Air leak and intraoperative bleeding in thoracic surgery: a Delphi consensus among the members of Italian society of thoracic surgery
Introduction
Video-assisted thoracoscopic surgery (VATS) approach has dramatically changed the field of thoracic surgery and represents the approach of choice in early-stage lung cancer according to international guidelines due to its clear advantages (1,2). Despite the advances made in surgical and perioperative techniques, in thoracic surgery the incidence of complications, remains too high (24–41%) (3).
An ever-present and most-common threat in thoracic surgical practice is the intraoperative air leakage, despite the adoption of surgical techniques and the use of mechanical staplers (3-5). Intraoperative air leaks have usually not clinical relevance, but while most of them resolve spontaneously within 48 hours, others may persist for days and are called prolonged air leaks (PALs) (5,6). The burden of persistent air leaks consists of complications such as longer drainage times, greater postoperative pain, increased risk of infections, empyema, thromboembolism, and increased length of hospitalization with a subsequent increase in direct and indirect costs (4,5,7,8). With the aim to classify the air leaks and establish, based on their intensity, the best therapeutic choices to be followed Zaraca and colleagues have defined a method of measurement and classification of air leaks (9). Another issue that must be considered is the management of intraoperative blood loss: it shows great importance in video thoracoscopy to reduce the need for thoracotomy conversion but also in the prevention of infection and in the reduction of in hospital stay (10,11).
Topical hemostats and surgical sealants together with new procedures, technology and materials represent a constantly updating toolkit for the control of bleeding and the management of air leaks (12).
Although there is sometimes an overlap of use between topical hemostats and surgical sealants they differ according to the chemical nature and physical characteristics, which affect their condition and anatomical site of use, therefore indications and contraindications. In addition, topical hemostats and surgical sealants follow different evaluation, regulatory/registration (licensing, pricing and, if applicable, reimbursement) and vigilance paths depending on whether they are listed as drugs or medical devices (13). Topical hemostats classified as medical devices are sterile products that can derive from the vegetable field (polysaccharides, derived from cellulose), animal (collagen and gelatins) or mineral (zeolite: removable only surgically). The mechanism of action is chemical and/or mechanical, the products favour the aggregation of platelets on the surface, creating a substrate for the coagulation cascade and therefore should not be administered in platelet-deficient patients (14). On the other hand, topical haemostats, identified as drugs, include products that may be of human or animal origin acting with a metabolic action on the coagulation cascade and exerting a mechanical action as an adhesive haemostatic (13). Based on the composition and regulatory/registration aspects, the products can be divided into 3 macro categories: haemostats classified as medical devices (topical haemostats), haemostats classified as drugs (adhesive haemostats), surgical sealants classified as medical devices (pure adhesives or sealants) (Table 1) (12,14-16).
Table 1
| Group | Regulatory category | Active principles |
|---|---|---|
| Topical haemostats | Medical device | Regenerated oxygenated cellulose |
| Bovine collagen + bovine thrombin | ||
| Porcine gelatin | ||
| Porcine gelatin + human thrombin | ||
| Adhesive haemostats | Drug | Fibrinogen + thrombin |
| Collagen + fibrinogen + thrombin | ||
| Pure adhesives or sealants | Medical device | Cyanoacrylate |
| Bovine albumina and glutaraldehyde | ||
| PEG | ||
| Recombinant human albumin + PEG |
PEG, polyethylene glycol.
At European level a modified Delphi survey agreed that the use of sealants for intraoperative air leak at the end of the lung surgery is considered a clear support for the efficiency of healthcare provision and therefore to reduce costs, mainly in selected high-risk patients (17). Very recently a steering group of multidisciplinary European surgeons, has evaluated the use of hemostatic powder in various surgical specialties: in thoracic surgery some specific applications were considered such as oozing during lymphadenectomy, decortication of the pleura. lung transplantation with patients under extracorporeal membrane oxygenation (ECMO) (18).
In Italian thoracic surgery practice, there are no ultimate indications with regards to the appropriate use of topical haemostatic products and sealants and often the need of an unambiguous and shared terminology among the experts arises.
Now a Delphi consensus was set to highlight the different points of view on the use of topical haemostatic products and sealants among the members of Italian Society of Thoracic Surgery (SICT). We present the following article in accordance with the COREQ reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-619/rc).
Methods
The Delphi is a well-established iterative method of investigation, particularly used in scientific research, that has been used for over 30 years in the clinical health field, which takes place through several phases of expression and evaluation of the opinions of a group of experts and which aims to bring together the most complete and shared opinion in a single “consent” (19-22).
The Delphi allowed to reach a consensus on current challenges related to the use of topical haemostatic products and sealants in thoracic surgery.
The board consisted of a group of five Italian experts, with known interest and high skills in thoracic surgery from a selection of the Italian Society of Thoracic Surgeons and based on the expressions of interests. The group met in September 2020 to review the current landscape and identify key topics in the care pathway through discussion, which were as follows:
- Major problems in thoracic surgery and technical solutions.
- Criteria for choosing topical hemostats and sealants, and their use.
- Risks and benefits of using topical hemostats and sealants.
- Clinical consequences and administrative impact of hemorrhages and air leaks.
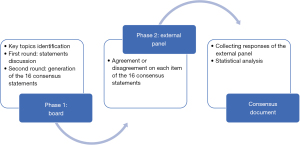
The Delphi process was divided into two phases each consisting of several rounds, as shown in Figure 1.
In the first phase, after a careful review of the scientific literature, a first version of the questionnaire with 17 statements was produced. During the first round, in January 2021, the 5 experts were invited in a live meeting to discuss and comment on the statements. Responses were collected and analysed, then common and conflicting viewpoints were identified. If consensus is not reached, the process continues through thesis and antithesis, to gradually work towards synthesis, and building consensus.
Following the first round, the statements were re-examined to eliminate any parameters that were deemed repetitive or lacking clinical relevance. In the second round, the board finally generated 16 consensus statements (Table 2) for testing across a wider audience.
Table 2
| Item 1 | The most problematic post-operative complications (unresolved issues) in thoracic surgery are in order of importance: |
| 1. Air leaks | |
| 2. Cardiovascular disorders | |
| 3. Bleeding | |
| 4. Infections | |
| Renal failure and respiratory failure are also relevant to consider | |
| Item 2 | The types of topical hemostats or sealants most used in thoracic surgery and their purpose of use are in order of importance: |
| 1. Topical hemostats (used for hemostasis) | |
| 2. Adhesive hemostats (they are mainly used for hemostasis but are also used for the treatment of air leaks) | |
| 3. Pure adhesives or sealants based on polyethylene glycol or polyethylene glycol polymers (used for the treatment of air leaks) | |
| Item 3 | The optimal procedures for the management of intraoperative bleeding in thoracic surgery are in sequence: |
| 1. Compression | |
| 2. Clipping or suturing | |
| 3. Use of topical hemostats | |
| Before applying hemostats, always evaluate the possibility of performing a topical surgical suture, even in the presence of a pulmonary artery lesion | |
| Item 4 | The most frequently adopted solutions for the treatment of intra-operative air leaks consist of a series of procedures, which depend on the extent of the air leak and range from surgical suturing to the use of sealants and specific instruments. The following are to be considered: surgical approaches for the prevention of air leaks, such as fissureless techniques, direct suturing and the use of surgical sealants |
| Item 5 | The differences that have the greatest impact on the choice between topical hemostats and surgical sealants are primarily the differences in the chemical composition/origin and in the mechanism of action, secondly the bureaucratic/administrative differences (e.g., authorization process, costs) and surgical context |
| Item 6 | The surgical situations in which topical hemostats are routinely used are: in the case of modest bleeding/hematoma of the pulmonary artery and ooze bleedings; in the hemostasis of vascular sutures or in involuntary vascular lesions when the continuation of the traditional surgical action with sutures risks worsening the situation; in hemostasis of particularly “sensitive” areas (e.g., close to the esophagus or phrenic or recurrent nerves); in case of parenchymal losses, tumor residue, lymphorrhagia and bleeding from the parenchymal suture line; after mediastinal lymphectomy, in small bleeding during lymphadenectomy; in the case of large areas of chest wall after decortication, particularly in patients with defective hemostasis; after parietal pleurectomy |
| Item 7 | The surgical situations in which sealants are usually used are in order of importance: |
| 1. Parenchymal air leaks between 150 and 300 cc recorded at the end of the operation with the Ventilation Mechanical Test | |
| 2. In VATS when other aerostatic methods are difficult | |
| 3. In the presence of modest lymphatic losses | |
| Item 8 | The possible criteria favoring the indication for the use of a surgical sealant are: |
| 1. Preoperative FEV1 reduction, presence of COPD | |
| 2. Prediction of the patient’s need for mechanical ventilation for long postoperative periods | |
| 3. Frail patient (e.g., elderly, with comorbidities) | |
| 4. Patient in whom the postoperative course is to be accelerated | |
| Item 9 | The preferential characteristics for the choice of a sealant in thoracic surgery are in order of importance: |
| 1. Adhesive power | |
| 2. Persistence suitable for pulmonary expansion | |
| 3. Speed of action, mechanism of action | |
| 4. Origin/derivation (e.g., synthetic, semi-synthetic, human, animal, etc.) | |
| 5. Specific indications given by the manufacturer | |
| 6. Simplicity of preparation | |
| 7. Publication of clinical studies | |
| 8. Easiness of use | |
| 9. Absorption times | |
| Item 10 | The benefits deriving from the use of a surgical sealant can be a decrease in complications and a reduction in costs. The decrease in complications consists in (in order of importance): |
| 1. Lower occurrence of postoperative air leakage | |
| 2. Reduced permanence of drainage and reduction in hospitalization times | |
| 3. Reduction of re-operations for persistent air leaks | |
| Item 11 | The possible problems to be addressed in the use of a surgical sealant are in order of importance: |
| 1. High costs or disadvantageous cost/effectiveness ratio | |
| 2. Insufficient expansion | |
| Item 12 | The possible parameters for evaluating and measuring the benefits deriving from the use of a surgical sealant are in order of importance: |
| 1. Incidence and intensity of intraoperative and postoperative air leaks | |
| 2. Incidence of postoperative complications related to prolonged air leakage (pleuropulmonary infections) | |
| 3. Maintenance times of drainage | |
| 4. Length of postoperative hospital stay | |
| Item 13 | The consequences of intraoperative hemorrhage that have the greatest impact on the postoperative course are in order of importance: |
| 1. Lengthening of operating times, of drainage maintenance and, consequently, of hospital stay times | |
| 2. Surgical site infections | |
| 3. Empyema | |
| It is also important to evaluate the impact of a possible reoperation and that of transfusions for the possible negative effects on the immune setting of the neoplastic patient | |
| Item 14 | The consequences of intraoperative hemorrhage that have the greatest impact on healthcare costs are in order of importance: |
| 1. Lengthening of operating times, of drainage duration and, consequently, of hospitalization times | |
| 2. Possible hospitalization in intensive care | |
| 3. More frequent monitoring/control visits | |
| 4. Lengthening of hospitalization times linked to surgical site infections and/or empyemas | |
| It is also important to evaluate the impact of any reoperation and the use of blood components | |
| Item 15 | The consequences of the air leak that have the greatest impact on the postoperative course are in order of importance: |
| 1. Lengthening of the drainage duration | |
| 2. Lengthening of hospital stay times | |
| 3. Lengthening of hospitalization times linked to surgical site infections and/or empyema | |
| Item 16 | The consequences of the air leak that have the greatest impact on health care costs are in order of importance: |
| 1. Lengthening of hospital stay times linked to longer drainage duration | |
| 2. Lengthening of operating times | |
| 3. Lengthening of hospitalization times due to surgical site infections and/or empyemas | |
| 4. Use of sealants | |
| It is also important to evaluate the impact of a possible re-intervention |
VATS, video-assisted thoracoscopic surgery; FEV1, forced expiratory volume in the 1st second; COPD, chronic obstructive pulmonary disease.
During the second phase, the statements were collated into a questionnaire, which was electronically sent to Italian surgeons, and experts in the field, respondents were engaged by an independent agency using a third-party database. The questionnaire was submitted in April–May 2021 to a panel of 46 surgeons working in thoracic surgery through an online platform for a Delphi round. Survey dissemination and response was completed electronically using LimeSurvey software, with three reminder emails circulated for each iteration. No patients were involved in this study and Committee Ethics approval was not necessary, and implied consent was assumed by voluntary response. The participating experts were invited to express their level of agreement or disagreement on each statement using a Likert-type scale from 1 to 5 (1 = strongly disagree to 5 = strongly agree).
Mean and standard deviation, Median value, the 25th (Q1) and 75th (Q3) percentiles and the interquartile range (IQR) of each statement were calculated. The consensus was defined as achieved with a threshold of 66% or greater of participants.
Statistical analysis
Analysis of surveys in rounds used descriptive statistics for the panel responses to each question. IQR is the absolute value of the difference between the Q3 and Q1, with smaller values indicating higher degrees of consensus. Statistical analyses were performed using R Software.
Results
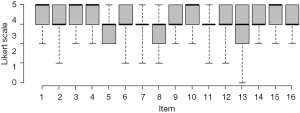
Out of 46 Italian surgeons, 33 (72%) panel members responded to the Delphi questionnaire. All the items reached a positive consensus, with elevated levels of agreement, as demonstrated by the presence of a 100% consensus for nine items, as depicted in Figure 2.
For the remaining 7 statements the minimum level of consent was 88% (29 participants approved the statement and 4 disagreed) and the maximum was 97% (32 participants approved the statement and 1 was in disagreement), with an average of at least 3.7 on the Likert scale. In details, the minimum level of consent (88%) was observed for item 8, item 13 obtained 91% and items 7 and 11 achieved a level of consensus equal to 94%. Item 2, 6 and 12 recorded a level of consensus of 97%.
The final consensus for each item was summarized in Table 3.
Table 3
| Items | Value (N=33) | Consensus agreement score ≥3 | Consensus achieved |
|---|---|---|---|
| Item 1 | 33 (100%) | Yes | |
| Mean (SD) | 4.39 (0.75) | ||
| Median (25–75%) | 5.00 (4.00–5.00) | ||
| Min–Max | 3.00–5.00 | ||
| Item 2 | 32 (97%) | Yes | |
| Mean (SD) | 4.12 (0.70) | ||
| Median (25–75%) | 4.00 (4.00–5.00) | ||
| Min–Max | 2.00–5.00 | ||
| Item 3 | 33 (100%) | Yes | |
| Mean (SD) | 4.33 (0.78) | ||
| Median (25–75%) | 5.00 (4.00–5.00) | ||
| Min–Max | 3.00–5.00 | ||
| Item 4 | 33 (100%) | Yes | |
| Mean (SD) | 4.52 (0.67) | ||
| Median (25–75%) | 5.00 (4.00–5.00) | ||
| Min–Max | 3.00–5.00 | ||
| Item 5 | 33 (100%) | Yes | |
| Mean (SD) | 3.94 (0.75) | ||
| Median (25–75%) | 4.00 (3.00–4.00) | ||
| Min–Max | 3.00–5.00 | ||
| Item 6 | 32 (97%) | Yes | |
| Mean (SD) | 4.06 (0.75) | ||
| Median (25–75%) | 4.00 (4.00–5.00) | ||
| Min–Max | 2.00–5.00 | ||
| Item 7 | 31 (94%) | Yes | |
| Mean (SD) | 3.94 (0.83) | ||
| Median (25–75%) | 4.00 (4.00–4.00) | ||
| Min–Max | 2.00–5.00 | ||
| Item 8 | 29 (88%) | Yes | |
| Mean (SD) | 3.70 (0.98) | ||
| Median (25–75%) | 4.00 (3.00–4.00) | ||
| Min–Max | 2.00–5.00 | ||
| Item 9 | 33 (100%) | Yes | |
| Mean (SD) | 4.12 (0.65) | ||
| Median (25–75%) | 4.00 (4.00–5.00) | ||
| Min–Max | 3.00–5.00 | ||
| Item 10 | 33 (100%) | Yes | |
| Mean (SD) | 4.48 (0.71) | ||
| Median (25–75%) | 5.00 (4.00–5.00) | ||
| Min–Max | 3.00–5.00 | ||
| Item 11 | 31 (94%) | Yes | |
| Mean (SD) | 3.85 (0.76) | ||
| Median (25–75%) | 4.00 (4.00–4.00) | ||
| Min–Max | 2.00–5.00 | ||
| Item 12 | 32 (97%) | Yes | |
| Mean (SD) | 4.27 (0.76) | ||
| Median (25–75%) | 4.00 (4.00–5.00) | ||
| Min–Max | 2.00–5.00 | ||
| Item 13 | 30 (91%) | Yes | |
| Mean (SD) | 3.94 (1.06) | ||
| Median (25–75%) | 4.00 (3.00–5.00) | ||
| Min–Max | 1.00–5.00 | ||
| Item 14 | 33 (100%) | Yes | |
| Mean (SD) | 4.24 (0.75) | ||
| Median (25–75%) | 4.00 (4.00–5.00) | ||
| Min–Max | 3.00–5.00 | ||
| Item 15 | 33 (100%) | Yes | |
| Mean (SD) | 4.52 (0.71) | ||
| Median (25–75%) | 5.00 (4.00–5.00) | ||
| Min–Max | 3.00–5.00 | ||
| Item 16 | 33 (100%) | Yes | |
| Mean (SD) | 4.36 (0.60) | ||
| Median (25–75%) | 4.00 (4.00–5.00) | ||
| Min–Max | 3.00–5.00 |
SD, standard deviation.
Discussion
Positive consensus was reached for all proposed statements, with elevated levels of agreement, as demonstrated by the presence of a 100% consensus for nine items and at least 88% for the remaining seven, with an average of at least 3.7 and a median of at least 4 (Likert scores). Considering that the Likert-type scale used in this Delphi survey included the assignment of a score from 1 to 5, where approval was expressed from 3 onwards, this means that the statements proposed are largely representative, in broad terms, of the clinical approach of Italian thoracic surgery to several aspects. Above all, the awareness about major surgical problems such as air leak and intraoperative bleeding (statement No. 1), their classical, surgical solutions (No. 3 and 4), and their consequences on patients and the healthcare system (No. 14–16). The maximum consensus was achieved also about the benefits deriving from the use of a surgical sealant as lower occurrence of postoperative air leakage, reduced permanence of drainage and reduction in hospitalization times, reduction of re-operations for persistent air leaks (statement No. 10) and the need to choose a surgical sealant mainly on the basis of its adhesive power and its persistence suitable for lung parenchyma and pulmonary expansion (statement No. 9).
The point about which the greatest level of disagreement emerged (88% of consensus, with 4 out of 33 participants not approving the proposed statement) is the one related to possible criteria to be used for the use of a surgical sealant (No. 8). Two other items related to sealants (No. 7 and No. 11) obtained an extremely high level of agreement, but however lower than the others.
Therefore, the results confirm the premises on which this investigation was developed and conducted, highlighting a significant overlap of surgical contexts and purposes of use of topical hemostats and sealants, regardless of their specific indications or their technical characteristics.
Air leaks are considered the most problematic post-operative complication in thoracic surgery, followed by cardiovascular disorders, hemorrhages and infections. However, the possibility of renal or respiratory failure also deserves attention.
In thoracic surgery, the optimal maneuvers for the management of intraoperative bleeding are, in sequence, compression, clipping or suturing, and the use of topical hemostats. However, before applying hemostats, it is always good to evaluate the possibility of performing a local surgical suture, even in the presence of a pulmonary artery lesion. Instead, the treatment of intra-operative air leaks involves a series of maneuvers depending on the extent of the air leak and ranging from surgical suturing to the use of sealing material and specific instruments. As a prevention, specific surgical choices should be considered, such as “fissureless” techniques, direct suturing and the use of sealants.
The types of topical haemostats or sealants most used in thoracic surgery are, in order of importance, topical haemostats (used to obtain haemostasis), adhesive haemostats (mainly used for haemostasis, but also for the treatment of air leaks), pure adhesives or sealants based on polyethylene glycol (PEG) or PEG polymers (used for the treatment of air leaks). The differences that have the greatest impact on the choice between topical hemostats and surgical sealants are the differences in the chemical composition/product origin and in the mechanism of action, and the bureaucratic/administrative differences (e.g., authorization process, costs) or surgical indication.
Topical haemostats are usually used in case of modest bleeding/hematoma of the pulmonary artery and in oozing bleedings, in the haemostasis of vascular sutures or in involuntary vascular lesions when the continuation of the traditional surgical action such as sutures may worsen the situation because in “sensitive” areas (e.g., close to the esophagus or phrenic or recurrent nerves). Moreover, topical haemostats can be used in presence of parenchymal losses, a residual tumor, lymphorrhages and bleeding of the parenchymal suture line, after mediastinal lymphectomy with small bleeding in the site of lymphadenectomy, after parietal pleurectomy in large areas of chest wall, especially in patients with defective hemostasis.
Meanwhile, the surgical situations in which sealants are usually used are: the presence of parenchymal air leaks of between 150 and 300 cc, recorded at the end of the operation with the ventilation mechanical test, in VATS when other methods of aerostasis are difficult and, finally, in the presence of modest lymphatic losses. Possible criteria supporting the indication for the use of surgical sealant are the reduction of preoperative forced expiratory volume in the 1st second (FEV1), the presence of chronic obstructive pulmonary disease, the estimate of the need for mechanical ventilation for long postoperative periods, the “frailty” condition (e.g., elderly, comorbidities) or a patient in whom the postoperative course is to be accelerated.
The preferential characteristics driving the choice of a sealant in thoracic surgery are, in order of importance, its adhesive power, a durability suitable for pulmonary expansibility, its speed, the mechanism of action, its origin/derivation (e.g., synthetic, semi-synthetic, human, animal, etc.), the specific indications given by the manufacturer, the simplicity of preparation, the availability of published clinical trials, the simplicity of use and, finally, the time of absorption.
The use of surgical sealants involves a number of benefits, such as the decrease in complications and the reduction of costs. With regards to the onset of complications, a lower occurrence of postoperative air leaks, a reduced permanence of drainage tubes and hospitalization times, and finally a reduction in re-operations for persistent air leaks can be noted. However, the use of sealants could also result in high costs, with a disadvantageous cost/effectiveness ratio or, rarely, in an insufficient expandability.
The consequences of intraoperative haemorrhage that have the greatest impact on the postoperative course are the lengthening of the operating times, the permanence of drainage and, consequently, the time spent in hospital, the possible hospitalization in intensive care, plus multiple monitoring visits/checkups, the infections of the surgical site and, finally, the risk of empyema. It is also important to evaluate the impact of a possible reoperation and that of transfusions. The same consequences have the greatest impact on healthcare costs.
Instead, the consequences of air leaks that have the greatest impact on the postoperative course and on healthcare costs are the permanence of the drainage, the lengthening of operating times, the lengthening of hospitalization times, and finally the lengthening of hospitalization times linked to infections of the surgical site and/or empyema. It is also important to evaluate the impact of a possible re-intervention.
The great interest in air leak is confirmed by the proposal of a new Prolonged Air Leak score from the Society of Thoracic Surgeons which has shown body mass index <25 kg/m2 to be the most important risk factor (23), and by a very recent Japanese prospective study on 2,200 patients which showed water-seal to be associated with a shorter postoperative stay compared to continuous suction and digital drain (24).
The authors, members of the SICT, wish that the level of knowledge about the new opportunities in the field of sealants and haemostats may increase among healthcare professionals, sharing scientific publications through all the dissemination channels.
Conclusions
From the results of this Delphi analysis, it can be argued that air leak and intraoperative bleeding are clinical problems well known within the members of SICT. Not only the awareness, but also many of the main points are shared. Nevertheless, the aim of the scientific societies and of the group of experts is to conduct the education activities in the surgery community. This Delphi survey suggest the need of wider and updated scientific information about technical and registration characteristics of most recent technologic solutions, such as the of topical hemostats and surgical sealants to provide healthcare and administrative staff with the opportunity to work and interact through a common and shared language and eventually to guarantee minimal requirements of assistance.
The important level of agreement reached in some points can facilitate optimizing treatment of air leak and intraoperative bleeding.
Acknowledgments
Funding: BD Medical Ltd. funded this project by supporting the costs of the methodological process.
Footnote
Reporting Checklist: The authors have completed the COREQ reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-619/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-619/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-619/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-619/coif). PC serves as an unpaid editorial board member of Journal of Thoracic Disease from November 2020 to October 2022. GM serves as an unpaid editorial board member of Journal of Thoracic Disease from February 2021 to January 2023. LV serves as an unpaid editorial board member of Journal of Thoracic Disease from October 2022 to September 2024. GC participated on an Advisory Board supported by BD in the past 36 months. MN participated on an Advisory Board supported by Bard Limited in the past 36 months. MS received honoraria by J&J, Medtronic and Medela for speakers fee, and participated on an Advisory Board supported by BD, and he was a member of ESTS board of directors in the past 36 months. MT participated on an Advisory Board supported by BD in the past 36 months. GC is an unpaid member of the Oncologic network of Sicilian Region in the past 36 months. AI is an unpaid member of Editorial Board of the “Monaldi Archives for Chest Disease” and unpaid member of Editorial Board of the Video-Assisted Thoracic Surgery in the past 36 months. GLP is an unpaid Councillor of the Italian Thoracic Surgery Society from 2021 to 2023 and an unpaid Scientific Coordinator Pulmonary Nodule Recommendations Group PNR of Italian Thoracic Surgery Society from 2021 to 2023. RC participated on an Advisory Board supported by BD in the past 36 months. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. No patients were involved in this study and approval from the Ethics Committee was not necessary, and implied consent was assumed by voluntary response.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bendixen M, Jørgensen OD, Kronborg C, et al. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol 2016;17:836-44. [Crossref] [PubMed]
- Lim E, Batchelor T, Dunning J, et al. In Hospital Clinical Efficacy, Safety and Oncologic Outcomes from VIOLET: A UK Multi-Centre RCT of VATS Versus Open Lobectomy for Lung Cancer. J Thorac Oncol 2019;14:S6. [Crossref]
- Feragalli B. Imaging integrato in terapia intensiva dopo chirurgia toracica. Journal of Radiological Review 2021;5:440-52.
- Ibrahim M, Pindozzi F, Menna C, et al. Intraoperative bronchial stump air leak control by Progel® application after pulmonary lobectomy. Interact Cardiovasc Thorac Surg 2016;22:222-4. [Crossref] [PubMed]
- Zaraca F, Vaccarili M, Zaccagna G, et al. Cost-effectiveness analysis of sealant impact in management of moderate intraoperative alveolar air leaks during video-assisted thoracoscopic surgery lobectomy: a multicentre randomised controlled trial. J Thorac Dis 2017;9:5230-8. [Crossref] [PubMed]
- Gologorsky RC, Alabaster AL, Ashiku SK, et al. Progel Use is Not Associated with Decreased Incidence of Postoperative Air Leak after Nonanatomic Lung Surgery. Perm J 2019;23:18-059. [Crossref] [PubMed]
- Fuller C. Reduction of intraoperative air leaks with Progel in pulmonary resection: a comprehensive review. J Cardiothorac Surg 2013;8:90. [Crossref] [PubMed]
- D'Andrilli A, Andreetti C, Ibrahim M, et al. A prospective randomized study to assess the efficacy of a surgical sealant to treat air leaks in lung surgery. Eur J Cardiothorac Surg 2009;35:817-20; discussion 820-1. [Crossref] [PubMed]
- Zaraca F, Vaccarili M, Zaccagna G, et al. Can a standardised Ventilation Mechanical Test for quantitative intraoperative air leak grading reduce the length of hospital stay after video-assisted thoracoscopic surgery lobectomy? J Vis Surg 2017;3:179. [Crossref] [PubMed]
- Igai H, Kamiyoshihara M, Yoshikawa R, et al. Algorithm-based troubleshooting to manage bleeding during thoracoscopic anatomic pulmonary resection. J Thorac Dis 2019;11:4544-50. [Crossref] [PubMed]
- Iyer A, Yadav S. Postoperative Care and Complications After Thoracic Surgery. In: Firstenberg MS. Editor. Principles and Practice of Cardiothoracic Surgery. Intechopen; 2013.
- Peverini D, Ciuti S, Di Fabrizio R, et al. Emostatici locali e sigillanti a uso chirurgico: clinica e appropriatezza d’uso. Bollettino SIFO 2008;54.
- Bettoni D. Farmaci e dispositivi medici: è possibile un confronto? Il caso degli emostatici e dei sigillanti in chirurgia. Clinico Economics Italian Articles on Outcomes Research 2015;10:61-72.
- Peghetti A, Carati D. Emostatici locali e sigillanti chirurgici. Dalle evidenze della letteratura alla pratica quotidiana. Delibera Giunta Regionale n. 1523/2008. 2014 Sep. Report No.: Delibera Giunta Regionale n. 1523/2008.
- Cupani C. Medicinali emostatici e sigillanti chirurgici: classificazione, indicazioni ed efficacia clinica. Catania: 2018. Available online: https://www.aifa.gov.it/documents/20142/1180150/2018-10-12_Cinzia_12_10_18.pdf
- Gruppo di lavoro Regionale permanente sui Dispositivi Medici (GR-DM) istituito con D n. 7468 del 17. 05 2018. Evidenze sull’appropriatezza di impiego e indicazioni sulla variabilita’ dei consumi degli emostatici e sigillanti. 2020.
- Brunelli A, Bölükbas S, Falcoz PE, et al. Exploring consensus for the optimal sealant use to prevent air leak following lung surgery: a modified Delphi survey from The European Society of Thoracic Surgeons. Eur J Cardiothorac Surg 2021;59:1265-71. [Crossref] [PubMed]
- Eden C, Buonomo OC, Busch J, et al. An international multidisciplinary peer-driven consensus on the optimal use of hemostatic powders in surgical practice. Updates Surg 2021;73:1267-73. [Crossref] [PubMed]
- Acone B, Urbani A. The Delphi Method: the methodology and its application. Journal of HIV and Ageing 2018;S1:9-14.
- Nair R, Aggarwal R, Khanna D. Methods of formal consensus in classification/diagnostic criteria and guideline development. Semin Arthritis Rheum 2011;41:95-105. [Crossref] [PubMed]
- Hasson F, Keeney S, McKenna H. Research guidelines for the Delphi survey technique. J Adv Nurs 2000;32:1008-15. [PubMed]
- Diamond IR, Grant RC, Feldman BM, et al. Defining consensus: a systematic review recommends methodologic criteria for reporting of Delphi studies. J Clin Epidemiol 2014;67:401-9. [Crossref] [PubMed]
- Seder CW, Basu S, Ramsay T, et al. A Prolonged Air Leak Score for Lung Cancer Resection: An Analysis of The Society of Thoracic Surgeons General Thoracic Surgery Database. Ann Thorac Surg 2019;108:1478-83. [Crossref] [PubMed]
- Adachi H, Wakimoto S, Ando K, et al. Optimal Chest Drainage Method After Anatomic Lung Resection: A Prospective Observational Study. Ann Thorac Surg 2022; [Epub ahead of print]. [Crossref] [PubMed]