
Risk factors contributing to impaired cough-specific quality of life at the time of admission for coronavirus disease 2019 treatment
Introduction
Cough is the most common symptom in patients seeking medical care worldwide. Most cases involve acute cough caused by viral respiratory infections (1). Although it usually resolves spontaneously within 3 weeks without specific therapy (1), the acute cough related to the common cold is associated with impaired quality of life (QoL) (2) due to the lack of specific pharmacological and non-pharmacological treatments (3). The United States Attitudes of Consumers Toward Health, Cough, and Cold survey emphasized that the cough from common cold affects the quality of sleeping, daily activity, labor productivity, and work absenteeism (4). Moreover, a post infectious cough lasting for ≥3 weeks after viral infection can further diminish the QoL (5).
The coronavirus family, together with the picornavirus family, is known as the most frequent cause of upper respiratory tract infection (URI) (6). The coronavirus disease 2019 (COVID-19) caused by severe acute syndrome coronavirus 2 (SARS-CoV-2) has been expanding worldwide since December 2019 (7). Cough is one of the most common presenting symptoms of COVID-19, along with fever and fatigue (7-9). Although cough itself does not contribute to disease severity, improvement, or mortality in COVID-19 (10,11), it is often present during hospitalization due to COVID-19 (12). Therefore, in patients with COVID-19, cough is one of the most common inconvenient symptom. However, to date, the impact of COVD-19-related acute cough on patient’s QoL has not been investigated.
In this study, we sought to identify the factors contributing to impaired cough-specific QoL at the time of admission for COVID-19 treatment using the Japanese version of acute cough with the Leicester Cough Questionnaire (LCQ-acute). We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-358/rc).
Methods
Participants
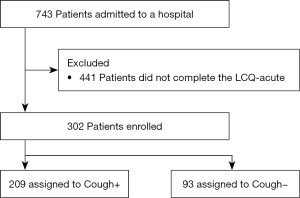
This was a retrospective cohort study. We reviewed the medical records of 743 patients admitted to Aichi Hospital, a specialized hospital for the treatment of COVID-19, from October 15, 2020 to October 31, 2021. Patients were eligible if they completed the LCQ-acute at the time of admission. As shown in Figure 1, 302 of the 743 patients completed the LCQ acute and their data were analyzed to identify factors contributing to impaired cough-specific QoL at the time of admission for COVID-19 treatment. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the ethical board of Aichi Hospital (approval number: 2021-2). The need for written informed consent was waived by the ethical board due to the retrospective nature of this study. Instead, we posted an opt-out document regarding this study on the website of Aichi Prefectural Government to offer patients the opportunity to deny participation in the study (https://www.pref.aichi.jp/soshiki/iryo-keikaku/aichikenritsuaichibyoin.html).
Measurements
We reviewed the medical records to assess presenting symptoms and clinical findings at the time of admission due to COVID-19. Fever (defined as a body temperature ≥37.5 ℃), cough, sputum, dyspnea (at rest or on effort), fatigue, appetite loss, gastrointestinal (GI) symptoms (nausea, vomiting, and diarrhea), musculoskeletal pain, headache, dysgeusia and/or dysosmia, rhinorrhea and/or nasal congestion, and sore throat were evaluated. We also evaluated patient characteristics {sex, smoking status, body mass index (BMI), and comorbidities [asthma (including a history of asthma), chronic obstructive pulmonary disease (COPD) or emphysema, diabetes mellitus, obstructive sleep apnea, hypertension, hyperlipidemia, hyperuricemia, cardiovascular diseases, and psychological disorders]}, clinical information regarding COVID-19 (time from onset to admission, length of hospital stay, and disease severity at the time of admission), and biomarkers [white blood cells (neutrophils, lymphocytes, monocytes, and eosinophils), C-reactive protein (CRP), lactate dehydrogenase (LDH), ferritin, hemoglobin A1c (HbA1c), immunoglobulin (Ig) G, IgA, IgM, procalcitonin, and D-dimer], along with the LCQ-acute score. Some information was lacking from the medical records (BMI: n=294; ferritin: n=293; HbA1c: n=281; IgG, IgA, and IgM: n=279; procalcitonin: n=286; and D-dimer: n=298). In addition, radiological findings and oxygen supplementation were used to determine disease severity.
LCQ-acute
The LCQ-acute consists of 19 items in three domains (physical, social, and psychological), and provides the patient’s cough-related QoL within 24 h before admission. The total scores range from 3 to 21, with higher scores indicating better cough-specific QoL (2). The Japanese version of the LCQ-acute (13) was used in this study. Patients completed the LCQ-acute at the time of admission for COVID-19 treatment.
Disease severity
Disease severity at the time of admission was classified based on the national guidelines for COVID-19 as follows (unpublished in English. https://www.mhlw.go.jp/content/000936623.pdf): (I) mild; patients did not have pneumonia on the chest computed tomography (CT) scan without oxygen supplication, (II) moderate I; patients had pneumonia on the chest CT scan but no oxygen supplementation [saturation of percutaneous oxygen (SpO2) ≥94% at room air], and moderate II; patients had pneumonia on the chest CT scan and hypoxia (SpO2 ≤93% at room air). Meanwhile, our cohort did not include severe cases requiring >5 L/min of oxygen supplementation or non-invasive or invasive mechanical ventilation at the time of admission because these patients did not meet the admission criteria of Aichi Hospital, which does not have an intensive care unit.
Statistical analysis
Statistical analyses were performed using JMP version 14.3 (SAS Institute Japan, Tokyo, Japan). Values are expressed as mean (standard deviation) for continuous variables and as n (%) for categorical variables. The number of comorbidities and presenting symptoms other than cough are expressed as median (range). We performed log-transformation for non-normally distributed biomarkers, and are expressed as geometric mean (standard deviation). The primary outcome was to determine factors associated with impaired cough-specific QoL at the time of admission for COVID-19 treatment. All presenting symptoms were divided into cough positive (+) and cough negative (−) groups. Patient characteristics such as sex, BMI, smoking status, disease severity, prevalence of presenting symptoms, biomarkers, and LCQ-acute scores were compared as well between the cough (+) and cough (−) groups. The LCQ-acute scores were compared among groups according to sex, BMI, smoking status, disease severity, and all presenting symptoms using the unpaired t-test and analysis of variance followed by the Tukey honestly significant difference test as appropriate. The correlation between biomarkers and LCQ-acute scores was assessed using Pearson’s correlation coefficient. Significant variables affecting the impaired cough-specific QoL assessed by the LCQ-acute at the time of admission for COVID-19 treatment were adopted for multivariate analysis. We arbitrarily adopted serum CRP as a biomarker of systemic inflammation in multivariate analysis because: (I) serum CRP is a sensitive and well-known biomarker of inflammation; (II) the Pearson’s correlation coefficient was highest for serum CRP (R=−0.28) when compared to serum LDH, IgM and procalcitonin; (III) serum CRP levels were significantly higher in the cough (+) group than in the cough (−) group; and (IV) there was no missing serum CRP data in the present cohort. The results are presented as estimates with 95% confidence intervals (CIs) and standardized beta values. For an α error of 5%, statistical significance was set at P≤0.05.
Results
Prevalence of acute cough associated with COVID-19 at the time of admission
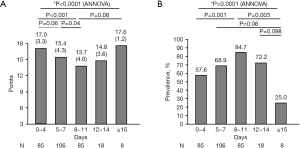
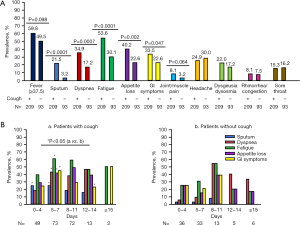
Patient characteristics are shown in Table 1. Two hundred and nine patients (69.2%) were coughing at the time of admission (Figure 1 and Table 1). Patients in the cough (+) group were younger and had greater BMI, disease severity, and more presenting symptoms than those in the cough (−) group (Table 1). Meanwhile, there was no difference in sex, time from onset to admission, and length of hospital stay between groups. Cough-specific QoL at the time of admission due to COVID-19 was lower in the cough (+) group than in the cough (−) group (Table 1). When arbitrarily stratified according to time from COVID-19 onset to admission, the LCQ-acute scores were lowest, and the cough prevalence was highest at 8–11 days after onset (Figure 2A,2B). Sputum, dyspnea, fatigue, appetite loss, and GI symptoms were more prevalent in the cough (+) group than in the cough (−) group (Figure 3A), particularly at 5–7 days after onset (Figure 3B). Interestingly, cough frequently presented with GI symptoms at 12–14 days after onset (Figure 3B). Meanwhile, the prevalence of other symptoms, including dysgeusia and/or dysosmia, was comparable between groups (Figure 3A).
Table 1
| Indices | All patients (n=302) | Cough (+) (n=209) | Cough (−) (n=93) | P value |
|---|---|---|---|---|
| Age, years | 52.1 (16.1) | 49.5 (14.3) | 58.0 (18.1) | <0.0001 |
| Sex (male) | 191 (63.2%) | 131 (62.7%) | 60 (64.5%) | 0.76 |
| Time from onset to admission, days | 6.8 (3.5) | 6.9 (3.0) | 6.4 (4.3) | 0.24 |
| The length of hospital stay, days | 10.8 (5.1) | 10.9 (4.4) | 10.7 (6.5) | 0.81 |
| BMI, kg/m2 | 24.4 (5.1)† | 24.8 (5.5)‡ | 23.4 (3.8)§ | 0.02 |
| Smoking, never/ex-/current | 157 (52%)/83 (27%)/62 (21%) | 108 (52%)/54 (26%)/47 (22%) | 49 (53%)/29 (31%)/15 (16%) | 0.37 |
| Severity, mild/moderate I/moderate II | 50 (16%)/156 (52%)/96 (32%) | 27 (13%)/109 (52%)/73 (35%) | 23 (25%)/47 (50%)/23 (25%) | 0.02 |
| Numbers of comorbidities | 1 (0–6) | 1 (0–6) | 2 (0–6) | 0.08 |
| Presenting symptoms except for cough | 3 (0–8) | 3 (0–8) | 2 (0–7) | <0.0001 |
| Cough specific QoL (LCQ-acute) points | ||||
| Physiological | 5.1 (1.1) | 4.8 (1.1) | 6.0 (0.6) | <0.0001 |
| Psychological | 5.1 (1.5) | 4.5 (1.5) | 6.2 (0.6) | <0.0001 |
| Social | 5.2 (1.7) | 4.6 (1.8) | 6.5 (0.6) | <0.0001 |
| Total | 15.4 (4.2) | 13.9 (4.2) | 18.6 (1.6) | <0.0001 |
Data are presented as mean (standard deviation), n (%) or median (range). +, cough symptom positive; −, cough symptom negative; †, n=294; ‡, n=205; §, n=89. COVID-19, coronavirus disease 2019; BMI, body mass index; QoL, quality of life; LCQ-acute, acute cough with the Leicester Cough Questionnaire.
Impact of cough-specific QoL on clinical indices during COVID-19 infection
Using the LCQ-acute, we evaluated the impact of cough-specific QoL on clinical indices at the time of admission for COVID-19 treatment stratified according to sex (male and female), age (≥50 and <50 years groups) (14), BMI (≥30 and <30 kg/m2 groups) (15), smoking status (never, ex-smoking, and current), disease severity (mild, moderate I, and moderate II), presenting symptoms, and comorbidities (Figure 4A,4B, Table 2). Similar to the difference between the cough (+) and cough (−) groups, young age (<50 years), higher BMI (≥30 kg/m2), higher disease severity, sputum, dyspnea, fatigue, appetite loss, and GI symptoms impaired cough-specific QoL at the time of admission due to COVID-19 (Figure 4A,4B). In addition, female sex, fever (temperature ≥37.5 ℃), and dysgeusia and/or dysosmia had a negative impact on QoL at the time of admission for COVID-19 treatment (Figure 4B).
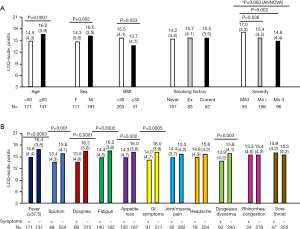
Table 2
| Comorbidities | (+) | (−) | P value |
|---|---|---|---|
| Asthma (including a history of asthma) | n=20 | n=282 | |
| 12.9 (4.9) | 15.6 (4.1) | 0.03 | |
| Allergic rhinitis/chronic rhinosinusitis | n=13 | n=289 | |
| 13.3 (4.7) | 15.5 (4.2) | 0.12 | |
| COPD/emphysema | n=9 | n=293 | |
| 17.3 (2.1) | 15.3 (4.2) | 0.02 | |
| Diabetes mellitus | n=44 | n=258 | |
| 16.7 (3.6) | 15.2 (4.7) | 0.01 | |
| Obstructive sleep apnea | n=8 | n=294 | |
| 14.5 (3.5) | 15.4 (4.2) | 0.51 | |
| Hypertension | n=80 | n=222 | |
| 15.9 (3.9) | 15.2 (4.3) | 0.16 | |
| Hyperlipidemia | n=52 | n=250 | |
| 16.7 (3.9) | 15.1 (4.2) | 0.01 | |
| Hyperuricemia | n=22 | n=280 | |
| 16.4 (3.4) | 15.3 (4.3) | 0.18 | |
| Cardiovascular diseases | n=17 | n=285 | |
| 17.1 (3.7) | 15.3 (4.2) | 0.06 | |
| Psychological disorders | n=14 | n=288 | |
| 15.2 (5.0) | 15.4 (4.2) | 0.91 |
Total LCQ-acute scores expressed as mean (standard deviation). +, comorbidities positive; −, comorbidities negative. QoL, quality of life; LCQ-acute, acute cough with the Leicester Cough Questionnaire; COVID-19, coronavirus disease 2019; COPD, chronic obstructive pulmonary disease.
With respect to comorbidities, patients with asthma (including six patients with a history of asthma, n=20) had significantly lower LCQ-acute scores than those without (Table 2). Conversely, LCQ-acute scores were significantly higher in patients with COPD, diabetes mellitus, and hyperlipidemia than in those without, respectively (Table 2). However, since patients with COPD, diabetes mellitus, and hyperlipidemia were significantly older than their counterparts (data not shown), we considered that the results were not affected by these three comorbidities, but by age.
Relationship between biomarkers and cough-specific QoL during COVID-19 infection
We also assessed the relationship between biomarkers and cough-specific QoL at the time of admission for COVID-19 treatment (Table 3). Higher serum LDH, IgM, CRP, and procalcitonin levels and lower blood eosinophil counts were significantly correlated with impaired cough-specific QoL at the time of admission for COVID-19 treatment (Table 3). However, all correlations were weak (Table 3). Additionally, serum LDH, CRP and IgM levels were significantly higher in the cough (+) group than in the cough (−) group (Table 3). Systemic inflammatory biomarkers were associated with impaired cough-specific QoL at the time of admission for COVID-19 treatment. On the other hand, the D-dimer plasma levels were significantly lower in the cough (+) group than in the cough (−) group. However, this difference may not be meaningful because both geometric mean values were within normal range (Table 3).
Table 3
| Biomarkers | Correlation between biomarkers and the LCQ-acute |
Comparison of biomarkers between cough (+) and cough (−) groups | |||||
|---|---|---|---|---|---|---|---|
| R (95% CI) | P value | All patients (n=302) | Cough (+) (n=209) | Cough (−) (n=93) | P value | ||
| WBC, /µL | −0.01 (−0.12, 0.10) | 0.85 | 5,178 [2,219] | 5,041 [1,884] | 5,485 [2,819] | 0.17 | |
| Neutrophils, /µL | −0.06 (−0.17, 0.06) | 0.32 | 3,536 [2,013] | 3,448 [1,723] | 3,734 [2,545] | 0.33 | |
| Lymphocytes, /µL | 0.05 (−0.06, 0.16) | 0.37 | 1,109 [474] | 1,103 [463] | 1,123 [502] | 0.74 | |
| Monocytes, /µL | 0.11 (−0.007, 0.22) | 0.07 | 467 [248] | 455 [236] | 495 [273] | 0.23 | |
| Eosinophils, /µL¶¶¶¶ | 0.16 (0.05, 0.27) | 0.006 | 8 [7] | 8 [6] | 10 [7] | 0.32 | |
| CRP, mg/dL¶¶¶¶ | −0.28 (−0.37, −0.17) | <0.0001 | 1.9 [4.1] | 2.3 [3.8] | 1.3 [4.6] | 0.002 | |
| LDH, IU/L | −0.20 (−0.31, −0.09) | 0.0005 | 289 [99] | 297 [101] | 271 [91] | 0.03 | |
| Ferritin, ng/mL¶¶¶¶ | −0.005 (−0.12, 0.11) | 0.94 | 325 [3]† | 328 [3]‡ | 321 [3]§ | 0.87 | |
| HbA1c, % | −0.02 (−0.13, 0.10) | 0.78 | 5.8 [1.0]¶ | 5.8 [1.1]†† | 5.7 [0.9]‡‡ | 0.42 | |
| IgG, mg/dL | 0.005 (−0.11, 0.12) | 0.93 | 1,235 [304]§§ | 1,216 [305]¶¶ | 1,281 [298]††† | 0.10 | |
| IgA, mg/dL | 0.09 (−0.03, 0.21) | 0.12 | 252 [110]§§ | 244 [91]¶¶ | 271 [144]††† | 0.12 | |
| IgM, mg/dL | −0.20 (−0.31, −0.08) | 0.0008 | 97 [53]§§ | 90 [57]¶¶ | 81 [37]††† | 0.0001 | |
| Procalcitonin, ng/mL¶¶¶¶ | −0.15 (−0.26, −0.04) | 0.01 | 0.13 [1.6]‡‡‡ | 0.13 [1.5]§§§ | 0.12 [1.8]¶¶¶ | 0.20 | |
| D-dimer, µg/mL¶¶¶¶ | 0.06 (−0.05, 0.17) | 0.27 | 0.8 [2.0]†††† | 0.7 [1.8]‡‡‡‡ | 0.9 [2.2]§§§§ | 0.02 | |
+, cough symptom positive; −, cough symptom negative; †, n=293; ‡, n=205; §, n=88; ¶, n=281; ††, n=197; ‡‡, n=84; §§, n=279; ¶¶, n=197; †††, n=82; ‡‡‡, n=286; §§§, n=202; ¶¶¶, n=84; ††††, n=298; ‡‡‡‡, n=208; §§§§, n=90; ¶¶¶¶, data expressed as geometric mean [standard deviation]. Remaining data are expressed as mean [standard deviation]. QoL, quality of life; COVID-19, coronavirus disease 2019; LCQ-acute, acute cough with the Leicester Cough Questionnaire; CI, confidence interval; WBC, white blood cell counts; CRP, C-reactive protein; LDH, lactate dehydrogenase; HbA1c, hemoglobin A1c; IgG, immunoglobulin G; IgA, immunoglobulin A; IgM, immunoglobulin M.
Risk factors contributing to impaired cough-specific QoL during COVID-19 infection
Finally, we conducted a multivariate analysis to determine the factors contributing to impaired cough-specific QoL at the time of admission for COVID-19 treatment (Table 4). The applied factors are listed in Table 4. We adopted serum CRP as a systemic inflammatory biomarker in multivariate analysis. Multivariate analysis revealed that young age, female sex, asthma, and dysgeusia and/or dysosmia, along with systemic and respiratory factors such as fever, higher CRP levels, sputum, and dyspnea, contributed to impaired cough-specific QoL at the time of admission for COVID-19 treatment (Model 1 of Table 4). GI symptoms in addition to young age, female sex, asthma, and dysgeusia and/or dysosmia also contributed to impaired cough-specific QoL at the time of admission for COVID-19 when the presence of pneumonia (i.e., Moderate I/II) was adopted as an applied factor instead of respiratory symptoms (Model 2 of Table 4). The presence of pneumonia was not related to impaired cough-specific QoL during COVID-19 infection (Model 2 in Table 4).
Table 4
| Indices | Model 1 | Model 2 | |||||
|---|---|---|---|---|---|---|---|
| Estimates (95% CI) | P value | Standardized β | Estimates (95% CI) | P value | Standardized β | ||
| Age, years | 0.03 (0.005, 0.06) | 0.02 | 0.12 | 0.04 (0.009, 0.07) | 0.004 | 0.14 | |
| Sex (female) | −1.01 (−1.45, −0.58) | <0.0001 | −0.23 | −1.00 (−1.45, −0.55) | <0.0001 | −0.23 | |
| BMI, kg/m2 | −0.02 (−0.10, 0.07) | 0.66 | −0.02 | −0.03 (−0.12, 0.05) | 0.45 | −0.04 | |
| Asthma, + | −0.93 (−1.78, −0.07) | 0.03 | −0.11 | −1.02 (−1.88, −0.15) | 0.02 | −0.12 | |
| Fever (≥37.5 ℃), + | −0.45 (−0.90, −0.01) | 0.045 | −0.11 | −0.36 (−0.81, 0.10) | 0.13 | −0.08 | |
| Fatigue, + | −0.27 (−0.71, 0.16) | 0.22 | −0.06 | −0.34 (−0.79, 0.11) | 0.14 | −0.08 | |
| Appetite loss, + | −0.23 (−0.69, 0.23) | 0.33 | −0.05 | −0.28 (−0.76, 0.19) | 0.24 | −0.06 | |
| GI symptoms, + | −0.46 (−0.93, 0.006) | 0.053 | −0.10 | −0.57 (−1.06, −0.08) | 0.02 | −0.13 | |
| Dysgeusia/dysosmia, + | −0.80 (−1.231, −0.29) | 0.002 | −0.16 | −0.72 (−1.25, −0.19) | 0.008 | −0.14 | |
| Log10 CRP, mg/dL | −1.23 (−1.97, −0.48) | 0.001 | −0.18 | −1.64 (−2.43, −0.86) | <0.0001 | −0.24 | |
| Sputum, + | −0.77 (−1.34, −0.20) | 0.009 | −0.14 | – | – | – | |
| Dyspnea, + | −0.90 (−1.38, −0.42) | 0.0003 | −0.20 | – | – | – | |
| Pneumonia, + | – | – | – | −0.18 (−0.69, 0.33) | 0.49 | −0.04 | |
+, positive for asthma or symptom. QoL, quality of life; COVID-19, coronavirus disease 2019; CI, confidence interval; BMI, body mass index; GI symptoms, gastrointestinal symptoms (nausea, vomiting, and diarrhea); CRP, C-reactive protein.
Discussion
Although this cohort consisted of patients with mild-to-moderate COVID-19 requiring hospitalization, we demonstrated for the first time that young age, female sex, asthma, GI symptoms such as nausea, vomiting, and diarrhea, and dysgeusia and/or dysosmia may impair cough-specific QoL at the time of admission for COVID-19 treatment, together with systemic condition and respiratory symptoms. Meanwhile, the presence of pneumonia with or without hypoxia was not related to impaired cough-specific QoL at the time of admission when considering only patients not requiring mechanical ventilation. Although this may indicate a stronger impact of other symptoms such as dyspnea, fever, and fatigue than that of cough in patients with pneumonia, cough might be a symptom of airway inflammation rather than lung injury. However, further researches are necessary to clarify how pneumonia affects cough-specific QoL and cough frequency in severe COVID-19 cases.
Inherently, cough plays a role in preventing the aspiration of aerosolized pathogens in the airways. Indeed, impaired cough reflex sensitivity and urge-to-cough are risk factors for recurrent (16) and aspiration pneumonia (17). Meanwhile, acute viral URI can transiently increase cough reflex sensitivity; an effect maintained for 4–8 weeks after infection (18). According to previous studies, both aging and sex differences alter cough reflex sensitivity. A previous study showed significantly lower cough frequency induced by inhaled distilled water in elderly healthy subjects than in younger subjects (19). Additionally, cough reflex sensitivity is heightened in healthy women than in men (20). Accordingly, a higher transient cough reflex sensitivity during COVID-19 infection may be more common in young individuals or females.
Cough is one of the typical symptoms of asthma, along with dyspnea and wheezing (21); asthma is the most common cause of chronic cough worldwide (22). Cough in asthma is thought to be provoked not only by classical asthmatic reactions, such as airway inflammation, obstruction, and hyper-responsiveness, but also by cough reflex hypersensitivity caused by airway neuronal dysfunction (23). Acute viral URI is a trigger of asthma exacerbation which activates immune responses, including granulocyte infiltration and interferon production (24). Respiratory viruses can upregulate the expression of several receptors associated with cough induction, including transient receptor potential vanilloid 1 and ankyrin 1, on sensory nerves and airways (TRPV1 and TRPA1, respectively) (25,26). Sensory nerves in the asthmatic airways may be more easily activated by viral infection than those on normal airways. Indeed, cough reflex sensitivity to inhaled capsaicin, an agonist for TRPV1, is greater in patients with asthma than in those without (27), and is associated with poor asthma control and frequent exacerbations in patients with severe asthma (28).
GI symptoms were more prevalent not only in the early phase (5–7 days from the onset) but also in the late phase (12–14 days from the onset) of COVID-19 infection in patients presenting with cough. This suggests that GI symptoms may affect cough-specific QoL for a longer time than other symptoms. Both cough and GI symptoms are exacerbated by gut dysmotility and a vagally mediated reflex between the airways and the upper GI tract (29). According to an epidemiological survey on the prevalence of chronic cough in the general population in the United Kingdom, GI symptoms such as regurgitation and irritable bowel syndrome are risk factors for chronic cough (30). However, we cannot confirm whether underlying GI disorders are associated with the worsening of cough and cough-specific QoL during COVID-19 infection. Additionally, the interaction between cough and GI symptoms remains unclear as we did not evaluate whether cough was preceded by GI symptoms or not in this study.
Despite the similar prevalence of dysgeusia and/or dysosmia between patients presenting with and without cough, they contributed to impaired cough-specific QoL at the time of admission for COVID-19 treatment. Dysgeusia and/or dysosmia were predictive symptoms of SARS-CoV-2 infection, particularly when coexisting with persistent cough, fatigue, and appetite loss (31). This relationship indicates the involvement of neurogenic inflammation in the development of both cough and dysgeusia and/or dysosmia in COVID-19 by sensory neuron sensitization (32). However, there is no evidence that SARS-CoV-2 can directly infect bronchopulmonary sensory nerves and olfactory and trigeminal nerves via angiotensin-converting enzyme 2 and transmembrane serine protease 2 receptors. Further studies are necessary to elucidate the mechanism of neuro-viral interaction in SARS-CoV-2, which might help treating dysgeusia and/or dysosmia and cough during COVID-19 infection.
This study has some limitations. First, this was a retrospective, single-center, cohort study. All presenting symptoms were retrospectively reviewed from medical records. Therefore, the prevalence of all symptoms may have been underestimated. On the other hand, the LCQ-acute scores were considered reliable because the patients completed it at the time of admission. Thus, we used the cough-specific QoL, but not cough prevalence, as a primary endpoint in this study. Second, we could not evaluate the severity of symptoms. Therefore, association between cough-specific QoL and symptom severity remains unclear. Third, we did not assess the improvement in cough-specific QoL after treatment. Cough prevalence at the time of hospital discharge remains unclear. Fourth, the present results are not applicable to all patients with COVID-19 during the acute phase. In addition, we could not recruit patients managed in the community or those with severe disease requiring mechanical ventilation. The factors contributing to impaired cough-specific QoL in the acute phase may differ depending on patient’s condition. Last, we could not measure objective cough frequency using a cough monitor. The factors contributing to increased cough frequency during the acute phase of COVID-19 remain unknown. However, using a cough monitor in patients with COVID-19 was not possible to prevent infection spread.
In summary, using the LCQ-acute, we showed for the first time the factors contributing to impaired cough-specific QoL at the time of admission for COVID-19 treatment. Although most factors such as female sex, asthma, and GI symptoms were consistent with risk factors of chronic cough, dysgeusia, and/or dysosmia were also associated with impaired cough-specific QoL at the time of admission for COVID-19 treatment. The sensory nerve dysfunction triggered by SARS-CoV-2 infection might be associated with the interaction between cough and the above factors. Further studies are necessary to clarify the neuro-viral association in the pathophysiology of cough associated with COVID-19.
Acknowledgments
We thank Drs Takuji Ichihashi (The chairman of Aichi hospital, Okazaki, Aichi, Japan), Yuichiro Shindo, Norihito Omote, Atsushi Suzuki, Toshinori Matsui, Ichidai Tanaka (Nagoya University, Nagoya, Aichi, Japan), Hiroyuki Tanaka, Shigehisa Kajikawa, Toshiyuki Yonezawa, Takuma Katano, Toshio Kato (Aichi Medical University, Nagakute, Aichi, Japan), Takahiro Inoue, Yuri Maeda, Shingo Maeda, Keita Kondo, and Toshikazu Watanabe (Fujita Health University, Toyoake, Aichi, Japan) and all staff (nurses, pharmacologists, radiological technologists, clinical laboratory technicians, and clerks) of Aichi Hospital for their helpful support to see patients with COVID-19 at Aichi Hospital, Okazaki, Aichi, Japan. We also thank Dr. Jennifer Yap Maries Go Yap, Department of Respiratory Medicine, Allergy, and Clinical Immunology for editing the English of this manuscript.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Woo-Jung Song and Kian Fan Chung) for the series “Novel insights into chronic cough” published in Journal of Thoracic Disease. The article has undergone external peer review.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-358/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-358/dss
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-358/coif). The series “Novel insights into chronic cough” was commissioned by the editorial office without any funding or sponsorship. AN serves as an unpaid editorial board member of Journal of Thoracic Disease. YK reports research grants from Novartis Pharma, MSD, and Sanofi corporations, and payment or honoraria for lectures, presentations, or educational events from GSK, AstraZeneca, Kyorin, Sanofi, and Novartis outside the submitted work. AN reports payment or honoraria for lectures, presentations, or educational events from Astellas, AstraZeneca, Kyorin, GSK, MSD, Shionogi, Bayer, Sanofi, Taiho, and Boehringer Ingelheim; and research grants from Astellas, Kyorin, Boehringer Ingelheim, Novartis, MSD, Daiichi Sankyo, Taiho, Teijin, Ono, Takeda, and Sanofi Pharmaceutical outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the ethical board of Aichi Hospital (approval number: 2021-2). Written informed consent was waived by the ethical board owing to the retrospective nature of this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Morice A, Kardos P. Comprehensive evidence-based review on European antitussives. BMJ Open Respir Res 2016;3:e000137. [Crossref] [PubMed]
- Yousaf N, Lee KK, Jayaraman B, et al. The assessment of quality of life in acute cough with the Leicester Cough Questionnaire (LCQ-acute). Cough 2011;7:4. [Crossref] [PubMed]
- Malesker MA, Callahan-Lyon P, Ireland B, et al. Pharmacologic and Nonpharmacologic Treatment for Acute Cough Associated With the Common Cold: CHEST Expert Panel Report. Chest 2017;152:1021-37. [Crossref] [PubMed]
- Dicpinigaitis PV, Eccles R, Blaiss MS, et al. Impact of cough and common cold on productivity, absenteeism, and daily life in the United States: ACHOO Survey. Curr Med Res Opin 2015;31:1519-25. [Crossref] [PubMed]
- Braman SS. Postinfectious cough: ACCP evidence-based clinical practice guidelines. Chest 2006;129:138S-46S. [Crossref] [PubMed]
- Footitt J, Johnston SL. Cough and viruses in airways disease: mechanisms. Pulm Pharmacol Ther 2009;22:108-13. [Crossref] [PubMed]
- World Health Organization. Coronavirus disease (COVID-19) Questions and answers. 2020. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/question-and-answers-hub/q-a-detail/coronavirus-disease-covid-19
- Fu L, Wang B, Yuan T, et al. Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: A systematic review and meta-analysis. J Infect 2020;80:656-65. [Crossref] [PubMed]
- Sonnweber T, Sahanic S, Pizzini A, et al. Cardiopulmonary recovery after COVID-19: an observational prospective multicentre trial. Eur Respir J 2021;57:2003481. [Crossref] [PubMed]
- Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020;323:1061-9. [Crossref] [PubMed]
- Zhang J, Wang X, Jia X, et al. Risk factors for disease severity, unimprovement, and mortality in COVID-19 patients in Wuhan, China. Clin Microbiol Infect 2020;26:767-72. [Crossref] [PubMed]
- Firouzabadi FD, Firouzabadi MD, Ghalehbaghi B, et al. Have the symptoms of patients with COVID-19 changed over time during hospitalization? Med Hypotheses 2020;143:110067. [Crossref] [PubMed]
- Sato R, Gui P, Ito K, et al. Effect of Short-Term Exposure to High Particulate Levels on Cough Reflex Sensitivity in Healthy Tourists: A Pilot Study. Open Respir Med J 2016;10:96-104. [Crossref] [PubMed]
- Biswas M, Rahaman S, Biswas TK, et al. Association of Sex, Age, and Comorbidities with Mortality in COVID-19 Patients: A Systematic Review and Meta-Analysis. Intervirology 2020; Epub ahead of print. [Crossref]
- Kalligeros M, Shehadeh F, Mylona EK, et al. Association of Obesity with Disease Severity Among Patients with Coronavirus Disease 2019. Obesity (Silver Spring) 2020;28:1200-4. [Crossref] [PubMed]
- Niimi A, Matsumoto H, Ueda T, et al. Impaired cough reflex in patients with recurrent pneumonia. Thorax 2003;58:152-3. [Crossref] [PubMed]
- Yamanda S, Ebihara S, Ebihara T, et al. Impaired urge-to-cough in elderly patients with aspiration pneumonia. Cough 2008;4:11. [Crossref] [PubMed]
- Dicpinigaitis PV. Effect of viral upper respiratory tract infection on cough reflex sensitivity. J Thorac Dis 2014;6:S708-11. [Crossref] [PubMed]
- Newnham DM, Hamilton SJ. Sensitivity of the cough reflex in young and elderly subjects. Age Ageing 1997;26:185-8. [Crossref] [PubMed]
- Fujimura M, Kasahara K, Kamio Y, et al. Female gender as a determinant of cough threshold to inhaled capsaicin. Eur Respir J 1996;9:1624-6. [Crossref] [PubMed]
- Mincheva R, Ekerljung L, Bjerg A, et al. Frequent cough in unsatisfactory controlled asthma--results from the population-based West Sweden Asthma study. Respir Res 2014;15:79. [Crossref] [PubMed]
- Niimi A, Ohbayashi H, Sagara H, et al. Cough variant and cough-predominant asthma are major causes of persistent cough: a multicenter study in Japan. J Asthma 2013;50:932-7. [Crossref] [PubMed]
- Niimi A, Fukumitsu K, Takeda N, et al. Interfering with airway nerves in cough associated with asthma. Pulm Pharmacol Ther 2019;59:101854. [Crossref] [PubMed]
- Matsumoto K, Inoue H. Viral infections in asthma and COPD. Respir Investig 2014;52:92-100. [Crossref] [PubMed]
- Abdullah H, Heaney LG, Cosby SL, et al. Rhinovirus upregulates transient receptor potential channels in a human neuronal cell line: implications for respiratory virus-induced cough reflex sensitivity. Thorax 2014;69:46-54. [Crossref] [PubMed]
- Omar S, Clarke R, Abdullah H, et al. Respiratory virus infection up-regulates TRPV1, TRPA1 and ASICS3 receptors on airway cells. PLoS One 2017;12:e0171681. [Crossref] [PubMed]
- Satia I, Tsamandouras N, Holt K, et al. Capsaicin-evoked cough responses in asthmatic patients: Evidence for airway neuronal dysfunction. J Allergy Clin Immunol 2017;139:771-9.e10. [Crossref] [PubMed]
- Kanemitsu Y, Fukumitsu K, Kurokawa R, et al. Increased Capsaicin Sensitivity in Patients with Severe Asthma Is Associated with Worse Clinical Outcome. Am J Respir Crit Care Med 2020;201:1068-77. [Crossref] [PubMed]
- Ing AJ. Cough and gastro-oesophageal reflux disease. Pulm Pharmacol Ther 2004;17:403-13. [Crossref] [PubMed]
- Ford AC, Forman D, Moayyedi P, et al. Cough in the community: a cross sectional survey and the relationship to gastrointestinal symptoms. Thorax 2006;61:975-9. [Crossref] [PubMed]
- Menni C, Valdes AM, Freidin MB, et al. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat Med 2020;26:1037-40. [Crossref] [PubMed]
- Song WJ, Hui CKM, Hull JH, et al. Confronting COVID-19-associated cough and the post-COVID syndrome: role of viral neurotropism, neuroinflammation, and neuroimmune responses. Lancet Respir Med 2021;9:533-44. [Crossref] [PubMed]


