting that the improvement of microcirculation and macrocirculation coincided. Nevertheless, microcirculatory perfusion did not successfully recover to baseline level, although CO and MAP were restored to baseline level using fluid resuscitation in the endotoxin shock model. Several studies have shown that microcirculation in patients with sepsis may still maintain hypoperfusion despite the recovery of macrocirculation with fluid resuscitation and vasoactive drug treatment. Dubin et al. (31) found that CO, CI, pulmonary circulatory resistance, systemic circulatory resistance, and the stroke index of left and right ventricles could be significantly improved with increased MAP using norepinephrine in patients with septic shock, but there was no change in sublingual microcirculatory MFI and PPV, and a downward trend was observed in PSVD. A randomized, double-blind crossover study of patients with septic shock showed the sublingual microcirculatory parameters did not change by infusing dobutamine and increasing oxygen delivery (32). Microcirculation plays a key role in delivering oxygen to cells and maintaining tissue perfusion. Circulatory dysfunction leads to the loss of hemodynamic correlation between microcirculation and macrocirculation during the progression of resuscitation, and microcirculation cannot be improved correspondently with the improvement of systemic hemodynamic indicators, resulting in the failure of organ-system perfusion and oxygen supply and eventually the development of multiple organ dysfunction or even death. The loss of consistency between macrocirculation and microcirculation during resuscitation from severe sepsis and traumatic hemorrhagic shock has been identified as a hemodynamic indicator associated with increased incidence and mortality due to multiple organ failure (33-36).
Due to the particularity of CPB-assisted cardiac surgery, patients often have extensive microcirculatory dysfunction after surgery. At present, there are very few reports on the consistency between microcirculation and macrocirculation after cardiac surgery. According to a study on children’s microcirculation by Scolletta et al. (20), there was no correlation between macroscopic hemodynamic indicators (MAP and venous oxygen saturation) and microcirculatory parameters (TVD, PPV, PSVD, HI, and MFI) at 0, 6, and 12 hours after CPB. However, these results may not be relevant for adult patients after cardiac surgery due to differences in microcirculatory changes between children and adults. Additionally, only children with cyanotic congenital heart disease were enrolled in this study. To our knowledge, there is no research on consistency between microcirculation and macrocirculation after cardiac surgery in adults. Herein, adult patients with CPB-assisted cardiac surgery were enrolled as study subjects, and the consistency between various microcirculatory parameters and macroscopic hemodynamic indicators during the early postoperative period (within 24 hours) after cardiac surgery was analyzed. The results indicated that microcirculatory parameters did not correlate with postoperative hemodynamic indicators (perfusion pressure, systolic blood pressure, MAP, CI, and systemic vascular resistance index), oxygen metabolic indicators (blood lactate, mixed venous oxygen saturation), and central body temperature at the 4-time intervals postoperatively. There was only a moderate correlation between microcirculatory parameters and DO2I at 24 hours (TVD: r=−0.5059, P=0.0456; PSVD: r=−0.5499, P=0.0273).
According to the calculation formula, DO2I = CI × CaO2 × 10, DO2I was positively correlated with CI, and as the increase of CI can improve microcirculation, the microcirculatory density indicators (TVD and PSVD) should positively correlate with DO2I. However, due to the loss of consistency between macrocirculation and microcirculation during the early postoperative period after cardiac surgery, there was no correlation between the CI and microcirculatory parameters in this study and TVD and PSVD were negatively correlated with DO2I. It is speculated that ischemia-reperfusion injury during the early postoperative period may lead to microcirculatory intolerance to high-level oxygen supply. Even normal-level oxygen supply may cause adverse effects on microcirculation due to the increased production of oxygen free radicals. Low-level oxygen supply may be more conducive to the recovery of microcirculatory function during the early postoperative period. Multiple studies on experimental cardiac arrest have shown that high-concentration oxygen ventilation after restoring spontaneous circulation is associated with the aggravation of cerebral nerve damage and a worse prognosis (37-39). However, due to the lack of reports on the correlation between microcirculatory parameters and the indicators of oxygen supply and oxygen consumption (VO2), this remains to be confirmed by larger-scale studies. In this study, there was no significant correlation between microcirculatory parameters and arterial lactate level, which is a common indicator of tissue perfusion. An increase in blood lactate levels after cardiac surgery is normally related to organic anaerobic metabolism caused by microcirculatory dysfunction. In addition, nonhypoxic mechanisms such as organic hypermetabolism may play a significant role in increasing blood lactate levels (40-42). However, the lack of statistical correlation between microcirculatory parameters and blood lactate level in our study may have been due to the limited sample size.
Pv-aCO2 is considered to be a good indicator for evaluating organic blood flow. According to the modified Fick’s formula, Pv-aCO2 = k × total carbon dioxide production (VCO2)/CO, Pv-aCO2 is inversely proportional to CO, which is supported by the results of clinical studies on patients with septic shock (43,44). However, some studies have reported no significant negative correlation between Pv-aCO2 and CO (45,46). In our study, Pv-aCO2 was not significantly negatively correlated with CI at 0 hour (r=−0.181, P=0.397) but showed a strong positive correlation with MFI (r=0.6496, P=0.0006). During the early postoperative period following cardiac surgery, microcirculatory perfusion is often restored. The hydrogen ions generated by anaerobic glycolysis and adenosine triphosphate (ATP) hydrolysis accumulated in the interstitial space will enter the bloodstream in large quantities with the increase of microcirculatory flow and is buffered by bicarbonate in the bloodstream, resulting in the output of a large amount of anaerobic carbon dioxide, thus significantly increasing VCO2 (47). It can be seen from the modified Fick’s formula that Pv-aCO2 is inversely proportional to CO and also proportional to VCO2. An increase in VCO2 will also cause PCO2gap, which can explain the strong positive correlation between Pv-aCO2 and MFI at the time of admission to the ICU (at 0 hour) postcardiac surgery. On the other hand, the replacement of carbon dioxide content by PCO2 is due to the near-linear correlation between the two under physiological conditions. In fact, the relationship between PCO2 and total blood carbon dioxide content is affected by many factors, including blood oxygen saturation, hematocrit, temperature, and the degree of metabolic acidosis (48). Patients after cardiac surgery are not under normal physiological conditions, so the relationship between PCO2 and total blood carbon dioxide content is not necessarily an ideal linear relationship. Ospina-Tascón et al. (47) suggested that VCO2 calculated according to Fick’s formula, VCO2 = CO × (CvCO2 − CaCO2), was effective under steady-state conditions, while the recovery of blood flow after organic ischemia would overstimulate VCO2, resulting in an increase in VCO2/VO2, which is consistent with the conclusion of our study. Herein, Pv-aCO2 was no longer correlated with MFI at 6 and 16 hours, suggesting that PCO2 could not completely replace carbon dioxide content under nonphysiological conditions to some extent.
There has been no research on the correlation between microcirculatory parameters and PCO2gap after cardiac surgery. A study on the correlation between PCO2gap and microcirculatory parameters in patients with sepsis (49) suggested that PCO2gap was the best predictor of uneven microvascular flow distribution and was significantly related to changes in PPV. In this study, we found that PPV moderately correlated with PCO2gap (r=0.4370, P=0.327) at 0 hour. This may be because there were so many factors affecting PCO2gap after cardiac surgery that PCO2gap could not reflect the postoperative distribution of microvessels. It is also possible that this resulted from a limited sample size.
The value of HI, which is used to evaluate differences in the adjacent blood flow of microcirculation, is calculated by MFI, so its correlation with carbon dioxide-related indicators is similar to that of MFI.
The shortcomings of this study are as follows: (I) the sample size was small, and increasing the number of patients enrolled may make the relationship between microcirculatory parameters and macrocirculatory changes clearer and more explicit. (II) The trend of dynamic change of microcirculatory parameters after CPB-assisted cardiac surgery was not clarified. (III) The value of early microcirculatory parameters after CPB-assisted cardiac surgery in determining the prognosis of patients was not analyzed.
Conclusions
Taken together, the primary finding of this study was that TVD and PSVD might be two most sensitive indicators affected by CPB-assisted cardiac surgery that changed significantly during the first 24 hours postoperatively. We detected no consistency between microcirculation and macrocirculation during the early postoperative period after cardiac surgery, meaning the improvement of systemic hemodynamic indicators does not guarantee correspondently improvement in microcirculation. However, the consistency between the two parameters was restored 24 hours postoperatively, mainly manifested by the negative correlation between DO2I and microcirculatory parameters. It is suggested that early controlled oxygen supply after CPB-assisted cardiac surgery may be conducive to the resuscitation of patients to a certain extent. On the other hand, there was a certain correlation between carbon dioxide-related indicators and microcirculatory flow parameters (MFI and HI) during the early postoperative period after cardiac surgery, and this correlation changed dynamically following the resuscitation process. Further studies with larger sample sizes are warranted to clarify the relationship between early microcirculatory parameters and macrocirculatory changes following CPB-assisted cardiac surgery. Moreover, future studies should aim to evaluate the value of microcirculatory parameters in determining the prognosis of patients and improve microcirculatory parameters through clinical interventions for better patient outcomes.
Acknowledgments
Funding: This work was supported by the National Key Research and Development Program (No. 2018YFC1002600), the Science and Technology Planning Project of Guangdong Province (Nos. 2019B020230003 and 2018B090944002), and Guangdong Peak Project (No. DFJH201802).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1159/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1159/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1159/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the ethics committee of Guangdong Provincial People’s Hospital (No. 2018-584H-1). The ethics committee of Guangdong Provincial People’s Hospital waived the need for patient’s written informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nam K, Jeon Y. Microcirculation during surgery. Anesth Pain Med (Seoul) 2022;17:24-34. [Crossref] [PubMed]
- Chalkias A, Papagiannakis N, Mavrovounis G, et al. Sublingual microcirculatory alterations during the immediate and early postoperative period: A systematic review and meta-analysis. Clin Hemorheol Microcirc 2022;80:253-65. [Crossref] [PubMed]
- Mohamed H, Hosny H, Tawadros Md P, et al. Effect of Dexmedetomidine Infusion on Sublingual Microcirculation in Patients Undergoing On-Pump Coronary Artery Bypass Graft Surgery: A Prospective Randomized Trial. J Cardiothorac Vasc Anesth 2019;33:334-40. [Crossref] [PubMed]
- Vandenbulcke L, Lapage KG, Vanderstraeten KV, et al. Microvascular reactivity monitored with near-infrared spectroscopy is impaired after induction of anaesthesia in cardiac surgery patients: An observational study. Eur J Anaesthesiol 2017;34:688-94. [Crossref] [PubMed]
- Abrard S, Fouquet O, Riou J, et al. Preoperative endothelial dysfunction in cutaneous microcirculation is associated with postoperative organ injury after cardiac surgery using extracorporeal circulation: a prospective cohort study. Ann Intensive Care 2021;11:4. [Crossref] [PubMed]
- Greenwood JC, Jang DH, Spelde AE, et al. Low Microcirculatory Perfused Vessel Density and High Heterogeneity are Associated With Increased Intensity and Duration of Lactic Acidosis After Cardiac Surgery with Cardiopulmonary Bypass. Shock 2021;56:245-54. [Crossref] [PubMed]
- den Os MM, van den Brom CE, van Leeuwen ALI, et al. Microcirculatory perfusion disturbances following cardiopulmonary bypass: a systematic review. Crit Care 2020;24:218. [Crossref] [PubMed]
- Atasever B, Boer C, Goedhart P, et al. Distinct alterations in sublingual microcirculatory blood flow and hemoglobin oxygenation in on-pump and off-pump coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth 2011;25:784-90. [Crossref] [PubMed]
- Raia L, Zafrani L. Endothelial Activation and Microcirculatory Disorders in Sepsis. Front Med (Lausanne) 2022;9:907992. [Crossref] [PubMed]
- Dubin A, Henriquez E, Hernández G. Monitoring peripheral perfusion and microcirculation. Curr Opin Crit Care 2018;24:173-80. [Crossref] [PubMed]
- Kiyatkin ME, Bakker J. Lactate and microcirculation as suitable targets for hemodynamic optimization in resuscitation of circulatory shock. Curr Opin Crit Care 2017;23:348-54. [Crossref] [PubMed]
- Jongman RM, Zijlstra JG, Kok WF, et al. Off-pump CABG surgery reduces systemic inflammation compared with on-pump surgery but does not change systemic endothelial responses: a prospective randomized study. Shock 2014;42:121-8. [Crossref] [PubMed]
- Bienz M, Drullinsky D, Stevens LM, et al. Microcirculatory response during on-pump versus off-pump coronary artery bypass graft surgery. Perfusion 2016;31:207-15. [Crossref] [PubMed]
- Dekker NAM, Veerhoek D, Koning NJ, et al. Postoperative microcirculatory perfusion and endothelial glycocalyx shedding following cardiac surgery with cardiopulmonary bypass. Anaesthesia 2019;74:609-18. [Crossref] [PubMed]
- Bakker J, Ince C. Monitoring coherence between the macro and microcirculation in septic shock. Curr Opin Crit Care 2020;26:267-72. [Crossref] [PubMed]
- Bakker J. Lactate levels and hemodynamic coherence in acute circulatory failure. Best Pract Res Clin Anaesthesiol 2016;30:523-30. [Crossref] [PubMed]
- Murkin JM. Monitoring and optimization of the microcirculation during CPB. J Thorac Dis 2019;11:S1489-91. [Crossref] [PubMed]
- Prestes I, Riva J, Bouchacourt JP, et al. Microcirculatory changes during cardiac surgery with cardiopulmonary bypass. Rev Esp Anestesiol Reanim 2016;63:513-8. [Crossref] [PubMed]
- Flick M, Duranteau J, Scheeren TWL, et al. Monitoring of the Sublingual Microcirculation During Cardiac Surgery: Current Knowledge and Future Directions. J Cardiothorac Vasc Anesth 2020;34:2754-65. [Crossref] [PubMed]
- Scolletta S, Marianello D, Isgrò G, et al. Microcirculatory changes in children undergoing cardiac surgery: a prospective observational study. Br J Anaesth 2016;117:206-13. [Crossref] [PubMed]
- Özarslan NG, Ayhan B, Kanbak M, et al. Comparison of the effects of sevoflurane, isoflurane, and desflurane on microcirculation in coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth 2012;26:791-8. [Crossref] [PubMed]
- Boly CA, Venhuizen M, Dekker NAM, et al. Comparison of Microcirculatory Perfusion in Obese and Non-Obese Patients Undergoing Cardiac Surgery with Cardiopulmonary Bypass. J Clin Med 2021;10:469. [Crossref] [PubMed]
- Koning NJ, Atasever B, Vonk AB, et al. Changes in microcirculatory perfusion and oxygenation during cardiac surgery with or without cardiopulmonary bypass. J Cardiothorac Vasc Anesth 2014;28:1331-40. [Crossref] [PubMed]
- Koning NJ, Simon LE, Asfar P, et al. Systemic microvascular shunting through hyperdynamic capillaries after acute physiological disturbances following cardiopulmonary bypass. Am J Physiol Heart Circ Physiol 2014;307:H967-75. [Crossref] [PubMed]
- Koning NJ, Vonk AB, Meesters MI, et al. Microcirculatory perfusion is preserved during off-pump but not on-pump cardiac surgery. J Cardiothorac Vasc Anesth 2014;28:336-41. [Crossref] [PubMed]
- Shih CC, Liu CM, Chao A, et al. Matched Comparison of Microcirculation Between Healthy Volunteers and Patients with Sepsis. Asian J Anesthesiol 2018;56:14-22. [PubMed]
- Sharawy N, Mukhtar A, Islam S, et al. Preliminary clinical evaluation of automated analysis of the sublingual microcirculation in the assessment of patients with septic shock: Comparison of automated versus semi-automated software. Clin Hemorheol Microcirc 2017;67:489-98. [Crossref] [PubMed]
- Yao B, Liu DW, Chai WZ, et al. Effect of N-acetyl-L-cysteine on vascular heterogeneity of microcirculation in endotoxemia rabbits and its mechanism. Zhonghua Yi Xue Za Zhi 2018;98:1869-72. [PubMed]
- van Iterson M, Bezemer R, Heger M, et al. Microcirculation follows macrocirculation in heart and gut in the acute phase of hemorrhagic shock and isovolemic autologous whole blood resuscitation in pigs. Transfusion 2012;52:1552-9. [Crossref] [PubMed]
- van Genderen ME, Klijn E, Lima A, et al. Microvascular perfusion as a target for fluid resuscitation in experimental circulatory shock. Crit Care Med 2014;42:e96-e105. [Crossref] [PubMed]
- Dubin A, Pozo MO, Casabella CA, et al. Increasing arterial blood pressure with norepinephrine does not improve microcirculatory blood flow: a prospective study. Crit Care 2009;13:R92. [Crossref] [PubMed]
- Hernandez G, Bruhn A, Luengo C, et al. Effects of dobutamine on systemic, regional and microcirculatory perfusion parameters in septic shock: a randomized, placebo-controlled, double-blind, crossover study. Intensive Care Med 2013;39:1435-43. [Crossref] [PubMed]
- Kuiper JW, Tibboel D, Ince C. The vulnerable microcirculation in the critically ill pediatric patient. Crit Care 2016;20:352. [Crossref] [PubMed]
- Libert N, Harrois A, Duranteau J. Haemodynamic coherence in haemorrhagic shock. Best Pract Res Clin Anaesthesiol 2016;30:429-35. [Crossref] [PubMed]
- Erdem Ö, Ince C, Tibboel D, et al. Assessing the Microcirculation With Handheld Vital Microscopy in Critically Ill Neonates and Children: Evolution of the Technique and Its Potential for Critical Care. Front Pediatr 2019;7:273. [Crossref] [PubMed]
- Kara A, Akin S, Ince C. Monitoring microcirculation in critical illness. Curr Opin Crit Care 2016;22:444-52. [Crossref] [PubMed]
- Nolan JP, Sandroni C, Böttiger BW, et al. European Resuscitation Council and European Society of Intensive Care Medicine guidelines 2021: post-resuscitation care. Intensive Care Med 2021;47:369-421. [Crossref] [PubMed]
- Pilcher J, Weatherall M, Shirtcliffe P, et al. The effect of hyperoxia following cardiac arrest - A systematic review and meta-analysis of animal trials. Resuscitation 2012;83:417-22. [Crossref] [PubMed]
- Brücken A, Kaab AB, Kottmann K, et al. Reducing the duration of 100% oxygen ventilation in the early reperfusion period after cardiopulmonary resuscitation decreases striatal brain damage. Resuscitation 2010;81:1698-703. [Crossref] [PubMed]
- Minton J, Sidebotham DA. Hyperlactatemia and Cardiac Surgery. J Extra Corpor Technol 2017;49:7-15. [PubMed]
- Renew JR, Barbara DW, Hyder JA, et al. Frequency and outcomes of severe hyperlactatemia after elective cardiac surgery. J Thorac Cardiovasc Surg 2016;151:825-30. [Crossref] [PubMed]
- Andersen LW. Lactate Elevation During and After Major Cardiac Surgery in Adults: A Review of Etiology, Prognostic Value, and Management. Anesth Analg 2017;125:743-52. [Crossref] [PubMed]
- Bakker J, Vincent JL, Gris P, et al. Veno-arterial carbon dioxide gradient in human septic shock. Chest 1992;101:509-15. [Crossref] [PubMed]
- Mecher CE, Rackow EC, Astiz ME, et al. Venous hypercarbia associated with severe sepsis and systemic hypoperfusion. Crit Care Med 1990;18:585-9. [Crossref] [PubMed]
- Ospina-Tascón GA, Bautista-Rincón DF, Umaña M, et al. Persistently high venous-to-arterial carbon dioxide differences during early resuscitation are associated with poor outcomes in septic shock. Crit Care 2013;17:R294. [Crossref] [PubMed]
- Du W, Liu DW, Wang XT, et al. Combining central venous-to-arterial partial pressure of carbon dioxide difference and central venous oxygen saturation to guide resuscitation in septic shock. J Crit Care 2013;28:1110.e1-5. [Crossref] [PubMed]
- Ospina-Tascón GA, Hernández G, Cecconi M. Understanding the venous-arterial CO2 to arterial-venous O2 content difference ratio. Intensive Care Med 2016;42:1801-4. [Crossref] [PubMed]
- Mallat J, Lemyze M, Tronchon L, et al. Use of venous-to-arterial carbon dioxide tension difference to guide resuscitation therapy in septic shock. World J Crit Care Med 2016;5:47-56. [Crossref] [PubMed]
- Ospina-Tascón GA, Umaña M, Bermúdez WF, et al. Can venous-to-arterial carbon dioxide differences reflect microcirculatory alterations in patients with septic shock? Intensive Care Med 2016;42:211-21. [Crossref] [PubMed]
(English Language Editor: A. Muijlwijk)


