
Utility of single-port laparoscopic retrograde gastric mobilization during McKeown esophagectomy for esophageal cancer: a 2-year experience with 120 cases in a single institution
Introduction
Surgery is the mainstay treatment for resectable esophageal cancer. In addition to ensuring the same radical treatment outcomes, minimally invasive esophagectomy (MIE) significantly limits surgical trauma, reduces the incidence of postoperative complications, and improves patients’ postoperative quality of life (1). At present, thoracoscopy combined with laparoscopic esophagectomy is routinely performed at most centers (2,3). However, controversy continues as to the optimal operative position, number of trocars, and layout (4,5).
Single-port thoracic technology has been successfully applied in lung operations (6,7) and reduces postoperative pain and improves aesthetics. However, few institutions have performed esophagectomy through single-port thoracoscopy, as esophageal mobilization and lymph node dissection are difficult through single-port thoracoscopy, and most esophagectomies are performed in the thoracic procedure (8-10). Yuan et al. (11) made preliminary comparisons between the single-port and 4-port esophagectomy approaches. The average surgical time of the single-port group was 30–40 min longer than that of the multi-port group. The performance of MIE with a single-incision approach using thoracoscopic and laparoscopic procedures simultaneously is even rarer (12). To reduce the difficulty of surgery, surgeons (13,14) often an additional auxiliary port outside the abdominal cavity in the single-port laparoscopic procedure.
Since March 2020, our center has innovatively adopted a pure single-port without an auxiliary port to complete abdominal procedures. However, as the reduction of incisions increases the difficulty of the endoscopic operation, the protocol of gastric mobilization had to be rearranged. We call this procedure single-port laparoscopic retrograde 3-step gastric mobilization (SLRM).
We report our preliminary findings on the use of SLRM in esophageal reconstruction during MIE to treat esophageal cancer and analyze the procedure details and feasibility. The results of this study should provide insights for local hospitals planning to adopt this procedure. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1193/rc).
Methods
Study design
The study was a case series study. We analyzed the clinical data of 120 patients with esophageal cancer who received a single-port laparoscopy combined with thoracoscopic McKeown esophagectomy from March 2020 to November 2021. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Fujian Medical University (No. [2021]300) and individual consent for this retrospective analysis was waived. Preoperative esophagogastroduodenoscopy and pathological examinations confirmed the diagnosis of esophageal squamous cell carcinoma in these patients. All the patients received computed tomography and ultrasound for staging studies. For patients with advanced disease (T3+ or N+), neoadjuvant chemoradiotherapy and postoperative chemoradiotherapy were performed, followed by surgery 4–6 weeks later. All the procedures were performed by the same surgical team. Following hospital discharge, patients were seen every 3 months during the first 2 years. Follow-up included recording of late complications (such as trachaeostomal stenosis and anastomotic stricture), functional outcomes, quality of life and survival rates. Gastroscopy and computed tomography (CT) of the neck, thorax, and upper abdomen were performed annually; all the patients were followed-up until June 2022.
Surgical procedures
All the surgeries were performed under general anesthesia and a single-lumen endotracheal tube with an enclosed bronchial blocker was inserted. The thoracic procedure was performed first.
Thoracic procedure—MIE and mediastinal lymph node dissection
Each patient was placed in the left lateral-prone position. The thoracic area of the operation was carried out under left single-lung ventilation [for details of the position and the port design, see our previous report (15)]. The esophagus was mobilized from the side of the spine upward to the thoracic inlet and downward to the diaphragmatic hiatus. Next, the lymph nodes and soft tissues of the recurrent laryngeal nerve (RLN) were dissected. As the esophagus and trachea were being pressed anteriorly using a special trachea retractor, lymph node dissection was performed along the left RLN up to the thoracic inlet. The remaining mediastinal lymph nodes in the tracheobronchial region and the upper, middle, and lower paraesophageal regions were dissected. Finally, the whole thoracic part of the esophagus was mobilized. A 22-French thoracic tube was inserted into the 6th costal cavity.
Abdominal procedure—SLRM
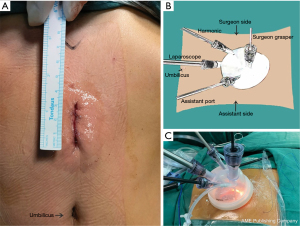
After the patient was placed in the supine position, a 3–4 cm vertical incision (see Figure 1A) was made in the middle between the umbilicus and the xiphoid process. Next, a single-port incision protector (Disposable casing puncture, kit BA, Hangzhou Kangji Medical Devices Co., Ltd., Hangzhou, China) was placed, through which 2 or 3 surgical instruments and a standard 10-mm 30° laparoscope were inserted. The laparoscope was at the 6 o’clock position, and the chief ports were at the 9 and 12 o’clock positions, respectively. The assistant port was at the 3 o’clock position (see Figure 1B,1C). Carbon dioxide was insufflated at 13 mmHg. To expose the surgical field by the gravity retractions from the body weight, the patient’s position was changed frequently from the Trendelenburg to the reverse Trendelenburg position and by tilting from the right to the left side. The lymph nodes were dissected, and the stomach was mobilized during the SLRM (see Figure 2), which is shown in Video 1.
In brief, first, the hepatogastric ligament was dissected to the esophageal hiatus. The left liver lobe was retracted by a modified combined suture technique using 2–0 polypropylene stitches on a straight needle (Prolene®; Ethicon, Somerville, NJ, USA) and 5-mm hemoclips (Weck®; Teleflex, Morrisville, NC, USA) with a self-retaining fixing stitch were passed through the diaphragmatic hiatus and tightened extracorporeally (see Figure 3A). The lymph nodes at the lesser curvature, left gastric artery, celiac trifurcation, and hiatus were dissected during the mobilization of the stomach. The left gastric vessels were then dissected (see Figure 3B). Second, the esophageal hiatus and the abdominal esophagus were dissociated (see Figure 3C,3D). The cardia was cutoff using a 60-mm linear stapler (EndoGIA 60–3.5 mm, Covidien, Mansfield, MA, USA). Finally, the cardia was pulled down by the assistant.
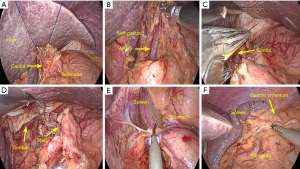
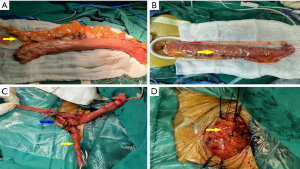
The gastric fundus and greater curvature were mobilized by dividing the short gastric vessels and gastrosplenic ligament, preserving the right gastric and gastroepiploic artery (see Figure 3E,3F). The mobilization continued toward the left colon. The pylorus and duodenum were released. The lymph nodes at the greater curvature were dissected. During this step, a part of the pedicled omentum from the mid-to upper third of the greater curvature was kept intact to wrap around the esophagogastric anastomosis (see Figure 4A). After the stomach was pulled out through the abdominal incision, a 3–4-cm-diameter gastric conduit was constructed using 60-mm linear staplers. The gastric conduit was placed in a plastic protective sleeve and put back into the abdominal cavity (see Figure 4B).
Cervical procedure
An incision of approximately 4 cm was made along the anterior border of the left sternocleidomastoid muscle. The gastric conduit was pulled up to the left neck through the posterior mediastinum. During this process, the gastric conduit was protected by a plastic bag, and the pulling was facilitated by laparoscopic manipulations.
A side-to-side anastomosis of esophagogastric anastomosis was performed by the linear stapler. After being reinforced by interrupted 5-0 silk sutures, the anastomotic area was encircled by the pedicled fat (see Figure 4C,4D), and a closed suction drain was placed in the anastomotic region. 12-French nasogastric tubes were inserted into the gastric conduit for decompression and 14-French nasogastric tubes were inserted into the duodenum for nutrition. No abdominal drain tube was placed.
Statistical analysis
The continuous data are presented as the mean and standard deviation. All the analyses were performed using SPSS software, version 21.0 (SPSS, Inc., Chicago, IL, USA).
Results
A total of 120 patients (88 male and 32 female) were included in the present study. The patients had a median age of 64±2.3 years. The clinical characteristics of the patients are shown in Table 1. None of the patients had to be converted to thoracotomy or laparotomy. There was no additional port placement. No death occurred during the surgeries and hospital stays. Due to an accidental intraoperative puncture by a straight needle, 1 patient suffered from abdominal wall bleeding, but the bleeding stopped following electrocoagulation. The perioperative outcomes and surgical complications are shown in Table 2.
Table 1
| Characteristics | Results |
|---|---|
| Age, years (mean ± SD) | 64±2.3 |
| Sex (male/female) | 88/32 |
| Smoking (yes/no) | 67/53 |
| Hypertension | 32 |
| Diabetes | 26 |
| Neoadjuvant therapy | |
| Chemoradiotherapy/no treatment | 59/61 |
| Location of the tumor | |
| Upper/middle/lower | 16/68/36 |
| Preoperative clinical stage | |
| cT1N0-1M0 | 26 |
| cT2N0-1M0 | 37 |
| cT3N0-1M0 | 57 |
SD, standard deviation.
Table 2
| Outcome measurements | Results |
|---|---|
| Operating time (min), mean ± SD | |
| Total time | 195±20 |
| Chest | 60±18 |
| Abdominal | 43±6 |
| Blood loss (mL), mean ± SD | 65±16 |
| Abdominal blood loss (mL), mean ± SD | 15±5 |
| Harvested lymph nodes (n), mean ± SD | |
| Chest | 13.2±2.7 |
| Abdominal | 10.2±2.5 |
| Postoperative hospital stay (days), mean ± SD | 8.5±4.6 |
| The most common complications, n (%) | |
| Pneumonia | 10 (8.3) |
| Temporary vocal cord paralysis | 20 (16.6) |
| Anastomotic leakage | 3 (2.5) |
SD, standard deviation.
The numbers of mediastinal and abdominal lymph nodes harvested were 13.2±2.7, and 10.2±2.5, respectively. The mean time for the entire procedure was 195±20 min, including the thoracic procedure (60±18 min) and the abdominal procedure (43±6 min). The mean entire blood loss was 65±16 mL, including abdominal blood loss of 15±5 mL. Ambulation was commenced on the 2nd to 3rd day after surgery. On average, the thoracic and cervical drainage tubes were removed 2 days after the operation. The thoracic Abel drainage tube was removed about 5 days after the operation.
Postoperative pneumonia occurred in 10 patients (8.3%), which was successfully treated by antibiotics. Anastomotic leakages were reported in 3 patients (2.5%), who were treated by fully draining the neck incision and applying a wet dressing, and subsequently recovered. Temporary vocal cord paralysis occurred in 20 patients (16.6%), who recovered within 6 weeks. No anastomotic stenosis was recorded in this study. All the patients were discharged after they tolerated the semi-fluid diet. The mean postoperative hospital stay was 8.5±4.6 days.
Detailed outcomes were reviewed in these patients at a mean follow-up duration of 13 months (range, 3–20 months) by hospital visits or telephone. No tumor recurrence occurred, and none of the patients had dysphagia. None of the patients died during the follow-up period.
Discussion
Due to the narrow operating space, a lack of triangular vision in traditional endoscopy, and difficult cooperation between the surgeon and his assistant, very few studies (16-18) have been conducted examining single-port laparoscopic esophagectomy. Additionally, the surgical approach of SLRM has rarely been mentioned previously. In the present study, we sought to evaluate the effectiveness and feasibility of the novel procedure and to share our experience.
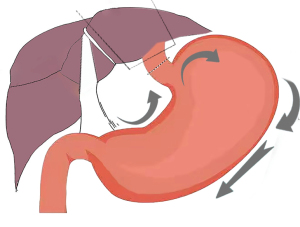
In 2020, SLRM was developed at our center. All the patients underwent the McKeown procedure. This study focused on the abdominal step, operative advantages, and perioperative complications. The procedure can be summarized into the following three steps: (I) the right operation, which includes liver lobe retraction, lesser curvature mobilization, left gastric vessel dissection, and lymph node dissection; (II) the top operation, which involves the dissection of the cardio-esophageal junction. (It should be noted that in patients with a tumor at the lower location, we cut off the lower esophagus in the thoracic cavity and a band was attached to the broken ends of the esophagus); and (III) the left operation, which includes the mobilization of the gastric fundus and the hilum of the spleen, along with lymph node dissection of the greater curvature.
SLRM has a number of obvious advantages. First, compared to the traditional 5-port laparoscope, the single-port laparoscope reduces the number of abdominal incisions to 1. The nerve and muscle compressions caused by the instrument operation are also limited to a 3-cm-incision, which results in less trauma and has better cosmetic effects. Second, we optimized the surgical procedure. Conventional laparoscopic gastric mobilization starts from the greater curvature (19,20). The space is limited by the upper diaphragm. The risk of bleeding from the spleen is high, especially in patients with obesity or bloating. However, SLRM mobilizes the gastric tissue reversely from the lesser curvature to the gastric fundus and the greater curvature. The omental adipose tissue on the greater curvature can be avoided, and the operation is not affected even if there is bloating and the gastrosplenic ligament is clearly exposed. The operation of the upper pole of the spleen, such as the cutting of the gastrosplenic ligaments and short gastric vessels, is unimpeded. Third, when the gastric conduit is constructed and placed back in the abdominal cavity, it is easier to re-establish the pneumoperitoneum with the single-port operation. The gastric conduit can be pulled up through the mediastinum under laparoscopic surveillance. Conversely, the re-establishment of traditional laparoscopic pneumoperitoneum is slightly troublesome.
We have several observation to share about this novel technique. First, our approach benefits from the use of a multichannel single-port incision protector that contains 4 moderately sized ports that are flexible and interchangeable. The base is locked in the abdominal wall. The upper part can be disassembled and assembled freely. Second, the skilled implementation of single-port abdominal surgery also requires a period of training and experience. After the surgical procedure was standardized, the laparoscopic time was reduced to 40 min, while the time of the multi-port laparoscopic procedure is about 60 min (15). Third, some of the surgical techniques are worthy of special attention. The first of these is the site of incision. The most appropriate location for a 3-cm-long incision is in the middle between the umbilicus and the xiphoid process. If the incision location is too low, the view is far away, and the operating instruments can interfere with each other frequently. If the incision location is too high, retracting the liver lobe will be difficult, which can obstruct the surgical field. The second important technique is the retraction process of the left liver lobe. Lakdawala et al. (21) routinely used grasping forceps to elevate the left lobe. Huang et al. (22) recommended inserting needles into the edge of the liver for retraction. We used a 2-0 prolene needle and thread to enter from the left margin of the costal arch, then removed the needle through the abdominal wall on the left of the falciform ligament. The left liver lobe was then retracted and fixed at the hiatus, which provided better exposure to the lesser curvature and the cardia. Fourth, the other important element relates to the skill of the laparoscope holder. The laparoscope should be placed at the lower pole of the incision and pressed down as far as possible. Other surgical instruments should be kept parallel and leveled up without crossing. A small field of vision aperture can enlarge the overall field of vision and reduce the limitation of the narrow surgical space.
In our series, the average number of harvested abdominal lymph nodes was about 10.2, which is similar to that reported in previous meta-analyses (23-25). In terms of intraoperative complications, only 1 patient suffered from abdominal wall bleeding as the result of an accidental needle puncture. For postoperative complications, the report on the McKeown MIE from Japan stated that the median incidences of pneumonia, anastomotic leakage, and RLN palsy were 12.0%, 7.2%, and 19.8%, respectively (26) while our results were 8.3%, 2.5%, and 16.8%, respectively, all of which were lower than those previously reported.
Limitations
We note that this article expresses our personal opinion, and currently there is a lack of sufficient data to compare SLRM with traditional laparoscopic surgery. To address this issue, a single-center randomized controlled study is being conducted at our institution and has been registered at Clinical Trials Registry (No. ChiCTR 2100043730). We look forward to reporting our future results.
Conclusions
The preliminary results demonstrate that SLRM is a technically feasible and safe procedure with an optimal operating process and a single incision. SLRM can be considered an alternative method for patients with esophageal cancer, especially those with obesity and gastric distension. However, it is still in the exploratory stage and requires further investigation.
Acknowledgments
Funding: This study was funded by the Natural Science Foundation of Fujian Province, China (grant No. 2020J01953), and the Scientific Research Project from the Education Department of Fujian Province, China (grant No. JAT190212).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1193/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1193/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1193/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Fujian Medical University (No. [2021]300)
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Straatman J, van der Wielen N, Cuesta MA, et al. Minimally Invasive Versus Open Esophageal Resection. Ann Surg 2017;266:232-6. [Crossref] [PubMed]
- Tanishima Y, Nishikawa K, Yuda M, et al. Feasibility of enhanced recovery protocol in minimally invasive McKeown esophagectomy. Esophagus 2021;18:537-47. [Crossref] [PubMed]
- Wang C, Pan C, Mao W, et al. Thoracolaparoscopic McKeown esophagectomy. J Thorac Dis 2019;11:2564-6. [Crossref] [PubMed]
- Li NL, Peng WL, Liu CC, et al. Comparison of the short-term postoperative results of prone positioning and lateral decubitus positioning during thoracoscopic esophagectomy. Wideochir Inne Tech Maloinwazyjne 2015;10:37-43. [Crossref] [PubMed]
- Kawasaki K, Oshikiri T, Kanaji S, et al. Thoracoscopic esophagectomy in prone position: advantages of five ports over four ports. Hepatogastroenterology 2015;62:69-72. [PubMed]
- Ji C, Xiang Y, Pagliarulo V, et al. A multi-center retrospective study of single-port versus multi-port video-assisted thoracoscopic lobectomy and anatomic segmentectomy. J Thorac Dis 2017;9:3711-8. [Crossref] [PubMed]
- Wang Y, Wang Z, Yao F. The safety and feasibility of three-dimension single-port video-assisted thoracoscopic surgery for the treatment of early-stage lung cancer. J Thorac Dis 2020;12:7257-65. [Crossref] [PubMed]
- Caronia FP, Arrigo E, Failla AV, et al. Uniportal thoracoscopy combined with laparoscopy as minimally invasive treatment of esophageal cancer. J Thorac Dis 2018;10:E265-9. [Crossref] [PubMed]
- Wang Q, Ping W, Cai Y, et al. Modified McKeown procedure with uniportal thoracoscope for upper or middle esophageal cancer: initial experience and preliminary results. J Thorac Dis 2019;11:4501-6. [Crossref] [PubMed]
- Hu W, Yuan Y, Chen L. Single-Port Thoracoscopic Minimally Invasive Esophagectomy for Esophageal Cancer. World J Surg 2019;43:567-70. [Crossref] [PubMed]
- Yuan Y, Liu L, Chen L. Single-port thoracoscopic minimally invasive esophagectomy for esophageal cancer: better for patients and more comfortable for surgeons. Proceedings of the 25th European Conference on Thoracic Surgery. Innsbruck-Austria, 2017:104, 2017.
- Lee JM, Chen SC, Yang SM, et al. Comparison of single- and multi-incision minimally invasive esophagectomy (MIE) for treating esophageal cancer: a propensity-matched study. Surg Endosc 2017;31:2925-31. [Crossref] [PubMed]
- Xin N, Wei R, Huang K, et al. Comparative study on short-term efficacy of single incision plus one (SI+1) port and multiportal 3D laparoscopic minimally invasive esophagectomy. J Gastrointest Oncol 2021;12:1277-84. [Crossref] [PubMed]
- Guo W, Ma L, Zhang Y, et al. Totally minimally invasive Ivor-Lewis esophagectomy with single-utility incision video-assisted thoracoscopic surgery for treatment of mid-lower esophageal cancer. Dis Esophagus 2016;29:139-45. [Crossref] [PubMed]
- Li X, Lai FC, Qiu ML, et al. Minimally Invasive Esophagectomy in the Lateral-prone Position: Experience of 226 Cases. Surg Laparosc Endosc Percutan Tech 2016;26:60-5. [Crossref] [PubMed]
- Huang YH, Chen KC, Lin SH, et al. Robotic-assisted single-incision gastric mobilization for minimally invasive oesophagectomy for oesophageal cancer: preliminary results. Eur J Cardiothorac Surg 2020;58:i65-9. [Crossref] [PubMed]
- Omar MA, Redwan AA, Mahmoud AG. Single-incision versus 3-port laparoscopic cholecystectomy in symptomatic gallstones: A prospective randomized study. Surgery 2017;162:96-103. [Crossref] [PubMed]
- Evers L, Bouvy N, Branje D, et al. Single-incision laparoscopic cholecystectomy versus conventional four-port laparoscopic cholecystectomy: a systematic review and meta-analysis. Surg Endosc 2017;31:3437-48. [Crossref] [PubMed]
- Cheng C, Tsai CY, Chao YK. Laparoscopy versus laparotomy gastric pull-up following thoracoscopic esophagectomy: A propensity score-matched analysis. Asian J Surg 2022;45:468-72. [Crossref] [PubMed]
- Constantinoiu S, Achim F, Constantin A. Use of the Stomach in Esophageal Reconstructive Surgery in Era of Minimally Invasive Approach. Chirurgia (Bucur) 2018;113:809-25. [Crossref] [PubMed]
- Lakdawala M, Agarwal A, Dhar S, et al. Single-incision sleeve gastrectomy versus laparoscopic sleeve gastrectomy. A 2-year comparative analysis of 600 patients. Obes Surg 2015;25:607-14. [Crossref] [PubMed]
- Huang CK, Tsai JC, Lo CH, et al. Preliminary surgical results of single-incision transumbilical laparoscopic bariatric surgery. Obes Surg 2011;21:391-6. [Crossref] [PubMed]
- Coelho FDS, Barros DE, Santos FA, et al. Minimally invasive esophagectomy versus open esophagectomy: A systematic review and meta-analysis. Eur J Surg Oncol 2021;47:2742-8. [Crossref] [PubMed]
- Chowdappa R, Dharanikota A, Arjunan R, et al. Operative Outcomes of Minimally Invasive Esophagectomy versus Open Esophagectomy for Resectable Esophageal Cancer. South Asian J Cancer 2021;10:230-5. [Crossref] [PubMed]
- Wang J, Hu J, Zhu D, et al. McKeown or Ivor Lewis minimally invasive esophagectomy: a systematic review and meta-analysis. Transl Cancer Res 2020;9:1518-27. [Crossref] [PubMed]
- Ozawa S, Koyanagi K, Ninomiya Y, et al. Postoperative complications of minimally invasive esophagectomy for esophageal cancer. Ann Gastroenterol Surg 2020;4:126-34. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)


