
The impact of tubeless anesthesia versus intubated anesthesia on cerebral oxygen saturation and postoperative cognitive function in patients undergoing video-assisted thoracoscopic surgery: a randomized trial
Introduction
Postoperative cognitive dysfunction (POCD) refers to the changes of cognitive function characterized by the impairment of orientation, long-term and short-term memory, concentration, and comprehension (1). POCD is associated with extended hospital stay and increased mortality, which affects the postoperative recovery and quality of life of patients and leads to increased social and family burden. One study showed the incidence of POCD in a population undergoing non-cardiac surgery was 25.8% after one week and 9.9% after three months (2). Therefore, it is essential to identify the factors that increase the risk of POCD in order to establish appropriate preventive measures.
Demographic, clinical, basic disease, operation type, preoperative cognitive function level, cerebral perfusion levels, anesthesia mode and medication, postoperative, and hospital-associated factors may contribute to the development of POCD (1-3). Near infrared spectroscopy (NIRS) is a noninvasive method for monitoring brain tissue oxygen saturation (StO2). Continuous monitoring of StO2 can effectively avoid cerebral hypoperfusion and imbalance between cerebral oxygen supply and demand, which plays a key role in preventing POCD (4-7). The correlation between the decrease of cerebral oxygen saturation and POCD has been confirmed in many types of surgery (8-11). Video-assisted thoracoscopic surgery (VATS) is often accompanied by a variety of physiological changes during one-lung ventilation (OLV), which is closely related to clinical anesthesia management and postoperative rehabilitation of patients. Previous studies have shown that OLV may impair the cerebral oxygen balance and increase the incidence of POCD during thoracoscopic surgery (12,13).
In order to reduce the intraoperative and postoperative complications associated with double-lumen bronchial intubated and minimize the impact of OLV. The non-endotracheal intubated anesthesia (tubeless anesthesia) has been gradually used in thoracic surgery procedures such as lung tissue biopsy, bulla resection, palmar hyperhidrosis treatment, wedge resection, lobectomy, resection, segmental resection, and mediastinal tumor resection (14-17). However, patients undergoing tubeless general anesthesia still suffer from insufficient minute ventilation (MV) due to the combined effects of artificial pneumothorax and opioid-related respiratory depression, resulting in frequent intraoperative hypercapnia. Related studies have shown hypercapnia is associated with prolonged reaction time, attention disorder, memory loss, logical thinking decline, and localization dysfunction (18-20). At present, the effect of hypercapnia on postoperative cognitive function is controversial. Mild hypercapnia [partial pressure of carbon dioxide (PaCO2) at 45–55 mmHg] increased regional cerebral oxygen saturation, improved cerebral oxygenation, and reduced the incidence of POCD, and the mechanism may be that hypercapnia can increase cerebral blood flow perfusion and raise the threshold of cerebral ischemic injury under surgical stress (20). Other studies have confirmed that hypercapnia can up-regulate the expression of IL-1β, thereby activating the inflammatory response in the central nervous system, leading to apoptosis of hippocampal neurons and aggravating cognitive impairment (21-23). Therefore, further studies are needed to determine whether tubeless anesthesia and its induced hypercapnia can offer a good benefit-to-risk ratio for patients.
At present, there is no relevant study comparing the cerebral oxygen saturation and POCD incidence of VATS patients under tubeless anesthesia and double-lumen bronchial intubated anesthesia. The purpose of this study was to investigate the effects of tubeless anesthesia and double-lumen bronchial intubated anesthesia on intraoperative cerebral oxygen saturation and POCD in patients undergoing VATS. We hypothesized that compared with double-lumen bronchial intubated anesthesia, the use of tubeless anesthesia would increase the incidence of intraoperative hypercapnia, enhance intraoperative cerebral oxygen saturation, and affect the incidence of POCD. We present the following article in accordance with the CONSORT reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1165/rc).
Methods
Study design
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This prospective, randomized, controlled trial was approved by the Ethics Committee of The First People’s Hospital of Yunnan Province (KHLL2021-KY024), and patients or their families signed an informed consent form. Patients who underwent VATS were enrolled in The First People’s Hospital of Yunnan Province from May to October 2021. They were randomly divided into two groups: non-intubated spontaneous ventilation group (SV group) and double-lumen bronchial intubated mechanical ventilation group (MV group). The clinical research design was showed in Figure 1.
Sample size calculation
The sample size was based on a previous study in thoracic surgery (24). We assumed that the incidence of POCD could be as high as 46% in the MV group and 12% in the SV group (19), 25 patients would be needed in each group to achieve a statistical power of 0.80 with a type I error of 0.05. Anticipating a 15% dropout rate, 30 subjects should be enrolled in Group SV, and 30 in Group MV, to obtain final group sample sizes of 25 and 25, respectively. To prevent excessive withdrawal, 69 patients who underwent VATS at The First People’s Hospital of Yunnan Province between May and October 2021 were enrolled.
Inclusion criteria
- Age <60 years old, unlimited gender;
- Patients with an American Society of Anesthesiologists Standard (ASA) classification of I–II, New York Heart Association (NYHA) classification I–II;
- Body mass index (BMI) <30 kg/m2;
- The operation time was less than 4 hours, and the amount of bleeding was less than 300 mL;
- Normal cardiopulmonary function [predicted forced expiratory volume in the first second (FEV1%)] >50% and ejection fraction (EF) >50%; resting blood gas analysis showing arterial partial pressure of oxygen (PaO2) ≥60 mmHg; and arterial PaCO2 <45 mmHg
- No contraindications related to paravertebral nerve block and intercostal nerve block, and patients with no clinically significant history of cardiac or nervous system problems.
Exclusion criteria
- Severe chronic obstructive pulmonary disease or pulmonary infection;
- Patients with an ASA classification ≥III, NYHA classification ≥III;
- BMI ≥30 kg/m2;
- Preoperative complications included coagulation dysfunction, hypoxemia, hypercapnia, or hepatorenal insufficiency;
- Difficult airway management;
- Extensive pleural adhesion;
- Change the anesthetic or surgical protocol;
- Significant lacunar cerebral infarction, ischemic stroke, or a history of other neurological disease;
- Preoperative Mini-Mental State Examination (MMSE) score <27.
Randomization
We use the SPSS software to generate random numbers in a 1:1 ratio and divided them into two groups. The group results were sealed in envelopes and kept at the beginning of the study until the end. Study members distributed the randomized results of the recruited patients. For each enrolled patient, anesthesia was administered by a dedicated anesthesiologist and intraoperative data collection was performed by researchers. Postoperative follow-up will be conducted by investigators not involved in the management of anesthesia. There was no communication between the anesthesiologist and the researcher during data collection.
Anesthesia methods
Preoperative management and anesthesia monitoring setup
All patients fasted for 8 hours and were water deprived for 2 hours before surgery. After the patient entered the operating room, a peripheral venous access was established, and a face mask was placed for oxygen (4–5 L/min). Electrocardiogram (ECG), heart rate (HR), pulse, respiratory rate (RR), pulse oxygen saturation (SpO2), noninvasive blood pressure (BP), StO2, end-tidal CO2 pressure (PETCO2), and invasive blood pressure (IBP) readings were monitored before the induction of anesthesia.
Thoracic paravertebral block (TPVB)
TPVB was performed under ultrasound guidance (Philips CX50, USA). Patients in both groups were placed in the healthy lateral position, and according to the operation site and placement position of the drainage tube, the T5 or T7 spinous processes were palpated and marked. The long axis of the probe was placed parallel to the spine to clearly display the sound image of the T5 spinous process then translated to the affected side along the rib to show the tile shaped joint process, before translating outward to reveal the cribriform transverse process, the rib, the superior costotransverse ligament, and the pleura. The deep layer of the superior costotransverse ligament and the shallow layer of the pleura are in the paravertebral space, and the fixed probe adopted in-plane technology to place the needle. When the needle tip reached the paravertebral space, it was drawn back without blood and gas, and 10 mL of local anesthetic 0.5% ropivacaine was slowly injected. During injection, the parietal pleura could be seen under pressure, and the local anesthetic diffused into the paravertebral space. Using the method described above, the T7 spinous process was located, and a paravertebral nerve block was performed on the affected side. After 15 minutes, the sensation and block segments of the affected side were evaluated and recorded by acupuncture and compared with the healthy side. If the block was successful, the pain degree of the affected side was lighter than that of the healthy side, and if the comparison of bilateral pain was not obvious, block failure was considered to have occurred.
Anesthesia stages of the SV group
Induction of anesthesia
Midazolam 0.05 mg/kg, dexamethasone 10 mg, sufentanil 0.1 µg/kg, and etomidate 0.2–0.4 mg/kg were administered induce anesthesia. A muscle relaxant was not used. When the bispectral index (BIS) dropped to 60, the laryngeal mask was placed to maintain BP fluctuations within 20% of the preoperative basic value. If hypotension occurred (the BP dropped more than 20% of the preoperative basic value or fell to less than 90/60 mmHg), ephedrine, dopamine, or noradrenaline were given. If hypertension occurred (BP increased by more than 20% of the preoperative basic value or the BP increased to more than 160/95 mmHg), nitroglycerin was administered.
Maintenance of anesthesia
Propofol 2–3 mg/kg/h, remifentanil 0.03–0.08 µg/kg/min, dexmedetomidine 0.3–1 µg/kg/h, and flurbiprofen axetil 50 mg were adopted to maintain anesthesia, and incremental adjustment was provided to maintain a BIS value between 40 and 60. Before skin incision, local anesthesia was administered with 1% lidocaine 5ml at the incision site, then under the guidance of thoracoscopy, intercostal nerve block, pleural surface anesthesia, and intrathoracic vagus nerve block were performed. The intercostal nerve block consisted of a 1:1 mixture of 1% ropivacaine and 2% lidocaine and was used to block the intercostal nerve innervating the surgical incision and drainage tube, at a dosage at each location of 1 mL. The pleural surface anesthesia consisted of 5 mL of 2% lidocaine which was sprayed on the lung surface, while the intrathoracic vagus nerve block consisted of a 2.5 mL mixture of 2% lidocaine and 1% ropivacaine to inhabit the cough reflex and stabilize the surgical field.
Ventilation
Spontaneous breathing was sought, with laryngeal mask ventilation and an oxygen flow of 2–3 L/min to maintain an oxygen concentration of 50–100%. During the anesthesia period, if the SpO2 dropped below 90% or PETCO2 continuously increased by more than 80 mmHg, the ventilation mode was adjusted to SIMV mode or manual ventilation to improve the ventilation quality and oxygenation. If hypoxemia persisted despite the above maneuvers, a conversion to intubation was required.
Conversion conditions
- Excessive bulging of the surgical field which made the procedure difficult and did not improve after medical treatment, and had a duration of >5 minutes;
- Severe bleeding in the thoracic cavity obscuring the surgical field;
- Significant increase in tracheal or bloody secretions, leading to difficulty breathing and increased airway obstruction;
- Persistent cough (>2 times/min).
Anesthesia stages of the MV group
Induction of anesthesia
Midazolam 0.05 mg/kg, sufentanil 0.5 mg/kg, dexamethasone 10 mg, etomidate 0.2–0.4 mg/kg, and rocuronium 1 mg/kg were administered to induce anesthesia, mask pressurized oxygen, and full oxygen and nitrogen removal. A double-lumen endotracheal tube was inserted, and the correct position confirmed using a flexible bronchoscope when the BIS dropped to 60. Fluctuation of intraoperative BP was maintained within 20% of the preoperative basic value, and the treatment methods for hypotension and hypertension were as described previously.
Maintenance of anesthesia
Propofol 4–12 mg/kg/h, remifentanil 0.03–0.08 µg/kg/min, and dexmedetomidine 0.3–1 µg/kg/h, in addition to an intravenous drip of flurbiprofen axetil 50 mg were adopted to maintain anesthesia. Incremental adjustment of anesthetics was provided to maintain a BIS value between 40 and 60, while rocuronium was added intermittently during the operation according to the basic condition of the patient. Dexmedetomidine was stopped 30 min before the end of the operation, and propofol and remifentanil were stopped 10 min before the end. Neostigmine 1mg and atropine 0.5 mg were injected intravenously to antagonize the effect of residual muscle relaxants according to the recovery of respiratory muscle strength.
Ventilation
Oxygen flow rate was 2–3 L/min, oxygen concentration was maintained 50–100%. Volume-controlled ventilation with PEEP 4–5 cmH2O was used during operation. For total lung ventilation, TV was 6–8 mL/kg (predicted body weight), respiratory ratio was 1:2–1:1.5, peak inspiratory pressure was <25 cmH2O, RR was 12–14 breaths/min, PETCO2 was 35–45 mmHg. When the operation began, ventilation was performed with OLV, TV was 5–6 mL/kg (predicted body weight), respiratory ratio was 1:2–1:1.5, peak inspiratory pressure was <35 cmH2O, RR was 12–20 breaths/min, PETCO2 was 35–45 mmHg.
Postoperative management
All patients were transferred to the post-anesthesia care unit after surgery and returned to the ward after being fully awake and evaluated. All patients used a patient-controlled intravenous analgesia pump for postoperative analgesia (sufentanil 1 µg/mL, mixed with normal saline to a total volume of 100 mL), load 2 mL, background dose 2 mL/h, additional dose 3 mL, locking time 15 min. A numerical rating scale (NRS) score was used for postoperative pain score, and if it was persistently >5 or the pain was unbearable, intravenous flurbiprofen axetil 50 mg was given.
Regional cerebral oxygen saturation monitoring
StO2 was monitored continuously by an oxygen saturation monitor (FORE-SIGHT, MC-2000 Series), with the sensor positioned on each side on the forehead. The normal range of StO2 of the instrument is 60–80%, and the StO2 value was set when breathing air as baseline.
POCD
An MMSE was conducted after enrolment in the trial, but instructions were given before the cognitive test to ensure that patients could understand the test. Patients with an MMSE score <27 were not tested further. Cognitive function was evaluated on the 1st day before surgery, and the 4th, 7th, 14th, and 30th day postoperatively. The scale assesses orientation, immediate memory, attention and calculation, memory ability, and language ability, with a total score of 30 points. An MMSE score of 27–30 is normal, <27 is cognitive dysfunction, 21–26 is mild cognitive dysfunction, 10–20 is moderate cognitive dysfunction, and 0–9 is severe cognitive dysfunction.
Analgesia assessment
Analgesia was assessed by the NRS score, from 0 (no pain) to 10 (maximum pain) and was performed at 2, 6, 12, 24, and 48 h after surgery.
Blood sample
Leukocyte, neutrophil, and lymphocyte counts were measured before surgery and 72 hours after surgery. Blood samples were taken 20 min before anesthesia, at the end of surgery, and 16 hours then 72 hours after surgery. The serum concentration of S100β, interleukin (IL)-6, IL-1β, and tumor necrosis factor α (TNF-α) was measured by enzyme linked immunosorbent assay (Human S100β, IL-6, IL-1β, TNF-α ELISA Kit, FANKEWEI).
Indicator recording
The primary outcome included the incidence of POCD and MMSE scores in all patients on the 1st day before surgery, and the 4th, 7th, 14th, and 30th day postoperatively, and StO2 at different points during surgery. The secondary outcome included respiratory and hemodynamic parameters, serum concentration of S100β, IL-6, IL-1β, and TNF-α, number of leukocytes, neutrophils and lymphocytes. Hypercapnia was observed by continuous monitoring of PETCO2 and blood gas analysis at different times. Postoperative recovery time, endotracheal intubation/ laryngeal mask extubating time, and NRS score at 2, 6, 12, 24, and 48 h after operation recorded. The occurrence of perioperative complications, rest time before the first out of bed activity, drainage volume and indwelling duration of the chest tube, and length of hospital stay recorded in order to assess the postoperative recovery status.
Statistical analysis
Subjects who completed the study according to the study protocol were analyzed. Patients with missing values were excluded from the trial. The primary analysis will be by per-protocol (PP). Statistical analysis was performed using SPSS 26.0 software. Measurement data were described by mean ± standard deviation or median and interquartile spacing, and t-test and rank sum test were used for comparison between groups. For repeated measurement data, multivariate test repeated measures analysis of variance (ANOVA) or Friedman non-parametric repeated measures ANOVA was used for analysis. Categorical data are presented as numbers and percentages, and the comparison between groups was adopted χ2 test or Fisher exact test. Rank correlation analysis or multivariate regression analysis was used for correlation analysis. P value <0.05 was considered statistically significant. All P values are two sided.
Results
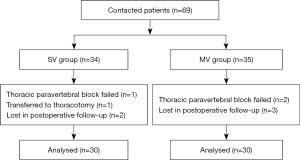
From May to October 2021, 69 patients who underwent VATS in The First People’s Hospital of Yunnan Province were included. One patient was transferred to thoracotomy, while in three the TPVB failed, and five patients were lost in postoperative follow-up. Finally, the data of 60 patients were analyzed, 30 in each group (Figure 2).
Patient’s baseline clinical characteristics
There was no significant difference in the clinical characteristics of patients in the two groups, including age, BMI, gender, ASA classification, NYHA classification, education, preoperative lung function, surgery type, and operation time (P>0.05) (Table 1).
Table 1
| Characteristics | All patients (n=60) | SV (n=30) | MV (n=30) | P value |
|---|---|---|---|---|
| Age (years), median (P25–P75) | 51 [46–56.5] | 51 [43–57] | 51 [44–54] | 0.60 |
| BMI (kg/m2), mean (standard deviation) | 22.9±2.4 | 22.1±3.0 | 22.8±2.6 | 0.31 |
| Gender (n, %) | 0.29 | |||
| Male | 24 [40] | 14 [47] | 10 [33] | |
| Female | 36 [60] | 16 [53] | 20 [67] | |
| ASA classification (n, %) | >0.99 | |||
| I | 9 [15] | 4 [13] | 5 [17] | |
| II | 51 [85] | 26 [87] | 25 [83] | |
| NYHA classification (n, %) | 0.28 | |||
| Normal | 52 [87] | 27 [90] | 25 [83] | |
| I | 3 [5] | 0 [0] | 3 [10] | |
| II | 5 [8] | 3 [10] | 2 [7] | |
| Education (n, %) | 0.79 | |||
| Elementary school | 11 [18] | 5 [17] | 6 [20] | |
| High school | 26 [43] | 12 [40] | 14 [47] | |
| College and above | 23 [38] | 13 [43] | 10 [33] | |
| Preoperative lung function | ||||
| FVC (% of predicted), median (25–75%) | 101.5 (93.1–113.4) | 96.7 (92.7–110.4) | 105 (94.2–116.6) | 0.23 |
| FEV1 (% of predicted), mean (standard deviation) | 96.9±15.7 | 96.3±18.3 | 97.4±13.3 | 0.81 |
| FEV1/FVC (%), mean (standard deviation) | 79.2±7.4 | 79.6±6.9 | 78.9±7.9 | 0.75 |
| Surgery type (n, %) | 0.22 | |||
| Wedge resection | 22 [37] | 12 [40] | 10 [33] | |
| Segment resection | 13 [22] | 6 [20] | 7 [23] | |
| Lobectomies | 15 [25] | 5 [17] | 10 [33] | |
| Bulla resection | 4 [7] | 4 [13] | 0 [0] | |
| Mediastinal goiter resection | 5 [8] | 3 [10] | 2 [7] | |
| Intrathoracic vagus nerve resection | 1 [2] | 0 [0] | 1 [3] | |
| Operative time (min), median (P25–P75) | 105 (71.3–143.8) | 95.0 (58.8–135.0) | 105.0 (75.0–141.3) | >0.99 |
SV group, spontaneous ventilation group; MV group, mechanical ventilation group; BMI, body mass index; ASA, American Society of Anesthesiologists; NYHA, New York Heart Association; FVC, forced vital capacity; FEV1, forced expiratory volume in the first second.
Respiratory and hemodynamic parameters
Respiratory parameters
There was no difference in the baseline values of respiratory parameters between the two groups (P>0.05). Compared with preoperative (T0), SpO2 increased from T1 to T6 in the SV group (P<0.05), and SpO2 in the MV group increased at T1, T2, and T6 (P<0.05) (Table 2). Compared with the induction of anesthesia (T1), TV and MV decreased from T2 to T5 in both groups (P<0.05) (Table 2). The SV group had lower TV at T3 and T4 (P<0.01), lower RR at T2, T4, and T5 (P<0.05), lower MV at T2, T3, T4, and T5 (P<0.01), and higher SpO2 at T2, T3, and T4 than the MV group (P<0.05) (Table 2).
Table 2
| Indicator | Group | T0 | T1 | T2 | T3 | T4 | T5 | T6 |
|---|---|---|---|---|---|---|---|---|
| TV (mL) | SV | – | 412 [400–454] | 310 [300–372]c | 200 [180–225]bc | 285 [155–388]bc | 312 [179–417]c | 443 [335–453] |
| MV | – | 439 [394–450] | 350 [324–375]c | 350 [296–353]c | 350 [300–369]c | 350 [300–360]c | 450 [425–450] | |
| RR (beats/min) | SV | – | 13 [12–14] | 12 [10–14]b | 12 [8–16] | 11 [7–14]b | 11 [8–15]b | 14 [11–16] |
| MV | – | 14 [12–15] | 14 [12–15] | 14 [12–15] | 14 [12–15] | 14 [12–15] | 14 [12–15] | |
| MV (mL/min) | SV | – | 5,765 [4,875–6,300] | 3,900 [3,397–4,500]bc | 2,400 [1,580–3,438]bc | 2,365 [1,600–4,506]bc | 3,330 [2,195–4,624]bc | 5,613 [3,998–6,750] |
| MV | – | 5,400 [4,937–6,750] | 4,550 [4,200–5,250]c | 4,500 [4,089–5,250]c | 4,550 [4,103–5,250]c | 4,655 [4,132–5,250]c | 6,080 [5,100–6,750] | |
| HR (times/min) | SV | 77 [72–81] | 70 [60–81] | 65 [57–79]a | 72 [65–84] | 76 [69–80]b | 77 [70–85]b | 77 [68–84]b |
| MV | 72 [66–78] | 69 [66–73] | 66 [56–71]a | 67 [63–75] | 68 [62–73] | 66 [61–75] | 63 [55–71]a | |
| SBP (mmHg) | SV | 134 [122–155] | 113 [104–127]a | 110 [101–126]a | 101 [93–122]ab | 95 [89–107]a | 104 [93–119]a | 118 [107–132] |
| MV | 146 [131–161] | 119 [109–125]a | 115 [109–129]a | 115 [99–130]a | 102 [90–119]a | 102 [90–112]a | 112 [103–121]a | |
| DBP (mmHg) | SV | 79 [72–85] | 71 [64–79] | 70 [64–75] | 62 [59–70]a | 58 [53–65]a | 65 [55–70]a | 69 [63–80] |
| MV | 83 [74–90] | 68 [62–74]a | 68 [63–80]a | 67 [54–76]a | 61 [53–68]a | 61 [54–71]a | 67 [59–75]a | |
| MAP (mmHg) | SV | 99 [89–108] | 83 [77–93] | 83 [77–95] | 74 [69–83]ab | 71 [65–78]a | 77 [69–85]a | 86 [79–96] |
| MV | 103 [91–114] | 83 [80–92]a | 84 [78–96]a | 82 [70–94]a | 73 [64–85]a | 75 [66–89]a | 82 [75–90]a | |
| SpO2 (%) | SV | 96 [95–97] | 100 [100–100]a | 100 [100–100]ab | 100 [100–100]ab | 100 [100–100]ab | 100 [99–100]a | 100 [100–100]a |
| MV | 96 [95–98] | 100 [100–100]a | 100 [99–100]a | 100 [96–100] | 98 [95–100] | 100 [96–100] | 100 [100–100]a |
a, P<0.05, compared to T0; b, P<0.05, compared to group MV; c, P<0.05, compared to T1. SV group, spontaneous ventilation group; MV group, mechanical ventilation group; TV, tidal volume; RR, respiratory rate; MV, minute ventilation; HR, heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; SpO2, pulse oxygen saturation; T0, preoperative; T1, anesthesia induction intubation; T2, beginning of operation; T3, 15 min after the operation began; T4, 30 min after the operation began; T5, 45 min after the operation began; T6, the end of operation.
Hemodynamic parameters
There was no difference in preoperative hemodynamic parameters between the two groups. Compared with that observed preoperatively (T0), the HR of patients in the SV group decreased at T2 (P<0.05), while that of the MV group decreased at T2, T6 (P<0.05) (Table 2). Systolic blood pressure (SBP), diastolic blood pressure (DBP), and mean arterial pressure (MAP) decreased during operation in both groups (P<0.05) (Table 2), while the SV group had a higher HR at T4, T5, and T6 than the MV group (P<0.05) (Table 2).
Correlation analysis between PETCO2 and PaCO2
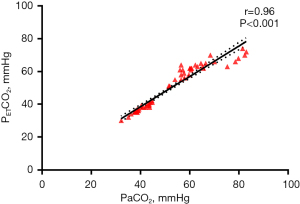
The PETCO2 in SV group at different time points during operation had significant difference (F=104.04, P<0.01), which gradually increased after the beginning of operation and decreased at the end of operation (Table 3, Figure 3). The PETCO2 in SV group was significantly higher than that in MV group (F=198.17, P<0.01) (Table 3, Figure 3). As arterial blood gas analysis is invasive, in this study, arterial blood was collected only before and during the operation (when PETCO2 is in a high state T5 time ±5 min) and after operation (before the patient left the post-anesthesia care unit). The results showed no significant difference in pH, PaO2, and PaCO2 between the two groups before and after operation (P>0.05) (Table 4). During the operation, the PaO2 increased (P<0.05) and the pH value decreased (P<0.01) in both groups (Table 4). The intraoperative pH value of the SV group was lower than that of the MV group (P<0.01), while PaCO2 and PETCO2 were significantly higher than that of the MV group (P<0.01), although there was no significant difference in PaO2 (P>0.05) (Table 4). PaCO2 >45 mmHg was defined as hypercapnia, and its incidence in the SV group was significantly higher than in the MV group (100% in the SV group vs. 0% in the MV group, P<0.001) (Table 5). Pearson correlation analysis found a strong correlation between PETCO2 and PaCO2 (R squared =0.92, R=0.96, 95% CI: 0.94–0.98, P<0.001) (Table 6, Figure 4).
Table 3
| Indicator | Group | T1 | T2 | T3 | T4 | T5 | T6 |
|---|---|---|---|---|---|---|---|
| PETCO2 (mmHg) | SV | 33.2±2.7 | 36.6±6.8ab | 54.3±10.1ab | 57.4±8.3ab | 54.4±12.6ab | 44.6±5.4ab |
| MV | 31.9±3.4 | 32.0±3.3 | 34.6±3.0 | 35.3±2.5 | 34.8±3.5 | 33.9±3.9 |
a, P<0.05, compare to T1; b, P<0.05, compared to group MV. SV group, spontaneous ventilation group; MV group, mechanical ventilation group; T1, anesthesia induction intubation; T2, beginning of operation; T3, 15 min after the operation began; T4, 30 min after the operation began; T5, 45 min after the operation began; T6, the end of operation; PETCO2, end-tidal CO2 pressure.
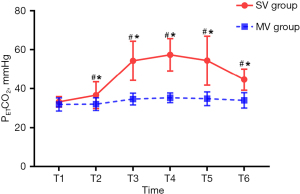
Table 4
| Indicator | Group | Preoperative | Intraoperative | Postoperative |
|---|---|---|---|---|
| pH | SV | 7.39±0.04 | 7.20±0.05*# | 7.35±0.05# |
| MV | 7.40±0.04 | 7.35±0.03# | 7.33±0.03# | |
| PaO2 (mmHg) | SV | 88.7 (81.50–105.13) | 139.45 (96.88–194.35)# | 96.90 (80.52–126.70) |
| MV | 96.1 (85.83–107.45) | 147.00 (93.25–273.95)# | 97.35 (87.33–144.70) | |
| PaCO2 (mmHg) | SV | 36.35±3.98 | 64.14±9.30*# | 40.09±5.46# |
| MV | 36.41±3.82 | 39.81±3.34 | 41.77±3.13# | |
| PETCO2 (mmHg) | SV | – | 62.30±5.53* | – |
| MV | – | 36.77±2.94 | – |
*, P<0.05, compared to the MV group; #, P<0.05, compared to preoperative. SV, spontaneous ventilation; MV, mechanical ventilation; PaO2, partial pressure of oxygen; PaCO2, partial pressure of carbon dioxide; PETCO2, end-tidal CO2 pressure.
Table 5
| Group | N | Present | Absent | χ2 test | |
|---|---|---|---|---|---|
| χ2 | P | ||||
| SV | 30 | 30 [100] | 0 [0] | 60 | <0.001 |
| MV | 30 | 0 [0] | 30 [100] | ||
SV, spontaneous ventilation; MV, mechanical ventilation.
Table 6
| Indicator | n=60 | R2 | Pearson r | P (two-tailed) | 95% confidence interval |
|---|---|---|---|---|---|
| PaCO2 (mmHg) | 52.0±14.1 | 0.92 | 0.96 | <0.001 | 0.94–0.98 |
| PETCO2 (mmHg) | 49.5±13.6 |
PETCO2, end-tidal CO2 pressure; PaCO2, partial pressure of carbon dioxide.
Correlation analysis of cerebral oxygen saturation
There was no difference in preoperative StO2 between the two groups. Compared with preoperative (T0), the StO2 of the SV group increased at T1, T3, T4, T5, and T6 (P<0.05), and that of the MV group increased at T1 and T6 (P<0.05) (Table 7). The SV group also had higher StO2 at T3, T4, and T5 than the MV group (P<0.01) (Table 7). Multiple linear regression analysis showed PETCO2 and HR effected the change of StO2 (P<0.05), and PETCO2 (R2=0.225, B=0.41, P<0.001) had a greater effect (Table 8).
Table 7
| Indicator | Group | T0 | T1 | T2 | T3 | T4 | T5 | T6 |
|---|---|---|---|---|---|---|---|---|
| StO2 (%) | SV | 70.3±5.2 | 72.4±4.2a | 72.8±3.8 | 76.0±3.9ab | 76.6±3.8ab | 77.1±3.3ab | 75.3±3.7a |
| MV | 70.7±2.7 | 72.7±3.4a | 71.8±4.4 | 71.8±5.1 | 71.7±4.7 | 72.2±5.0 | 73.9±4.3a |
a, P<0.05, compare to T0; b, P<0.05 compared to the MV group. StO2, brain tissue oxygen saturation; T0, preoperative; T1, anesthesia induction intubation; T2, beginning of operation; T3, 15 min after the operation began; T4, 30 min after the operation began; T5, 45 min after the operation began; T6, end of the operation; SV, spontaneous ventilation; MV, mechanical ventilation.
Table 8
| Variables | R-square | Non-standardized | Standardized | t value | P value | ||
|---|---|---|---|---|---|---|---|
| Beta | SE | Beta | |||||
| Constant | 0.225 | 61.841 | 14.397 | – | 4.296 | <0.001 | |
| SBP | −0.015 | 0.047 | –0.065 | −0.312 | 0.756 | ||
| DBP | 0.031 | 0.085 | 0.079 | 0.357 | 0.722 | ||
| MAP | 0.022 | 0.115 | 0.068 | 0.193 | 0.847 | ||
| PETCO2 | 0.137 | 0.026 | 0.41 | 5.328 | <0.001 | ||
| HR | 0.063 | 0.022 | 0.202 | 2.832 | 0.005 | ||
| SpO2 | −0.001 | 0.144 | 0 | −0.007 | 0.994 | ||
StO2, brain tissue oxygen saturation; SE, standard error; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; PETCO2, end-tidal CO2 pressure; HR, heart rate; SpO2, pulse oxygen saturation.
Hematologic and serum inflammatory parameters
There were no significant differences in the expressions of TNF-α, IL-6, IL-1β, and S100β between the two groups at each time point (P>0.05) (Table 9). In the SV group, TNF-α was lower at T2 than at T1, and IL-6 at T3 and T4 was lower than at T1 (P<0.05) (Table 9). The S100β level was lower in the SV group at T3 and T4 than at T1 (P<0.05) (Table 9), while in the MV group TNF-α, IL-6, and IL-1β decreased at T2, T3, and T4 compared with T1 (P<0.05) (Table 9). The S100β level was lower in the MV group at T2 and T3 than at T1 (P<0.05) (Table 9). Leukocyte, neutrophil, and lymphocyte counts increased significantly 72 h after the surgery in both groups (P<0.01) (Table 9) with postoperative leukocyte counts (P=0.044) and neutrophil counts (P=0.035) in the SV group lower than in the MV group (Table 9).
Table 9
| Indicator | Group | T1 | T2 | T3 | T4 |
|---|---|---|---|---|---|
| TNF-α (pg/mL) | SV | 468.3±125.0 | 441.0±95.6a | 449.8±105.2 | 444.2±115.9 |
| MV | 468.2±119.1 | 443.0±110.9a | 431.3±116.2a | 410.5±106.0a | |
| IL-6 (pg/mL) | SV | 97.9±30.6 | 91.9±32.3 | 87.4±27.1a | 84.6±24.6a |
| MV | 97.3±20.8 | 85.7±13.6a | 84.6±15.1a | 75.8±12.3a | |
| IL-1β (pg/mL) | SV | 50.0±16.2 | 49.5±16.0 | 47.8±13.2 | 48.9±13.0 |
| MV | 50.2±10.1 | 47.4±9.7a | 45.8±7.9a | 44.8±8.5a | |
| S100β (pg/mL) | SV | 710.0±140.0 | 686.9±152.0 | 647.8±142.4a | 657.4±138.8a |
| MV | 756.7±184.1 | 701.4±157.9a | 698.0±145.4a | 728.3±192.5 | |
| Leukocyte counts (×109/L) | SV | 5.59 (4.39–7.10) | – | – | 6.93 (5.38–8.74)ab |
| MV | 5.50 (4.25–6.64) | – | – | 8.09 (7.04–9.88)a | |
| Neutrophil counts (×109/L) | SV | 3.10 (2.78–4.11) | – | – | 4.61 (3.40–6.64)ab |
| MV | 3.08 (2.39–4.08) | – | – | 5.49 (4.57–7.49)a | |
| Lymphocyte counts (×109/L) | SV | 1.75 (1.39–2.33) | – | – | 1.45 (1.19–1.69)a |
| MV | 1.77 (1.47–2.19) | – | – | 1.40 (1.07–1.79)a |
a, P<0.05, compared to T1; b, P<0.05, compared to the MV group; T1, preoperative; T2, at the end of operation; T3, 16 h after operation; T4, 72 h after operation; SV, spontaneous ventilation; MV, mechanical ventilation; TNF, tumor necrosis factor; IL, interleukin.
Cognitive function assessment
There was no difference in preoperative MMSE scores between the two groups. The MMSE scores of patients in MV group on postoperative days 14 were higher than those on postoperative days 4 (P<0.05) (Table 10). The MMSE score of the SV group at 4 days after operation was higher than that of the MV group (P<0.05) (Table 10). According to established criteria for the diagnosis of POCD, more patients in the MV group developed POCD (four patients, 13.3%) than in the SV group (one patient, 3.3%) on the fourth postoperative day (P=0.35) (Table 11). However, there was no significant difference in the incidence of POCD in both groups at any time point (Table 11). A multiple logistic regression model was established and showed gender, age, education level, surgical plan, surgical duration, StO2 lower limit, blood loss, and hypercapnia had no statistical significance on the incidence of POCD (Table 12).
Table 10
| Group | M0 | M1 | M2 | M3 | M4 |
|---|---|---|---|---|---|
| SV | 29.5 [29–30] | 30 [28–30]b | 30 [28.75–30] | 30 [29–30] | 30 [29–30] |
| MV | 29.0 [28–30] | 28.5 [27–30] | 30 [28.75–30] | 30 [29–30]a | 30 [28–30] |
a, P<0.05, compared to M1; b, P<0.05, compared to group MV. M0, 1 d before surgery; M1, 4 d after surgery; M2, 7 d after surgery; M3, 14 d after surgery; M4, 30 d after surgery. MMSE, Mini-Mental State Examination; SV, spontaneous ventilation; MV, mechanical ventilation.
Table 11
| Group | M0 | M1 | M2 | M3 | M4 |
|---|---|---|---|---|---|
| SV | 0 (0) | 1 (3.3) | 0 (0) | 0 (0) | 0 (0) |
| MV | 0 (0) | 4 (13.3) | 2 (6.7) | 1 (3.3) | 1 (3.3) |
POCD, postoperative cognitive dysfunction; M0, 1 d before surgery; M1, 4 d after surgery; M2, 7 d after surgery; M3, 14 d after surgery; M4, 30 d after surgery; SV, spontaneous ventilation; MV, mechanical ventilation.
Table 12
| Variables | B | S.E. | Wals | P value | OR | 95% CI | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Gender | –2.47 | 1.35 | 3.36 | 0.06 | 0.09 | 0.01 | 1.19 |
| Age | 0.12 | 0.14 | 0.73 | 0.39 | 1.13 | 0.86 | 1.48 |
| Education | –1.62 | 1.14 | 2.02 | 0.16 | 0.2 | 0.02 | 1.85 |
| Surgery type | –0.26 | 0.65 | 0.16 | 0.69 | 0.77 | 0.22 | 2.77 |
| Operative time | 0.01 | 0.01 | 0.5 | 0.48 | 1.01 | 0.98 | 1.03 |
| Lowest StO2 | –0.12 | 0.12 | 1.01 | 0.31 | 0.88 | 0.7 | 1.12 |
| Blood loss | –0.03 | 0.03 | 1.38 | 0.24 | 0.97 | 0.92 | 1.02 |
| Hypercapnia | –0.27 | 1.76 | 2.35 | 0.13 | 0.07 | 0.002 | 2.12 |
POCD, postoperative cognitive dysfunction; S.E., standard error; OR, odd ratio; CI, confidence intervals; StO2, brain tissue oxygen saturation.
Perioperative complications
There was no difference in the incidence of arrhythmia or cough between the two groups (P>0.05) (Table 13), and no patients experienced hypotension, hypoxemia, or asphyxia (Table 13). Dizziness, nausea, vomiting, and pulmonary infection were lower in the SV group, but there was no significant difference in both groups (P>0.05) (Table 13). In the SV group, the incidence of sore throat was significantly less than in the MV group (P=0.001) (Table 13).
Table 13
| Indicator | SV | MV | P value |
|---|---|---|---|
| Intraoperative adverse reactions | |||
| Arrhythmia | 2 (6.7) | 1 (3.3) | >0.999 |
| Hypotension | 0 (0) | 0 (0) | – |
| Hypoxemia | 0 (0) | 0 (0) | – |
| Cough | 2 (6.7) | 3 (10.0) | >0.999 |
| Asphyxia | 0 (0) | 0 (0) | – |
| Postoperative adverse reactions | |||
| Dizziness | 8 (26.7) | 11 (36.7) | 0.58 |
| Nausea | 4 (13.3) | 5 (16.7) | >0.999 |
| Vomiting | 4 (13.3) | 10 (33.3) | 0.125 |
| Sore throat | 3 (10.0)* | 16 (53.3) | 0.001 |
| Pulmonary infection | 0 (0) | 1 (3.3) | >0.999 |
*, P<0.05, compared to the MV group. SV, spontaneous ventilation; MV, mechanical ventilation.
Anesthesia and operation effect
In both groups, NRS scores in the active state were significantly higher than in the rest state 48 hours after surgery (P<0.01) (Table 14). In both groups, NRS scores increased at 12 h after surgery in the rest state (P<0.05) (Table 14). In the active state, the NRS score of MV group increased from 6 to 48h, and that of SV group increased from 12 to 24 h (P<0.05) (Table 14). NRS scores in the rest state and active state were not different between the two groups at any time point (P>0.05) (Table 14). The SV group showed shorter postoperative awakening time (P<0.001), endotracheal intubation/laryngeal mask extubating time (P<0.001), rest time before the first out of bed activity (P<0.001), and indwelling duration of chest tube (P=0.015), as well as less drainage volume of the chest tube (P=0.039) (Table 15).
Table 14
| Time | SV | MV | |||
|---|---|---|---|---|---|
| Rest | Active | Rest | Active | ||
| T1 | 2.0 (1.0–3.0)c | 3.5 (2.0–4.0) | 2.0 (0.75–3)c | 2.5 (2.0–4.25) | |
| T2 | 3.0 (1.75–4.0)c | 5.0 (3.0–6.0) | 3.0 (2.0–4.25)c | 5.0 (3.0–7.0)a | |
| T3 | 3.0 (2.0–6.0)ac | 5.0 (3.0–8.0)a | 3.0 (2.0–5.0)ac | 5.0 (3.75–8.0)a | |
| T4 | 3.0 (2.0–4.0)c | 6.0 (5.0–7.0)a | 2.5 (2.0–5.0)c | 5.5 (3.75–8.0)a | |
| T5 | 2.0 (1.0–3.0)bc | 4.0 (3.0–5.0) | 2.5 (1.75–4.0)bc | 5.0 (3.75–8.0)a | |
a, P<0.05 compared to T1; b, P<0.05 compared to T3; c, P<0.05 compared to active. T1, 2 h after surgery; T2, 6 h after surgery; T3, 12 h after surgery; T4, 24 h after surgery; T5, 48 h after surgery. SV, spontaneous ventilation; MV, mechanical ventilation.
Table 15
| Outcomes | Total (n=60) | SV (n=30) | MV (n=30) | P value |
|---|---|---|---|---|
| Extubation time (min) | 11.0 (8.0–15.0) | 8.0 (7.0–11.0)* | 13.5 (11.8–15.0) | <0.001 |
| Awakening time (min) | 10.0 (7.0–12.0) | 7.0 (5.0–10.0)* | 11.5 (9.8–12.3) | <0.001 |
| Leaving bed time (h) | 11.5 (2.0–17) | 2.0 (2.0–2.6)* | 16.3 (14–18.3) | <0.001 |
| Chest tube drainage (mL) | 290 [100–675] | 150.0 (43.8–582.5)* | 392.5 (225.0–720.0) | 0.039 |
| Chest tube duration (day) | 3 [1–4] | 1.5 (1.0–4.0)* | 3.0 (2.0–4.0) | 0.015 |
| Post-operative hospital stay (day) | 7 [6–8] | 7 [6–8] | 7 [6–8] | 0.857 |
*, P<0.05 compared to the MV group. SV, spontaneous ventilation; MV, mechanical ventilation.
Discussion
Our results showed the incidence of intraoperative hypercapnia in the SV group was significantly higher than in the MV group (100% vs. 0%, P<0.001), and there was no hypoxemia. This suggests tubeless anesthesia techniques may induce hypercapnia. In patients under tubeless anesthesia, intraoperative StO2 increased after anesthesia induction, reached equilibrium 15 minutes after the beginning of the operation, and remained until the end of the operation. The SV group had higher MMSE scores 4 days after surgery than the MV group [30.0 (28.0–30.0) vs. 28.5 (27.0–30.0), P=0.047], but there was no significant difference in the incidence of POCD (13.3% vs. 3.3%, P>0.05) and serum levels of IL-6, TNF-α, IL-1β, and S100β. This suggests tubeless general anesthesia is more likely to induce hypercapnia and increase intraoperative regional cerebral oxygen saturation, but has no significant effect on POCD. The postoperative leukocyte, neutrophil, and lymphocyte counts in both groups were significantly higher than the preoperative levels. In addition, postoperative leukocytes and neutrophils in the MV group were higher than in the SV group (P<0.05). This shows tubeless anesthesia has a significant advantage in reducing postoperative inflammatory responses compared with double-lumen bronchial general anesthesia. Both arrhythmia and cough did not differ between the two groups (P>0.99), and no patient experienced hypotension, hypoxemia, or asphyxia in either group. The awakening and extubation times of patients were shortened, the first activity time of leaving bed was earlier, the retention time of the chest tube was shortened, the drainage volume of the chest tube was reduced, and postoperative throat pain all occurred less in the SV group. The results of our study indicate tubeless anesthesia has significant advantages in promoting the rapid rehabilitation of patients compared with double-lumen bronchial general anesthesia.
Tubeless anesthesia retains spontaneous breathing during surgery and has been reported to decrease postoperative complications (16,25-27). During VATS, and under the action of an artificial pneumothorax, the affected lung is compressed, the mediastinum swings, and the ventilation blood flow ratio of the healthy side decreases. When this occurs, it is necessary to increase the TV or RR of the healthy side lung to meet the gas exchange. However, under the action of opioids, the TV and RR of spontaneous breathing decreases, resulting in the instability of MV, and inhibiting reactivity of the respiratory center to carbon dioxide (CO2). Therefore, patients undergoing VATS with tubeless anesthesia often have insufficient ventilation of one-lung spontaneous respiration, which manifests as hypercapnia. In this study, intraoperative TV and RR of patients undergoing tubeless anesthesia were unstable, and the MV was significantly lower than that of patients undergoing double-lumen general anesthesia.
PETCO2 was continuously monitored by airway monitor and PaCO2 was measured by intermittent arterial blood sampling, and a high correlation was seen between the two (R squared =0.92, R=0.96, 95% CI: 0.94–0.98, P<0.001). PETCO2 can be used as a noninvasive monitoring index of PaCO2, and it was found that PETCO2 and PaCO2 increased significantly in the SV group and the incidence of intraoperative hypercapnia in the SV group was significantly higher than in the MV group (100% vs. 0%, P<0.001), but there was no hypoxemia. After anesthesia induction, patients inhaled 50–100% oxygen during the operation, and SpO2 increased in both groups. CO2 has a complex impact on physiological function and participates in the physiological activities of important organs such as the heart, brain, and lung (28,29). Hypercapnia can improve pulmonary oxygenation (30), regulate pulmonary compliance and airway resistance (31), and reduce pulmonary inflammation (32,33). At the same time, it increases coronary blood flow and cardiac output (29,34) and improves myocardial oxygen supply (35). However, severe hypercapnia can lead to pulmonary vasoconstriction and increase right heart load (36). In addition, it is associated with attention disorder, memory disorder, prolonged reaction time, decline of logical thinking, and positioning dysfunction (18). Therefore, the management of hypercapnia during tubeless anesthesia is of great significance in preventing serious intraoperative and postoperative complications.
A cerebral oxygen saturation monitor can monitor the oxygenation and metabolism of brain tissue continuously and noninvasively in real time, providing opportunities for the early detection of brain deoxygenation events and imbalance between cerebral oxygen supply and demand (7,37,38). It also plays an important role in preventing postoperative neurological complications. A multicenter study found monitoring cerebral oxygen saturation during cardiac surgery could guide its timely detection and prevention but could not reduce the incidence of perioperative neurocognitive changes (39). However, some studies have shown perioperative cerebral oxygen saturation below 50–60% is related to neurological complications and mortality (4), and its monitoring could reduce the occurrence of POCD (40). Kazan et al. reported that in 82% of patients receiving OLV, the cerebral oxygen saturation decreased by 15% compared with the baseline value, and intraoperative was related to the decline of cognitive function score after thoracic surgery (8). Therefore, the decrease of cerebral oxygen saturation may be one of the causes of POCD in patients undergoing thoracoscopy.
In both groups, StO2 levels increased after the induction of anesthesia. At this time, PETCO2 is within the normal range, which may be caused by the expansion of cerebral blood vessels and the reduction of cerebral metabolism by anesthetic drugs. Multivariate regression analysis showed that the change of cerebral oxygen saturation was related to PETCO2 (R2=0.225, B=0.41, P<0.001). There may be several reasons for this. First, every 1 mmHg increase of CO2 will increase cerebral blood flow (CBF) by 1–2 mL/100 g/min. When the PaCO2 increases to 80mmHg, the CBF increases by 100–200% under the action of cerebral vasodilation, increasing intracranial pressure (41,42). Second, when PaCO2 changes are in the range of 20–80 mmHg, the oxygen dissociation curve is shifted to the right, the affinity between hemoglobin and oxygen is reduced, the release of oxygen is increased, and the local partial pressure of oxygen is increased (43). Third, hypercapnia stimulates central chemoreceptors which excite the cardiac sympathetic system, resulting in an increase in HR, stroke volume, cardiac output, and SBP, which increases cerebral perfusion (44). In addition, the change of HR was also related to StO2 (R2=0.225, B=0.202, P=0.005), as hypercapnia stimulates cardiac sympathetic nerves, increases the HR, enhances myocardial contractility, increases cardiac output, and finally improves cerebral perfusion. Vasoactive drugs were used to regulate BP, and the intraoperative BP was maintained in a stable state, excluding the effect of arterial pressure on brain perfusion.
POCD is a complication of surgery and anesthesia, and includes cognitive changes characterized by the impairment of orientation, memory, attention, and language comprehension (1). Central nervous system inflammation plays an important role in the occurrence and development of POCD, and surgical trauma and postoperative pain can induce the body to release inflammatory mediators causing a systemic inflammatory response. On the one hand, inflammatory mediators activate peripheral inflammatory responses, while on the other, inflammatory mediators can cross the blood-brain barrier, activate microglia, and induce inflammation in the central nervous system. IL-1β, TNF-α, IL-6, and S100β play a key role in triggering the occurrence and development of the nervous system inflammatory response (45,46).
Hypercapnia can promote the activation of NLRP3 inflammatory bodies in hypoxic activated microglia, up-regulate the expression of IL-1β, then activate the inflammatory response of the central nervous system, leading to the apoptosis of hippocampal neurons and aggravating cognitive function (21). IL-6 is a key mediator leading to hippocampal injury, and its increase can cause inflammation related learning and memory damage (47). A meta-analysis showed the occurrence of POCD was related to the concentration of serum IL-6, indicating it can be used as an indicator to guide the prevention and treatment of POCD (48). The concentration of serum IL-6 usually increases within 30–60 min after surgery, changes significantly after 2–4 h, reaches its peak around 24 h, and remains up to 48–72 hours after surgery (45). In addition, TNF-α plays an important role in the development of inflammation, acting upstream of IL-1β (49). TNF-α starts the peripheral inflammatory cascade, resulting in the decline of cognitive function, while anti-TNF-α antibody can prevent the decline of postoperative cognitive function (45). Under physiological conditions, the serum concentration of S100β is low. In the early stage of brain injury, when permeability of the blood-brain barrier changes, glial cells are activated, and S100β is released into the blood circulation. This results in increased serum concentrations of S100β protein, which can reflect the degree of neuronal damage, and is considered to be a potential marker of POCD (50). Linstedt et al. showed the concentration of plasma S100β peaked at 30 min after surgery and returned to baseline about 18 hours after surgery (51). Therefore, in this study, venous blood was collected before anesthesia, at the end of surgery, and 16 and 72 hours after surgery, to measure the expression of IL-6, TNF-α, IL-1β, and S100β in the serum of the two groups.
Our results showed the MMSE score 4 days after double-lumen bronchial general anesthesia was lower than in the tubeless anesthesia group {30 [28–30] vs. 28.5 [27–30], P=0.047}, and the incidence of cognitive function was higher than that in the SV group (13.3% vs. 3.3%, P>0.05). However, there was no statistical significance between the two groups. Gender, age, education level, operation plan, operation time, the lower limit of StO2, blood loss, and hypercapnia had no significant effect on POCD. In addition, there was no difference in the expression of cognitive function related proteins between the two groups at each time point (P>0.05). The incidence of POCD in patients undergoing VATS in this study was lower than the 28% reported by Egawa et al. (13) and Li et al. (52), which may be because of the age in this study was younger (18–60 vs. 20–85/40–75 years). In the study of Li et al. (52), the intraoperative StO2 of POCD patients was significantly reduced {M [P25–P75], 59 [53–65]}, suggesting decreased cerebral blood perfusion accompanied by cerebral ischemia and hypoxia during OLV. This may be because of the imbalance of the ventilation blood flow ratio and increase of the intrapulmonary arteriovenous shunt during OLV, resulting in changes in physiological functions such as hypoxemia and pulmonary ischemia-reperfusion. In our study, intraoperative StO2 decline did not exceed the lower limit (<60%), and no patient developed hypoxemia. Our results are also inconsistent with the severe cognitive impairment reported by Ding et al. (21), which may be because in that study hypercapnia and hypoxemia coexisted in rats. In our study, pulse blood oxygen saturation and brain oxygen saturation were monitored throughout the entire process, and hypoxemia was not found. This shows that under the condition of adequate oxygen supply, hypercapnia will not cause serious damage to the central nervous system and does not promote cognitive dysfunction.
The postoperative leukocyte, neutrophil count in the two groups were significantly higher than those seen preoperatively, and were higher in the MV group than in the SV group (P<0.05). This shows tubeless anesthesia has advantages in reducing the postoperative inflammatory response compared with double-lumen bronchial general anesthesia. However, the trend of blood cells is opposite to that of serum inflammatory factors. This may be related to less inflammatory factors included in this study, and other inflammatory factors affecting the postoperative leukocyte count, neutrophil count, and lymphocyte count were not included. In addition, these counts were measured 2–3 days before operation and 72 hours after operation, which was different from the detection time of inflammatory factors.
Studies have shown postoperative acute pain can aggravate memory impairment (53,54), and to minimize its effect and complications in inflammation and POCD, a multimodal analgesia scheme was used to reduce postoperative pain and accelerate postoperative rehabilitation. In this study, NRS scores in the rest and active state did not differ between the two groups at any time point. In the active and resting states, with the disappearance of local nerve block, patients in the two groups gradually experienced obvious pain 12 h after operation, and the NRS score in the active state was significantly higher than in the resting state, which was mostly related to cough and the indwelling position of the drainage tube. Timely analgesia or other analgesic programs are required to provide a satisfactory analgesic effect. The shortening of postoperative recovery time [7.0 (5.0–10.0) vs. 11.5 (9.8–12.3) min, P<0.001], extubation time [8.0 (7.0–11.0) vs. 13.5 (11.8–15.0) min, P<0.001], and first leaving bed activity time [2.0 (2.0–2.6) vs. 16.3 (14–18.3) h, P<0.001] in patients receiving tubeless technology are related to the reduction of intraoperative opioid and muscle relaxant dosage. The duration of chest tube indwelling [1.5 (1.0–4.0) vs. 3.0 (2.0–4.0) d, P=0.015] was shortened, and the drainage volume of the chest tube [150.0 (43.8–582.5) vs. 392.5 (225.0–720.0) mL, P=0.039] was reduced, suggesting the rehabilitation of patients can be accelerated. In addition, such patients avoid stimulation of the pharynx caused by double-lumen endobronchial intubation, and postoperative sore throat rarely occurs.
Some limitations of our study should be mentioned. Firstly, the incidence of POCD is high in patients over 60 years old (specific proportion), while those in this study were mainly under 60 years old. Therefore, in the future, the sample should be further expanded and in-depth study should be conducted on patients over 60 years of age. Secondly, cognitive dysfunction is affected by multiple factors such as a patient’s educational level, nationality, and as this was a single center study, multi-centre studies are required. Thirdly, the evaluation of postoperative cognitive function in this study only lasted until 1 month after surgery, without long-term follow-up (more than 3 months).
Conclusions
The results of this study show that the use of tubeless anesthesia for VATS is more likely to induce hypercapnia and increase cerebral oxygen saturation during the operation, but has no significant difference in the incidence of POCD. In addition, tubeless anesthesia can reduce systemic inflammation, promote early mobilization after surgery, and accelerate the recovery of systemic multi-organ functions.
Acknowledgments
Funding: The study was supported by the Science and Technology Program of Kunming City (2020-1-H-003), Yunnan Clinical Medical Center for Respiratory Diseases (2021LCZXXF-HX02), Special Fund of the Applied Basic Research Programs of Yunnan Province associated with Kunming Medical University (202201AY070001-263), and Key Project of Basic Research Program of Yunnan Province (202201AS070009).
Footnote
Reporting Checklist: The authors have completed the CONSORT reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1165/rc
Trial Protocol: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1165/tp
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1165/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1165/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Ethical approval for the study was granted by the Ethics Committee of The First People’s Hospital of Yunnan Province (No. KHLL2021-KY024). In addition, written informed consent was obtained from all patients who participated. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Berger M, Nadler JW, Browndyke J, et al. Postoperative Cognitive Dysfunction: Minding the Gaps in Our Knowledge of a Common Postoperative Complication in the Elderly. Anesthesiol Clin 2015;33:517-50. [Crossref] [PubMed]
- Ackenbom MF, Butters MA, Davis EM, et al. Incidence of postoperative cognitive dysfunction in older women undergoing pelvic organ prolapse surgery. Int Urogynecol J 2021;32:433-42. [Crossref] [PubMed]
- Wu XM, Xu WC, Yu YJ, et al. Postoperative serum thioredoxin concentrations correlate with delirium and cognitive dysfunction after hip fracture surgery in elderly patients. Clin Chim Acta 2017;466:93-7. [Crossref] [PubMed]
- Ding L, Chen DX, Li Q. Effects of electroencephalography and regional cerebral oxygen saturation monitoring on perioperative neurocognitive disorders: a systematic review and meta-analysis. BMC Anesthesiol 2020;20:254. [Crossref] [PubMed]
- Murkin JM. Cerebral oximetry: monitoring the brain as the index organ. Anesthesiology 2011;114:12-3. [Crossref] [PubMed]
- Kim HS, Ha SO, Yu KH, et al. Cerebral Oxygenation as a Monitoring Parameter for Mortality During Venoarterial Extracorporeal Membrane Oxygenation. ASAIO J 2019;65:342-8. [Crossref] [PubMed]
- Peng A, Chua MJ, Chan B, et al. Tissue oxygenation indices of cerebrovascular autoregulation in healthy volunteers: a comparison of two NIRS devices. Neurol Res 2020;42:897-903. [Crossref] [PubMed]
- Kazan R, Bracco D, Hemmerling TM. Reduced cerebral oxygen saturation measured by absolute cerebral oximetry during thoracic surgery correlates with postoperative complications. Br J Anaesth 2009;103:811-6. [Crossref] [PubMed]
- Kim J, Shim JK, Song JW, et al. Postoperative Cognitive Dysfunction and the Change of Regional Cerebral Oxygen Saturation in Elderly Patients Undergoing Spinal Surgery. Anesth Analg 2016;123:436-44. [Crossref] [PubMed]
- Holmgaard F, Vedel AG, Rasmussen LS, et al. The association between postoperative cognitive dysfunction and cerebral oximetry during cardiac surgery: a secondary analysis of a randomised trial. Br J Anaesth 2019;123:196-205. [Crossref] [PubMed]
- Zhang J, Chen L, Sun Y, et al. Comparative effects of fentanyl versus sufentanil on cerebral oxygen saturation and postoperative cognitive function in elderly patients undergoing open surgery. Aging Clin Exp Res 2019;31:1791-800. [Crossref] [PubMed]
- Tang L, Kazan R, Taddei R, et al. Reduced cerebral oxygen saturation during thoracic surgery predicts early postoperative cognitive dysfunction. Br J Anaesth 2012;108:623-9. [Crossref] [PubMed]
- Egawa J, Inoue S, Nishiwada T, et al. Effects of anesthetics on early postoperative cognitive outcome and intraoperative cerebral oxygen balance in patients undergoing lung surgery: a randomized clinical trial. Can J Anaesth 2016;63:1161-9. [Crossref] [PubMed]
- Liu J, Cui F, Pompeo E, et al. The impact of non-intubated versus intubated anaesthesia on early outcomes of video-assisted thoracoscopic anatomical resection in non-small-cell lung cancer: a propensity score matching analysis. Eur J Cardiothorac Surg 2016;50:920-5. [Crossref] [PubMed]
- Szabó Z, Tanczos T, Lebak G, et al. Non-intubated anaesthetic technique in open bilobectomy in a patient with severely impaired lung function. J Thorac Dis 2018;10:E275-80. [Crossref] [PubMed]
- Wen Y, Liang H, Qiu G, et al. Non-intubated spontaneous ventilation in video-assisted thoracoscopic surgery: a meta-analysis. Eur J Cardiothorac Surg 2020;57:428-37. [PubMed]
- Zhang K, Chen HG, Wu WB, et al. Non-intubated video-assisted thoracoscopic surgery vs. intubated video-assisted thoracoscopic surgery for thoracic disease: a systematic review and meta-analysis of 1,684 cases. J Thorac Dis 2019;11:3556-68. [Crossref] [PubMed]
- Ozge C, Ozge A, Unal O. Cognitive and functional deterioration in patients with severe COPD. Behav Neurol 2006;17:121-30. [Crossref] [PubMed]
- Zhu L, Shi H, Zhu C, et al. Impact of permissive hypercapnia on regional cerebral oxygen saturation and postoperative cognitive function in patients undergoing cardiac valve replacement. Ann Palliat Med 2020;9:4066-73. [Crossref] [PubMed]
- Wong C, Churilov L, Cowie D, et al. Randomised controlled trial to investigate the relationship between mild hypercapnia and cerebral oxygen saturation in patients undergoing major surgery. BMJ Open 2020;10:e029159. [Crossref] [PubMed]
- Ding HG, Deng YY, Yang RQ, et al. Hypercapnia induces IL-1β overproduction via activation of NLRP3 inflammasome: implication in cognitive impairment in hypoxemic adult rats. J Neuroinflammation 2018;15:4. [Crossref] [PubMed]
- Barrientos RM, Frank MG, Hein AM, et al. Time course of hippocampal IL-1 beta and memory consolidation impairments in aging rats following peripheral infection. Brain Behav Immun 2009;23:46-54. [Crossref] [PubMed]
- Norozian FM, Leoncio M, Torbati D, et al. Therapeutic hypercapnia enhances the inflammatory response to endotoxin in the lung of spontaneously breathing rats. Crit Care Med 2011;39:1400-6. [Crossref] [PubMed]
- Wang KY, Yang QY, Tang P, et al. Effects of ulinastatin on early postoperative cognitive function after one-lung ventilation surgery in elderly patients receiving neoadjuvant chemotherapy. Metab Brain Dis 2017;32:427-35. [Crossref] [PubMed]
- Yu MG, Jing R, Mo YJ, et al. Non-intubated anesthesia in patients undergoing video-assisted thoracoscopic surgery: A systematic review and meta-analysis. PLoS One 2019;14:e0224737. [Crossref] [PubMed]
- Gonzalez-Rivas D, Bonome C, Fieira E, et al. Non-intubated video-assisted thoracoscopic lung resections: the future of thoracic surgery? Eur J Cardiothorac Surg 2016;49:721-31. [Crossref] [PubMed]
- Wang ML, Galvez C, Chen JS, et al. Non-intubated single-incision video-assisted thoracic surgery: a two-center cohort of 188 patients. J Thorac Dis 2017;9:2587-98. [Crossref] [PubMed]
- Masterson C, Horie S, McCarthy SD, et al. Hypercapnia in the critically ill: insights from the bench to the bedside. Interface Focus 2021;11:20200032. [Crossref] [PubMed]
- Pelletier-Galarneau M, deKemp RA, Hunter CRRN, et al. Effects of Hypercapnia on Myocardial Blood Flow in Healthy Human Subjects. J Nucl Med 2018;59:100-6. [Crossref] [PubMed]
- Goldenberg NM, Hare GM. From the Journal archives: Understanding the mechanism(s) regulating hypoxic pulmonary vasoconstriction: how an early study has led to novel translational approaches. Can J Anaesth 2014;61:195-9. [Crossref] [PubMed]
- Emery MJ, Eveland RL, Min JH, et al. CO2 relaxation of the rat lung parenchymal strip. Respir Physiol Neurobiol 2013;186:33-9. [Crossref] [PubMed]
- Contreras M, Ansari B, Curley G, et al. Hypercapnic acidosis attenuates ventilation-induced lung injury by a nuclear factor-κB-dependent mechanism. Crit Care Med 2012;40:2622-30. [Crossref] [PubMed]
- Sinclair SE, Kregenow DA, Lamm WJ, et al. Hypercapnic acidosis is protective in an in vivo model of ventilator-induced lung injury. Am J Respir Crit Care Med 2002;166:403-8. [Crossref] [PubMed]
- Beaudin AE, Brugniaux JV, Vöhringer M, et al. Cerebral and myocardial blood flow responses to hypercapnia and hypoxia in humans. Am J Physiol Heart Circ Physiol 2011;301:H1678-86. [Crossref] [PubMed]
- Chi L, Wang N, Yang W, et al. Protection of Myocardial Ischemia-Reperfusion by Therapeutic Hypercapnia: a Mechanism Involving Improvements in Mitochondrial Biogenesis and Function. J Cardiovasc Transl Res 2019;12:467-77. [Crossref] [PubMed]
- Crystal GJ. Carbon Dioxide and the Heart: Physiology and Clinical Implications. Anesth Analg 2015;121:610-23. [Crossref] [PubMed]
- Benni PB, MacLeod D, Ikeda K, et al. A validation method for near-infrared spectroscopy based tissue oximeters for cerebral and somatic tissue oxygen saturation measurements. J Clin Monit Comput 2018;32:269-84. [Crossref] [PubMed]
- Scheeren TW, Schober P, Schwarte LA. Monitoring tissue oxygenation by near infrared spectroscopy (NIRS): background and current applications. J Clin Monit Comput 2012;26:279-87. [Crossref] [PubMed]
- Deschamps A, Hall R, Grocott H, et al. Cerebral Oximetry Monitoring to Maintain Normal Cerebral Oxygen Saturation during High-risk Cardiac Surgery: A Randomized Controlled Feasibility Trial. Anesthesiology 2016;124:826-36. [Crossref] [PubMed]
- Ni C, Xu T, Li N, et al. Cerebral oxygen saturation after multiple perioperative influential factors predicts the occurrence of postoperative cognitive dysfunction. BMC Anesthesiol 2015;15:156. [Crossref] [PubMed]
- Curley G, Laffey JG, Kavanagh BP. Bench-to-bedside review: carbon dioxide. Crit Care 2010;14:220. [Crossref] [PubMed]
- Brian JE Jr. Carbon dioxide and the cerebral circulation. Anesthesiology 1998;88:1365-86. [Crossref] [PubMed]
- Turek Z, Kreuzer F. Effect of shifts of the O2 dissociation curve upon alveolar-arterial O2 gradients in computer models of the lung with ventilation-perfusion mismatching. Respir Physiol 1981;45:133-9. [Crossref] [PubMed]
- Delliaux S, Ichinose M, Watanabe K, et al. Cardiovascular responses to forearm muscle metaboreflex activation during hypercapnia in humans. Am J Physiol Regul Integr Comp Physiol 2015;309:R43-50. [Crossref] [PubMed]
- Kristek G, Radoš I, Kristek D, et al. Influence of postoperative analgesia on systemic inflammatory response and postoperative cognitive dysfunction after femoral fractures surgery: a randomized controlled trial. Reg Anesth Pain Med 2019;44:59-68. [Crossref] [PubMed]
- Subramaniyan S, Terrando N. Neuroinflammation and Perioperative Neurocognitive Disorders. Anesth Analg 2019;128:781-8. [Crossref] [PubMed]
- Marsland AL, Gianaros PJ, Abramowitch SM, et al. Interleukin-6 covaries inversely with hippocampal grey matter volume in middle-aged adults. Biol Psychiatry 2008;64:484-90. [Crossref] [PubMed]
- Peng L, Xu L, Ouyang W. Role of peripheral inflammatory markers in postoperative cognitive dysfunction (POCD): a meta-analysis. PLoS One 2013;8:e79624. [Crossref] [PubMed]
- Terrando N, Monaco C, Ma D, et al. Tumor necrosis factor-alpha triggers a cytokine cascade yielding postoperative cognitive decline. Proc Natl Acad Sci U S A 2010;107:20518-22. [Crossref] [PubMed]
- Li YC, Xi CH, An YF, et al. Perioperative inflammatory response and protein S-100β concentrations - relationship with post-operative cognitive dysfunction in elderly patients. Acta Anaesthesiol Scand 2012;56:595-600. [Crossref] [PubMed]
- Linstedt U, Meyer O, Kropp P, et al. Serum concentration of S-100 protein in assessment of cognitive dysfunction after general anesthesia in different types of surgery. Acta Anaesthesiol Scand 2002;46:384-9. [Crossref] [PubMed]
- Li XM, Li F, Liu ZK, et al. Investigation of one-lung ventilation postoperative cognitive dysfunction and regional cerebral oxygen saturation relations. J Zhejiang Univ Sci B 2015;16:1042-8. [Crossref] [PubMed]
- Chi H, Kawano T, Tamura T, et al. Postoperative pain impairs subsequent performance on a spatial memory task via effects on N-methyl-D-aspartate receptor in aged rats. Life Sci 2013;93:986-93. [Crossref] [PubMed]
- Zhang X, Xin X, Dong Y, et al. Surgical incision-induced nociception causes cognitive impairment and reduction in synaptic NMDA receptor 2B in mice. J Neurosci 2013;33:17737-48. [Crossref] [PubMed]
(English Language Editor: B. Draper)


