Subcostal thoracoscopic extended thymectomy for patients with myasthenia gravis
Introduction
Video-assisted thoracic surgery (VATS) has become a routine technique for extended thymectomy. But it has some drawbacks. When approaching from the side of the chest, it is difficult to identify the contralateral phrenic nerve or to secure a sufficient operative field in the neck region. In addition, intercostal nerve impairment develops along incision sites for port insertion. We previously developed a surgical technique in performing extended thymectomy through subcostal incisions. The aim of this study is to analyze the safety and feasibility of performing subcostal thoracoscopic extended thymectomy (STET) on patients with myasthenia gravis (MG).
Materials and methods
Between February 2015 to November 2015, 15 patients with MG received STET in the Department of Thoracic Surgery at Liuhuaqiao Hospital by the same surgical team. This study was approved by the Liuhuaqiao Hospital Ethics Committee. The operation time, blood loss, duration of postoperative hospital stay, pleural drainage volume in the first 24 hours, postoperatively thoracic drainage periods were retrospected.
Surgical technique
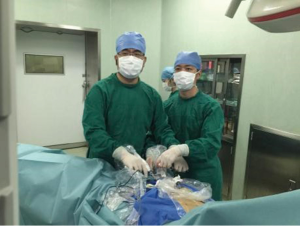
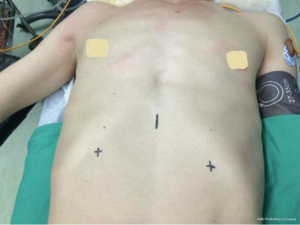
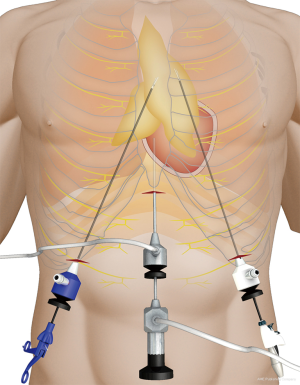
Under general anesthesia and intubation with a single-lumen endotracheal tube, the patient was placed in the type supine position with legs spread apart. The surgeon stood between the patient’s legs while the endoscopist stood on the right side of the patient to operate the camera (Figure 1). The monitor was placed on the right side of the patient. A 1.5-cm thoracoscope incision was made 0.5 cm distal to the inferior margin of the xiphoid process. A midline retrosternal tunnel was created by blunt finger dissection, and the pleura mediastinal was pushed to both sides at the costal arch. Two 0.5-cm operation incisions were created at the intersection point of bilateral midclavicular line and subcostal margin (Figure 2). The long trocars were penetrated into mediastinal cavity and did not go through the pleural cavity. Most dissection was performed by an endoscopic cautery introduced via the working channel or Harmonic scalpel. A long curved-tip ring forceps was introduced from the operation port for traction and dissection of the thymic tissues. These instruments should be guided by the surgeon’s finger to anterior mediastinum. A 10-mm 30 degree angled thoracoscope and artificial pneumothorax were used during the operation. Carbon dioxide (CO2) insufflation collapses the bilateral lungs and pericardium at the pressure of 8 mmHg, thus extending the working space behind the sternum and providing a wide operative field (Figure 3).
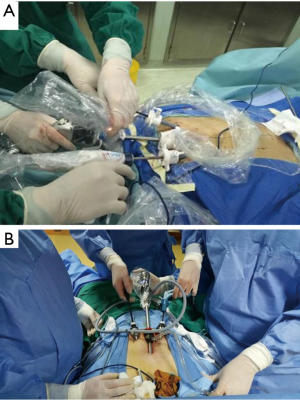
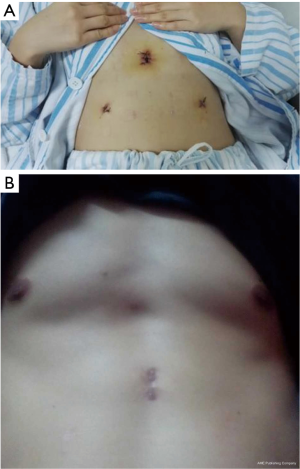
Substernal space was explored first, then right pleural cavity was entered, and the fat pad in the pericardiophrenic sinus was dissected from the pericardium. The direction of dissection was kept cephalad along the anterior border of phrenic nerve, and right lobe of thymus was separated from the underlying ascending aorta. With meticulous dissection, the conjunction of the brachiocephalic vein and superior vena cava was clearly identified. Right thymic horn was dissected and pulled downward to expose the thymic gland, which was pulled to left side using ring forceps, facilitating separation of the gland from its underlying brachiocephalic vein. Switch to left pleural cavity and rapid dissection along left phrenic nerve were performed. Left thymic horn was also dissected completely. Finally, the totally freed thymic gland and perithymic fat tissue could be brought out from the subxiphoid incision. En bloc resection of the thymus was performed by wielding a long curved-tip ring forceps in the left hand and a Harmonic scalpel in the right hand (Figures 4,5). A 20F chest tube was inserted into mediastinal cavity through the thoracoscopic port site for drainage for 13 patients, while for the 2 patients lung dilation was applied and no chest tube was placed. The thoracic tubes were usually removed on the 1st to 2nd postoperative day, and the patients were discharged the following day. Postoperative scars were small, with superior cosmesis as shown in Figure 6.

Results
STET was performed on 15 consecutive patients (Table 1). As shown in Table 2, the mean operation time was 157.53±40.31 min (range, 73–275 min). Mean blood loss was 56.33±7.07 mL (range, 10–200 mL). Mean duration of postoperative hospital stay was 6.13±0.71 days (range, 3–22 days). Mean pleural drainage volume in the first 24 hours was 72.67±17.68 mL (range, 0–250 mL). Mean postoperative thoracic drainage periods were 1.20±0.71 days (range, 0–3 days). One leptosomic patient needed to add one more 1.5 cm intercostal incision to use the vascular clips to control bleeding. All the other surgeries were finished successfully and no patients converted to sternotomy. Except for two patients who needed prolonged mechanical ventilatory support, there were no other perioperative complications or mortalities. The postoperative pathological diagnosis of the fifteen cases was as follows: Masaoka stage I thymoma in six cases (Type A Thymoma in one patients, B1 Thymoma in three patients, B2 Thymoma in two patients), thymic cyst in one case, and true thymic hyperplasia in eight patients (Table 2).

Full table

Full table
Comments
Since Blalock et al. first introduced thymectomy in 1939, median sternotomy has been the gold standard for treating myasthenia and anterior mediastinal tumor (2). With the development of endoscopic instrument technology and video-assisted surgery, the lateral intercostal approach in VATS thymectomy has been gradually applied in anterior mediastinal tumor resection. However, it is usually difficult for surgeons to make a complete mediastinal fatty tissue dissection and identify the phrenic nerve on the contralateral side. Uniportal bilateral video-assisted thoracoscopic extended thymectomy has been reported by Francesco Paolo Caronia, a procedure in which the thymus is removed through two ports (3). Although this transthoracic thoracoscopic thymectomy is minimally invasive, it still need to make two intercostal incisions and this intercostal approach from the sides of the chest is always associated with persistent postoperative pain and intercostal nerve damage. To achieve a better view of the bilateral pleural cavities and more radical thymectomy, in 2002 Hsu et al. first reported performing extended thymectomy through subxiphoid approach (4). Their approach needed to create two additional ports sitting at the bilateral intercostal space and it is possible cause intercostal nerve paralysis or neuralgia. Since Suda et al. described their first experiences of thymectomy without any intercostal incision in 2012, CO2 insufflation is applied to thymectomy through subxiphoid approach (5). The subxiphoid uniportal thoracoscopic approach has been proved feasibility for extended thymectomy (5-7). However this procedure needs to remove the xiphoid process and a 3–4 cm transverse incision, and it needs some special surgical instruments such as autonomy grasper (7). Differential lung ventilation was also needed in the subxipoid uniportal thoracoscopic thymectomy and that may damage lung function.
The STET approach does not cause postoperative intercostal neuropathy and postoperative pain was minimized as the intercostal space is bypassed. In addition, dual-operation-port thymectomy has eliminated the risk of interference between forceps operated both hands of surgeon and has thereby significantly simplified operative procedures. Since it is not required to go through the chest, it is not necessary to double lumen endotracheal intubation, nor a single lung ventilation, thereby avoiding the throat and lung injury. With the help of artificial pneumothorax and the thoracoscope incision which was made over the subxiphoid area, the visual operative field of this approach was obtained as same as that of sternotomy. Another advantage of this new technique is that identification of the cervical region and bilateral phrenic nerves is easier with this procedure than with VATS approached from the side chest. A surgical risk of thymectomy is vascular injury which needs emergency transit thoracotomy. Because the patient is in supine position, transit thoracotomy is extremely convenient. To learning curve, subxiphoid and subcostal approach for thoracoscopic thymectomy may be easier for the surgeon to complete the operation. After experiencing individual learning curves, about four patients, we now complete thymectomy in about 100 min in most patients. We think that surgeons should master this technique in order for patients to reap benefits such as pain reduction and excellent aesthetic outcome. The learning curve of this procedure is shorter in surgeons who are already well experienced in other thoracoscopic procedures. Because of the little volume of postoperative drainage of the early patients, we have attempted not to place chest tube for the three patients. At the end of the operation, the gas in the thoracic cavity was discharged by lung dilation, then the wound was sutured. Postoperative chest X-ray show there was no effusion, no pneumothorax, and no reintubation was happened.
The effect of bilateral artificial pneumothorax on the heart and lung function is still unknown. In our experience to date, low-pressure CO2 insufflation within the mediastinum does not alter the patients’ hemodynamic status regardless of continuity of the unilateral or bilateral pleura. Moreover, a large amount of data suggest that artificial pneumothorax under two-lung ventilation is beneficial for maintaining stable hemodynamics and oxygenation in thoracoscopic esophagectomy in prone position (8).
Conclusions
In conclusion, STET is a novel and minimally invasive approach that avoids the postoperative intercostal neuropathy and minimizes postoperative pain.
Acknowledgements
Funding: This work was supported by Medical Scientific Research Foundation of Guangdong Province, China (A2015405).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Tang Y, Ou ZA, Qiao GB, et al. The technique of subcostal thoracoscopic extended thymectomy (STET). Asvide 2016;3:147. Available online: http://www.asvide.com/articles/888
- Blalock A, Mason MF, Morgan HJ, et al. Myasthenia gravis and tumors of the thymic region: report of a case in which the tumor was removed. Ann Surg 1939;110:544-61. [Crossref] [PubMed]
- Caronia FP, Fiorelli A, Santini M, et al. Uniportal bilateral video-assisted thoracoscopic extended thymectomy for myasthenia gravis: A case report. J Thorac Cardiovasc Surg 2015;150:e1-3. [Crossref] [PubMed]
- Hsu CP, Chuang CY, Hsu NY, et al. Subxiphoid approach for video-assisted thoracoscopic extended thymectomy in treating myasthenia gravis. Interact Cardiovasc Thorac Surg 2002;1:4-8. [Crossref] [PubMed]
- Suda T, Sugimura H, Tochii D, et al. Single-port thymectomy through an infrasternal approach. Ann Thorac Surg 2012;93:334-6. [Crossref] [PubMed]
- Wu L, Lin L, Liu M, et al. Subxiphoid uniportal thoracoscopic extended thymectomy. J Thorac Dis 2015;7:1658-60. [PubMed]
- Suda T, Hachimaru A, Tochii D, et al. Video-assisted thoracoscopic thymectomy versus subxiphoid single-port thymectomy: initial results†. Eur J Cardiothorac Surg 2016;49 Suppl 1:i54-i58. [PubMed]
- Saikawa D, Okushiba S, Kawata M, et al. Efficacy and safety of artificial pneumothorax under two-lung ventilation in thoracoscopic esophagectomy for esophageal cancer in the prone position. Gen Thorac Cardiovasc Surg 2014;62:163-70. [Crossref] [PubMed]