Impact of viral infection on acute exacerbation of asthma in out-patient clinics: a prospective study
Introduction
Acute exacerbations are the source of substantial asthma healthcare costs, and severe asthma exacerbations may be life threatening (1). It was reported that the average cost for a patient with an acute exacerbation is 3.5 times higher than that for a patient without one (2). Although multiple factors, such as allergen exposure, infection, air pollution, medication, and weather change, have been reported to be involved in asthma exacerbations, viral respiratory tract infection is considered a very common trigger (3-5). One study detected respiratory viruses in 50% of adult asthma patients having an acute exacerbation (3). A study of non-hospitalized asthma exacerbations in children reported that the presence of a viral respiratory illness was associated with more severe asthma symptoms, but had no impact on the recovery time (6). However, there are inconsistencies between different study reports (7). Corne (8) reported in a study that people with atopic asthma had more severe and longer-lasting lower-respiratory-tract symptoms than healthy individuals. The purpose of the current study was to explore the prevalence and potential roles of viral respiratory tract infection as a trigger of asthma exacerbations using subjects from out-patient clinics.
Materials and methods
Subjects
Subjects with stable asthma or experiencing an asthma exacerbation were recruited from the out-patient clinics and the Emergency Department of the First Affiliated Hospital of Guangzhou Medical University between February 2012 and February 2013. The inclusion criteria were as follows: (I) diagnosis of asthma was confirmed with a clinical profile and spirometry results according to the criteria described by the Global Initiative for Asthma (GINA) guidelines (1); (II) aged between 16 and 70 years; and (III) agreed to provide informed consent to participate in this study. The exclusion criteria were as follows: (I) major systemic disorders or respiratory disorders other than asthma, such as chronic obstructive pulmonary disease or bronchiectasis; (II) a history of psychiatric disorders, alcoholism, drug abuse, or persistent use of β2 blockers; and (III) other disorders that can result in wheezing. Age-matched healthy volunteers, who had no respiratory symptoms within the previous 4 weeks, were also recruited as members of our control group during the same period (from February 2012 to February 2013).
Study design
A prospective controlled observational study was employed to compare asthma exacerbation in viral-positive subjects with that in viral-negative subjects. The estimated number of subjects needed for this study was calculated by assuming that the viral-positive rate would be 44% in subjects with asthma exacerbation (3-5) and 18% in subjects with stable asthma (9). With alpha set at 0.05, the estimated number of required subjects (10) was 61 cases.
Measurement
The presence of respiratory viruses in the nasal swab samples was detected with TaqMan real-time polymerase chain reaction (PCR) (11,12) using Respiratory Viruses Detection Kid (Guangzhou HuYanSuo Medical Technology Co., Ltd). DNA or RNA from respiratory samples was extracted using a QIAamp DNA Mini Kit or a QIAamp Viral RNA Mini Kit (Qiagen Co. Ltd., Shanghai, China), respectively, in accordance with the manufacturer’s protocols. The viruses assessed in this study were influenza A (FluA), influenza B (FluB), respiratory syncytial virus (RSV), adenovirus (ADV), human parainfluenza viruses (hPIVs), rhinoviruses (RV, including most serotypes of human rhinovirus groups A, B, and C), enterovirus (EV), human metapneumovirus (hMPV), human coronaviruses (hCoV-229E, OC43, NL63, and HKU1), and human bocavirus (hBoV). The virus detection was conducted by special technicians in the State Key Laboratory for Respiratory Disease, National Clinical Research Center for Respiratory Disease.
Asthma symptom scores were assessed with an Asthma Control Questionnaire (ACQ) (13), an Asthma Control Test (ACT) (14), and a Mini-Asthma Quality of Life Questionnaire (Mini-AQLQ) (15). Pulmonary function was assessed with spirometry.
Other symptoms, such as fever, headache, myalgia, pharyngalgia, nasal discharge, sneezing, cough, dyspnea, and night wakening, were also recorded and evaluated with arbitrary units from 0 (null) to 3 (severe).
The management of asthma exacerbation in each subject was consistent with the GINA recommendations (1,16). All of the patients were followed up for 4 weeks with an asthma diary and peak expiratory flow (PEF) monitoring (17-19). At the end of the 4-week follow-up, patients were re-evaluated with viral detection and spirometry.
This study was approved by the Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University [2012-30]. The research protocol was registered on the Registry Center of Chinese Clinical Research (chictr.org, ChiCTR-OCC-12002935). Written informed consent was obtained for every subject involved in this study.
Statistics
Statistical analyses were performed using an Statistical Product and Service Solutions (SPSS, IBM®) 16.0 package. Normally distributed data were compared by t-tests and are expressed as their mean (standard deviation, SD). The significance of changes in paired data was analyzed by paired t-tests. Skewed data were analyzed by Mann-Whitney U tests or Wilcoxon signed ranks tests and are expressed as the median (interquartile range). Two-tailed P values less than 0.05 are considered significant.
Results
Patient demographics
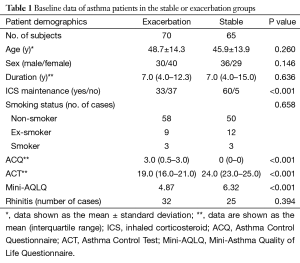
A total of 70 subjects with asthma exacerbation (exacerbation group) and 65 subjects with stable asthma (stable group) were recruited from February 2012 to February 2013. A total of 134 age-matched normal volunteers were recruited as controls (normal group). In the exacerbation group, 70 (100%) initial nasal swabs were obtained. The average number of days after exacerbation onset before sample collection was 5.5 (range, 3–7) days. Of the 70 exacerbation group cases, 34 (48.6%) completed the 4-week follow-up and follow-up nasal swab samples for use in viral detection. Nasal swab samples were obtained for viral detection in the 65 stable asthma group patients and the 134 normal control group patients.
The demographic data from the stable and exacerbation asthma groups are shown in Table 1. In the exacerbation group, the ACQ and ACT scores were higher (all P<0.001) and the rate of inhaled corticosteroid (ICS) maintenance therapy was lower than those in the stable group (47.1% vs. 92.3%, P<0.001). There were no significant differences in the duration of asthma history, smoking status, or comorbidity of rhinitis between the stable and exacerbation groups (all P>0.05).
Full table
Results of respiratory virus detection
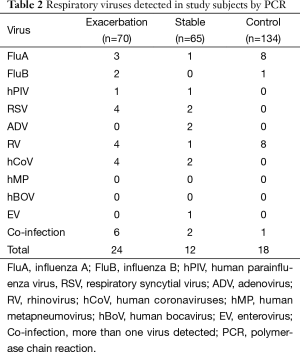
The presence of respiratory viruses in the patient samples was detected by PCR (Table 2). The viral-positive rate of samples from the exacerbation group was 34.2% (24/70), which is significantly higher than that for samples from the stable asthma group (12/65; 18.5%; P=0.038) or from the normal control group (18/134; 13.4%; P<0.001). There was no significant difference between the viral-positive rates of the stable and control groups (P=0.279). Of the tested viruses, the most common viral yields were HCoV (eight cases), Flu A (six cases), RSV (five cases), RV (four cases), and hPIV (three cases). In the exacerbation group, samples from six cases (8.5%) were positive for more than one virus, according to the PCR results. No human metapneumovirus or human bocavirus was detected in our samples.
Full table
Among the viral-positive subjects, the viral load (number of viral copies) was significantly higher in the acute asthma exacerbation group [(5.00±4.63) ×107 copies/L] (Mean ±SD) than in the stable asthma group [(1.24±1.44) ×106 copies/L; P<0.001] or in the healthy control group [(1.44±0.44) ×106 copies/L; P<0.001]. However, there was not a significant difference between the viral loads of the stable asthma and healthy control groups (P=0.774).
Clinical characteristics of asthma exacerbation
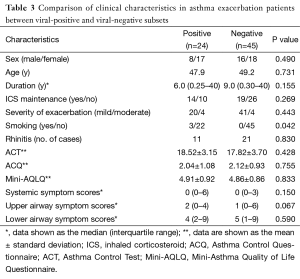
No significant differences were found between the virus-positive and virus-negative subjects within the asthma exacerbation group (all P>0.05) for their severity of exacerbation, ACT score, ACQ score, mini-AQLQ score, general symptoms score, upper respiratory tract symptoms score, and lower respiratory tract symptoms score, although the smoking rate was higher in the viral-positive subgroup (12%; 3/25) than in the viral-negative subgroup (0%; 0/45; P=0.042), (Table 3).
Full table
Effect of being viral positive on asthma convalescence
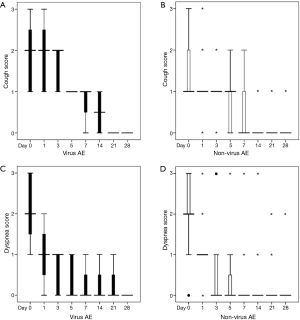
The dynamic symptom changes during the observation period are shown in Figure 1. In comparison with the viral-negative subgroup, the cough score in the viral-positive subgroup of asthma exacerbation was significantly higher on days 1, 3, and 14 {[day 1: 2 [1, 3] [median (interquartile range)] vs. 1 [1, 1]; day 3: 2 [1, 2] vs. 1 [1, 1]; day 14: 1 [0, 1] vs. 0 [0, 0]; P=0.016; P=0.004, and P<0.05, respectively}. However, no statistically significant differences were observed on any other days during the observation period. However, there were no significant differences in the other evaluated symptoms, such as dyspnea and total time for recovery (P>0.05) between these two subgroups. The total convalescence time was similar in each of the two subgroups {viral positive group: 9.5 days (range, 9–12 days) [median (interquartile range)] vs. viral-negative group: 8.5 days (range, 6–15 days); P>0.05}.
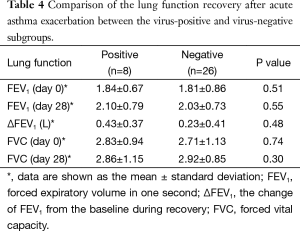
Of the 70 patients with asthma exacerbation, 34 (48.6%) completed a 4-week follow-up. The lung function recovery after exacerbation of these subjects is shown in Table 4. There was no difference in the recovery of lung function between the viral-positive and viral-negative subgroups. Although PEF monitoring was meant to be included in this study, only 62% (21/34) of the subjects actually performed PEF monitoring, and of those who did perform the PEF monitoring, poor compliance with the protocol was common. Because of this, we were unable to include PEF data in our analyses.
Full table
Discussion
Although many studies have investigated the connection between viruses and asthma, few studies have focused on the impact of respiratory viruses on the recovery from asthma exacerbation. Here, we investigated the potential impacts of respiratory viral infection on asthma symptoms and recovery by comparing the dynamic changes in symptoms and lung function during the observation period between groups of asthma exacerbation patients that were respiratory virus positive (viral positive) or virus negative. Additionally, this study describes the prevalence of respiratory viral infection as a cause of acute exacerbation of asthma in the local area of Guangzhou, which is in the southern part of China. The positive rate of respiratory viral detection for our subjects was 34.2%, which is similar to previous reports in the literature (4).
For this type of study, the use of reliable methodology for detecting respiratory viruses is crucial. Previous reports indicate that the PCR method can be used to detect common respiratory viruses with both good sensitivity and good specificity (11-12,20), and they recommend it as the first choice for clinical diagnosis. Therefore, we used PCR for respiratory virus detection in this study. Although we had originally planned to include the detection of convalescence antibodies and virus culture in our study protocol, we failed to acquire convalescent blood samples from the majority of patients owing to a low compliance rate for the follow-up clinical visit 4 weeks after exacerbation. Additionally, virus culture is well known for its low yield rate and is not recommended as a routine method for clinical detection (21,22).
Other important factors in the asthma exacerbation induced by respiratory viruses are the identity and number of viral species involved. A variety of viruses might be involved in the exacerbation of asthma. Several reports showing that viral infection is an important trigger for asthma exacerbation (3-5,23) included only five or six viral species. Here, we aimed to include as many respiratory viruses as possible; we detected 13 different viral species in our samples. In agreement with previous reports, the most commonly detected viruses in our study were influenza A, influenza B, human parainfluenza virus, RSV, human coronavirus, and rhinovirus (4). Although human metapneumovirus has been commonly detected during asthma exacerbation in children (24), we failed to find any human metapneumovirus in our cohort of subjects.
It remains controversial whether positive PCR results always represent true respiratory viral infections or if they could result from a bystander virus. To address this issue, we included stable asthma and healthy subjects as control groups in our study. Although respiratory viruses were detected by PCR in approximately 10–15% of the stable asthma and healthy subjects, the viral-positive rate was significantly higher in the asthma exacerbation group and there was no significant difference in this rate between the stable asthma and healthy control groups. These results are similar to those reported by Corne et al. (8). Furthermore, our results using the quantitative PCR method show that the viral load (viral copy number) was significantly higher in the exacerbation group than in the stable group. Further studies are still needed to establish a cut-off value for discriminating between true infection and bystander viruses.
Asthma patients are predisposed to infections with respiratory viruses because the epithelia damage caused by uncontrolled asthma makes them more susceptible to these infections (25). The use of ICS can restore intact epithelia and reduce the incidence of respiratory viral infections (26). One report indicates that a lack of ICS maintenance therapy is a risk factor for respiratory infection in asthma patients (25), which is consistent with the results of our study. Additionally, smoking has been shown as another important risk for susceptibility to respiratory viral infections (25), especially for rhinovirus infection (26), which is also consistent with our results.
The impact of viral infection on asthma exacerbation remains an important issue. Interestingly, there have been reports that viral infections interact with allergen exposure in triggering asthma exacerbation (23). Duff et al. (27) reported that the co-existence of high IgE levels and rhinovirus infection increased the risk of asthma exacerbation with an odds ratio of 10.8. Allergen exposure may increase the expression of intercellular adhesion molecule-1, which is the cell surface receptor for rhinovirus and facilitates its entry (28,29). However, rhinovirus infection has been shown to promote airway hypersensitivity and eosinophil, neutrophil, and lymphocyte inflammation in an ovalbumin-sensitized mouse model (30). A study by Message et al. (31) found that rhinovirus infection in an asthma patient increased both his asthma symptoms and airway eosinophil inflammation. However, in our study, which agrees with a similar report from Hongkong (6), there were no significant differences between the viral-positive and viral-negative asthma exacerbation subgroups in their overall asthma severity, CAT score, or respiratory symptoms at the first visit during acute exacerbation. The reasons for this discrepancy are unclear, but could include the differences in our study populations. In our study, we only included asthma exacerbations that could be managed in out-patient clinics, so we did not include hospitalized patients. Whether or not respiratory viral infection is more important in asthma exacerbations that require hospitalization or in children still remains unknown. However, our study found that cough symptoms were more prominent and persistent in the viral-positive subgroup than in the viral-negative subgroup, indicating that respiratory viral infection does have an impact on asthma exacerbation. Further investigations on the potential role of respiratory viral infection on asthma exacerbation should be conducted with larger sample sizes, on patients with severe asthma exacerbations, and on patients prone to infections with respiratory viruses, such as children or the elderly.
Conclusions
Respiratory viruses were detected in 34% of the asthma patients experiencing an acute exacerbation. The patients infected with these viruses had more prominent and persistent cough symptoms, suggesting that respiratory viruses are involved in the acute exacerbation of asthma. The potential effects of respiratory viral infection on severe asthma exacerbations and on asthma in children or in the elderly deserve further study.
Acknowledgements
This work was supported by the State Key Laboratory for Respiratory Disease (Guangzhou Medical University).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Global Initiative for Asthma (GINA). GINA report: Global strategy for asthma management and prevention 2011 (update) 2011. Available from: http://www.ginasthma.org/, accessed on December 13th, 2011.
- Hoskins G, McCowan C, Neville RG, et al. Risk factors and costs associated with an asthma attack. Thorax 2000;55:19-24. [Crossref] [PubMed]
- Tan WC, Xiang X, Qiu D, et al. Epidemiology of respiratory viruses in patients hospitalized with near-fatal asthma, acute exacerbations of asthma, or chronic obstructive pulmonary disease. Am J Med 2003;115:272-7. [Crossref] [PubMed]
- Teichtahl H, Buckmaster N, Pertnikovs E. The incidence of respiratory tract infection in adults requiring hospitalization for asthma. Chest 1997;112:591-6. [Crossref] [PubMed]
- Nicholson KG, Kent J, Ireland DC. Respiratory viruses and exacerbations of asthma in adults. BMJ 1993;307:982-6. [Crossref] [PubMed]
- Chang AB, Clark R, Acworth JP, et al. The impact of viral respiratory infection on the severity and recovery from an asthma exacerbation. Pediatr Infect Dis J 2009;28:290-4. [Crossref] [PubMed]
- Louis R, Demarche S, Schleich F. A historical perspective: Are inhaled corticoids sufficient to control asthma? J Transl Intern Med 2015;3:113-5.
- Corne JM, Marshall C, Smith S, et al. Frequency, severity, and duration of rhinovirus infections in asthmatic and non-asthmatic individuals: a longitudinal cohort study. Lancet 2002;359:831-4. [Crossref] [PubMed]
- Harju TH, Leinonen M, Nokso-Koivisto J, et al. Pathogenic bacteria and viruses in induced sputum or pharyngeal secretions of adults with stable asthma. Thorax 2006;61:579-84. [Crossref] [PubMed]
- Shi JP. The methods of Simple Size estimation in clinical study. Chin J Clin Rehabil 2003;7:1567-9.
- Liu WK, Chen DH, Liu Q, et al. Detection of human bocavirus from children and adults with acute respiratory tract illness in Guangzhou, southern China. BMC Infect Dis 2011;11:345. [Crossref] [PubMed]
- Liu WK, Liu Q, Chen DH, et al. Epidemiology and clinical presentation of the four human parainfluenza virus types. BMC Infect Dis 2013;13:28. [Crossref] [PubMed]
- Juniper EF, O'Byrne PM, Guyatt GH, et al. Development and validation of a questionnaire to measure asthma control. Eur Respir J 1999;14:902-7. [Crossref] [PubMed]
- Nathan RA, Sorkness CA, Kosinski M, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol 2004;113:59-65. n. [Crossref] [PubMed]
- Juniper EF, Guyatt GH, Cox FM, et al. Development and validation of the Mini Asthma Quality of Life Questionnaire. Eur Respir J 1999;14:32-8. [Crossref] [PubMed]
- Lu M, Yao W. Asthma and chronic obstructive pulmonary disease overlap syndrome: An update. J Transl Intern Med 2015;3:144-6.
- Li JT. Home peak expiratory flow rate monitoring in patients with asthma. Mayo Clin Proc 1995;70:649-56. [Crossref] [PubMed]
- Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005;26:319-38. [Crossref] [PubMed]
- Santanello NC, Barber BL, Reiss TF, et al. Measurement characteristics of two asthma symptom diary scales for use in clinical trials. Eur Respir J 1997;10:646-51. [PubMed]
- Yang ZF, Zhan YQ, Chen RC, et al. A prospective comparison of the epidemiological and clinical characteristics of pandemic (H1N1) 2009 influenza A virus and seasonal influenza A viruses in Guangzhou, South China in 2009. Jpn J Infect Dis 2012;65:208-14. [Crossref] [PubMed]
- Xiang X, Qiu D, Chan KP, et al. Comparison of three methods for respiratory virus detection between induced sputum and nasopharyngeal aspirate specimens in acute asthma. J Virol Methods 2002;101:127-33. [Crossref] [PubMed]
- Kuypers J, Campbell AP, Cent A, et al. Comparison of conventional and molecular detection of respiratory viruses in hematopoietic cell transplant recipients. Transpl Infect Dis 2009;11:298-303. [Crossref] [PubMed]
- Green RM, Custovic A, Sanderson G, et al. Synergism between allergens and viruses and risk of hospital admission with asthma: case-control study. BMJ 2002;324:763. [Crossref] [PubMed]
- Williams JV, Crowe JE Jr, Enriquez R, et al. Human metapneumovirus infection plays an etiologic role in acute asthma exacerbations requiring hospitalization in adults. J Infect Dis 2005;192:1149-53. [Crossref] [PubMed]
- Wark PA, Johnston SL, Bucchieri F, et al. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med 2005;201:937-47. [Crossref] [PubMed]
- Venarske DL, Busse WW, Griffin MR, et al. The relationship of rhinovirus-associated asthma hospitalizations with inhaled corticosteroids and smoking. J Infect Dis 2006;193:1536-43. [Crossref] [PubMed]
- Duff AL, Pomeranz ES, Gelber LE, et al. Risk factors for acute wheezing in infants and children: viruses, passive smoke, and IgE antibodies to inhalant allergens. Pediatrics 1993;92:535-40. [PubMed]
- Bianco A, Whiteman SC, Sethi SK, et al. Expression of intercellular adhesion molecule-1 (ICAM-1) in nasal epithelial cells of atopic subjects: a mechanism for increased rhinovirus infection? Clin Exp Immunol 2000;121:339-45. [Crossref] [PubMed]
- Canonica GW, Ciprandi G, Pesce GP, et al. ICAM-1 on epithelial cells in allergic subjects: a hallmark of allergic inflammation. Int Arch Allergy Immunol 1995;107:99-102. [Crossref] [PubMed]
- Almirall J, González CA, Balanzó X, et al. Proportion of community-acquired pneumonia cases attributable to tobacco smoking. Chest 1999;116:375-9. [Crossref] [PubMed]
- Message SD, Laza-Stanca V, Mallia P, et al. Rhinovirus-induced lower respiratory illness is increased in asthma and related to virus load and Th1/2 cytokine and IL-10 production. Proc Natl Acad Sci U S A. 2008;105:13562-7. [Crossref] [PubMed]


