Efficacy and safety of sivelestat sodium for the treatment of inflammatory response in acute Stanford type A aortic dissection: a retrospective cohort study
Introduction
With an in-hospital mortality rate of 5–30% (1), acute Stanford type A aortic dissection (ATAAD) is one of the most lethal emergency procedures. Surgery remains the mainstay of treatment for ATAAD, during which cardiopulmonary bypass (CPB) is often required. CPB disrupts homeostasis within the human body, causing disorders of the nervous and endocrine systems, which in turn result in systemic inflammatory response syndrome (SIRS). If not effectively controlled, SIRS can cause sepsis, multiple organ dysfunction syndrome, and even death (2). SIRS following surgery dramatically increases the postoperative complications and mortality rate of patients who have undergone CPB.
Neutrophil elastase (NE) plays an important role in the inflammatory response by activating and processing tumor necrosis factor alpha, interleukin-1 (IL-1), interleukin-6 (IL-6), interleukin-8 (IL-8), and other pro-inflammatory cytokines (3). Sivelestat sodium is a NE inhibitor that can selectively inhibit the injury of NE to lung tissue and reduce inflammatory response. The efficacy of sivelestat on post perfusion lung, ischemia-reperfusion and endothelial injuries has been demonstrated in several investigations. The report showed the protective effects of this drug on pulmonary function after CPB in experiments (4-6). It has been reported that post-operative sivelestat administration after surgical operation improved the pathophysiological condition of SIRS and the post-operative clinical course. There is currently no effective drug for improving the associated systemic inflammatory response. In this study, we explored the efficacy and safety of sivelestat sodium in treating postoperative SIRS in ATAAD patients. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1220/rc).
Methods
Subjects
The clinical data of 71 ATAAD patients who received surgical treatment at our center from January 2021 to December 2021 were retrospectively analyzed. This study was approved by the Institutional Review Board (IRB) of our hospital (ethical approval No. WAGHMEC-KT-2022012). As a retrospective study, the requirement of informed consent was waived. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Patients were divided into two groups according the date of surgery, September 2021, when we started sivelestat administration. The sivelestat sodium group (n=20), operated after September 2021, was administrated sivelestat. The control group (n=51), operated before September 2021, was not administrated. To be eligible for inclusion in this study, patients had to meet the following inclusion criteria: (I) have ATAAD as confirmed by coronary computed tomographic angiography; (II) have undergone surgical treatment of ATAAD via CPB; (III) received mechanical ventilation and met the ARDS Berlin criteria and (IV) be aged 25–75 years. Patients were excluded from the study if they met any of the following exclusion criteria: (I) had 3 or more damaged organs and/or organ failure; (II) had iatrogenic ATAAD; and/or (III) died within 24 hours of admission.
Study methods
The 71 patients were divided into the following two groups based on the use (or lack of use) of sivelestat sodium (HuilunBio, Shanghai, China): (I) the sivelestat sodium group (n=51); and (II) the control group (n=20). Sivelestat sodium, which was administered only in the sivelestat sodium group, was given as a continuous intravenous infusion at 0.2 mg/kg/h (300 mg sivelestat sodium dissolved in 50 mL of 0.9% normal saline) for 3–7 days. An ICU physician made a diagnosis of ARDS on admission to the ICU and also made the decision whether to use sivelestat. The following conventional treatments were provided to patients in both groups: (I) mechanical ventilation via a Dräger ventilator (tidal volume: 6–8 mL/kg; inspiratory oxygen concentration: 40–100%; ventilation frequency: 16–18 times/min; positive end-expiratory pressure: 5–12 cmH2O; and inspiratory/expiratory ratio: 1.0:2.0–1.0:2.3); prone ventilation was administered if necessary; (II) antimicrobial therapy: Blood, sputum, and other specimens were collected from the patients for microbial testing. Antimicrobials were administered based on the results of the etiological data, pathogen cultures, and drug susceptibility tests; (III) sufentanil and propofol were used for analgesia and sedation, and a sedation score of –2 to –3 was therapeutically targeted; (IV) nutritional support was provided to patients with a targeted NRS-2002 (Nutrition Risk Screening, 2002) score of >3 points, a target energy intake of 25–30 kcal/kg (ideal body weight), and a target protein level of 1.2–2.0 g/kg; (V) rehabilitation measures, including passive and active rehabilitation, were implemented as soon as possible; (VI) organ function support treatment: patients with acute kidney injury, such as anuria, hyperkalemia, and severe metabolic acidosis received continuous renal replacement therapy.
Main measures
The following basic data of patients were collected from the electronic medical record system: (I) general information: gender, age, height, weight, APACHE (Acute Physiology And Chronic Health Evaluation) II score when admitted to the intensive care unit (ICU), left ventricular ejection fraction (echocardiography) (%), routine blood test, liver and kidney function tests, and other laboratory measurements; (II) efficacy indicators: duration of postoperative ventilator use (hours), oxygenation index (PaO2/FiO2) at 4, 12, 24, 36, 48, 72, and 96 h after surgery, white blood cell (WBC) counts 1–7 days after surgery (×109/L), hemoglobin (Hb) (g/L), procalcitonin (PCT) level (ng/L), interleukin-6 (IL-6) level (pg/mL), alanine aminotransferase (ALT) (U/L), aspartate aminotransferase (AST) (U/L), total bilirubin (TBIL) (µmol/L), direct bilirubin (DBIL) (µmol/L), serum urea (SU) (mmol/L), serum creatinine (SCr) (µmol/L), glomerular filtration rate (GFR) (mL/min), and other indicators; and (III) efficacy endpoints: length of ICU hospitalization and the 28-day mortality rate.
Statistical analysis
The statistical analyses were completed using the SPSS 22.0 software package. Data were expressed as the mean ± standard deviation (SD), Student’s t-test was used to compare the distributions of normally distributed variables and Wilcoxon rank sum test was used to compare the distributions of non-normally distributed variables. The patient characteristics on admission to ICU were compared by Student’s t-test. The oxygenation index was analyzed by repeated two-way analysis of variance (ANOVA). A Fisher’s exact test or chi-squared test was used to compare the in-hospital mortality rate and length of ICU stay. All reported P values were two sided, and differences at P<0.05 were considered to be statistically significant in all tests.
Results
Clinical data
Based on the inclusion criteria, 71 patients were ultimately included in the analysis, including 51 patients (72%) in the control group and 20 patients (28%) in the sivelestat sodium group. There were no significant differences between the two groups in terms of age, gender, height, weight, APACHE II score, preoperative echocardiographic left ventricular ejection fraction, routine blood tests, liver function, and renal function (all P>0.05; see Table 1).
Table 1
| Clinical data | Control group (n=51) | Sivelestat sodium group (n=20) | P value |
|---|---|---|---|
| Age (years) | 56±12 | 57±12 | 0.637 |
| Gender (male/female) | 36/15 | 16/4 | 0.420 |
| Height (cm) | 167±9 | 168±6 | 0.816 |
| Weight (kg) | 70±15 | 67±12 | 0.544 |
| APACHE II score | 14±5 | 13±4 | 0.480 |
| Left ventricular ejection fraction (echocardiography) (%) | 51±4 | 51±4 | 0.927 |
| WBC (×109/L) | 10.02±4.67 | 12.78±4.44 | 0.026 |
| Hb (g/L) | 133±19 | 134±19 | 0.835 |
| ALT (U/L) | 25±28 | 44±47 | 0.113 |
| TBIL (µmol/L) | 12.0±5.6 | 14±12 | 0.248 |
| DBIL (µmol/L) | 5.0±2.6 | 5.2±2.0 | 0.676 |
| SU (mmol/L) | 6.38±2.46 | 7.23±3.04 | 0.231 |
| SCr (µmol/L) | 96±39 | 94±42 | 0.843 |
| GFR (mL/min) | 73±25 | 81±30 | 0.241 |
Data are presented as mean ± standard deviation. ICU, intensive care unit; APACHE, Acute Physiology and Chronic Health Evaluation; WBC, white blood cell; Hb, hemoglobin; ALT, alanine aminotransferase; TBIL, total bilirubin; DBIL, direct bilirubin; SU, serum urea; SCr, serum creatinine; GFR, glomerular filtration rate.
Changes in postoperative oxygenation index and inflammatory indicators
There was no statistically significant difference in the oxygenation index (PaO2/FiO2) between the control group and the sivelestat sodium group at 4, 12, 24, 36, 48, 72, and 96 h after surgery (all P>0.05; see Table 2). Additionally, the WBC count, PCT level, and IL-6 level did not differ significantly between these two groups (all P>0.05).
Table 2
| Group | 4 h | 12 h | 24 h | 36 h | 48 h | 72 h | 96 h | P value |
|---|---|---|---|---|---|---|---|---|
| Control group | 201±90 | 204±89 | 222±102 | 200±88 | 208±102 | 214±120 | 257±137 | >0.05 |
| Sivelestat sodium group | 187±60 | 188±84 | 164±53 | 198±75 | 191±75 | 200±103 | 205±90 |
Data are presented as mean ± standard deviation.
Subgroup analyses
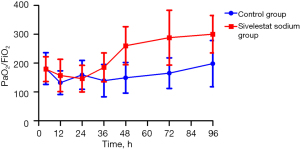
A further analysis of the patients showed that 16 patients (72.7%) from the control group and 6 patients (27.3%) from the sivelestat sodium group had a mechanical ventilation time >96 h; the oxygenation index (PaO2/FiO2) at 48 hours after surgery was 149±53 in the control group and 260±66 in the sivelestat sodium group (P=0.01, see Figure 1), and the oxygenation index (PaO2/FiO2) at 72 h after surgery was 165±66 in the control group and 288±95 in the sivelestat sodium group (P=0.02, see Figure 1). The WBC count, PCT level, and IL-6 level in the sivelestat sodium group decreased significantly compared with the control group; meanwhile, the differences in the WBC count and PCT level between the sivelestat sodium group and the control group in post-operative day 4 were statistically significant (P<0.05; see Figure 2).

Length of ICU stay and mortality rate
During ICU hospitalization, among the 71 patients enrolled in this study, 9 died, yielding an overall mortality rate of 12.68%. The mortality rate was 13.73% (7/51) in the control group and 10% (2/20) in the sivelestat sodium group. The mortality rate showed a downward trend in the sivelestat sodium group; however, the difference in mortality rates did not differ significantly between the two groups (P=0.393; see Table 3). There was no difference in the length of ICU stay between these two groups (sivelestat sodium group: 8.75±4.80 days; control group: 8.04±5.92 days; P>0.05).
Table 3
| Group | Control group | Sivelestat sodium group | P value |
|---|---|---|---|
| Number of deaths | 7 | 2 | >0.05 |
| Mortality rate | 13.73% | 10% | |
| Length of ICU stay (days) | 8.04±5.92 | 8.75±4.80 |
Data are presented as mean ± standard deviation. ICU, intensive care unit.
Adverse reactions
There were no significant differences between the two groups in terms of liver insufficiency (increased ALT, AST, TBIL, and DBIL) or renal insufficiency (increased SU and SCr, and a decreased GFR) 1–7 days after surgery (all P>0.05).
Discussion
ATAAD is the most lethal cardiovascular disease. Its incidence rate is increasing annually, the natural prognosis is extremely poor, and the rates of surgical complications and mortality are high (1). The 48-h mortality rate can reach 50% and the 2-week mortality as high as 75% in patients who do not undergo surgery (7,8). Its surgical treatment requires CPB and deep hypothermic circulatory arrest (DHCA). In patients undergoing surgery with DHCA, the occurrence of SIRS also increases the postoperative mortality rate and the incidence of cerebral infarction, infection, bleeding, and other adverse events (9,10).
NE can induce ALI or ARDS and aggravate the inflammatory response by promoting the production of neutrophil chemokines. Sivelestat sodium can selectively inhibit the release of NE from neutrophils and improve ALI/ARDS induced by the systemic inflammatory response (11). In the current study, we explored the efficacy of sivelestat sodium in treating systemic inflammatory response after surgery for ATAAD. We found that sivelestat sodium improves the oxygenation index and inflammatory response of patients after surgery for ATAAD, especially those with longer postoperative ventilator use.
Many studies have investigated the effect of sivelestat sodium on ALI/ARDS. In our current study, we found no difference in the oxygenation index (PaO2/FiO2) at 4, 12, 24, 36, 48, 72, and 96 h after surgery between the sivelestat sodium and the control group. Interestingly, the oxygenation index seemed to be higher in the control group than the sivelestat sodium group, which may be due to selection bias in this retrospective study. However, for patients with a longer mechanical ventilation time, the oxygenation index (PaO2/FiO2) was better in the sivelestat sodium group than the control group, which may be because these patients tended to have more severe inflammatory response, which resulted in a longer intubation time.
Sivelestat sodium has an inhibitory effect on the inflammatory response, which is more obvious in patients with long ventilator use. An open-label, non-randomized, multi-center controlled study in Japan showed that both the adjusted 28-day ventilator-weaning rate and ICU discharge rate were significantly higher in the sivelestat group than the control group (74.6% vs. 61.2%, 72.3% vs. 61.2%, respectively) (12). In a retrospective study of patients with ALI/ARDS who were given sivelestat sodium within 7 days of admission, the actual mortality rates in the sivelestat sodium group at 30, 60, and 90 days were 28.0%, 43.6%, and 52.8%, respectively, which can be compared to 35.0%, 52.7%, and 61.7% in the control group, respectively; thus, sivelestat sodium improved the outcomes (13). In a retrospective study of patients with severe bacterial pneumonia in Japan, the survival group had an earlier timing of sivelestat sodium use, along with improved oxygenation index, body temperature, heart rate, and respiratory rate (14). In patients undergoing total arch replacement under deep hypothermia for ATAAD, sivelestat sodium reduced lung injury after CPB, and the prophylactic use of sivelestat sodium before CPB improved postoperative lung function and shortened hospital stay (15).
Yamaguchi et al. (16) explored the effect of prophylactic use of sivelestat sodium on bronchial inflammation and found that the prophylactic use of sivelestat sodium during transthoracic esophagectomy alleviated bronchial inflammation and shortened the duration of SIRS after the surgery. In a prospective, double-blind, randomized, controlled study performed after pediatric cardiac surgery, the postoperative WBC count, neutrophil percentage, and C-reactive protein of the control group were significantly higher than those of the sivelestat sodium group, suggesting sivelestat sodium improves the perioperative inflammatory response in pediatric patients undergoing cardiac surgery via CPB (17). In a study of septic patients, the sivelestat group showed significant improvements in oxygenation, thrombocytopenia, and multiple organ dysfunction scores compared to the control group, and the sivelestat group also had significantly fewer days of ventilator use and shorter ICU stay, and a halved in-hospital mortality rate compared to the control group (18).
The retrospective data analysis showed that in patients with sepsis complicated with ARDS and disseminated intravascular coagulation (DIC), sivelestat sodium treatment improved the lung injury score, oxygenation index, DIC score, ICU stay, and survival (19,20). CPB triggers the generation of a large amount of tumor necrosis factor α and inflammatory mediators, thereby causing a systemic inflammatory response (9). In the current study, we compared the postoperative inflammatory response between the control group and the sivelestat sodium group based on the inflammatory mediators, including the WBC count, PCT level, and IL-6 level, and found that in patients with longer mechanical ventilation time (>96 h), the effect of sivelestat sodium on reducing inflammatory response was more prominent, as the WBC count, PCT level, and IL-6 level were significantly lower in the sivelestat sodium group than the control group. The sivelestat sodium group was not superior to the control group in terms of hospital stay; however, the sivelestat sodium group had a lower mortality rate than the control group, which suggests that sivelestat sodium improved patient outcomes.
The present study had some limitations. First, as a single-center retrospective study, it had a small sample size and biases. Second, we only analyzed the oxygenation index and a limited number of inflammatory mediators (e.g., the WBC count, PCT level, and IL-6 level) after surgery for aortic dissection. However, we did not analyze whether there were differences in other inflammatory mediators. Third, there might have been a bias in the selection of patients who received the sivelestat sodium treatment. Indeed, patients with severe postoperative inflammatory response were more likely to receive sivelestat sodium. As a result, the efficacy of sivelestat sodium might be more prominent in patients with longer ventilator use. Thus, prospective studies with large sample sizes urgently need to be conducted to examine the role of sivelestat sodium in improving postoperative inflammatory response.
In summary, sivelestat sodium inhibits the inflammatory response through a variety of pathways. It improves the oxygenation index and inflammatory response after surgery for ATAAD, especially in patients who require long mechanical ventilation. Given its prominent therapeutic effects on the inflammatory response, sivelestat sodium may be a potential treatment for various inflammatory diseases.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1220/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1220/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1220/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). As a retrospective study, the requirement of informed consent was waived. This study was approved by the Institutional Review Board (IRB) of our hospital (ethical approval No. WAGHMEC-KT-2022012).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lin FY, Tseng YH, Huang JW, et al. Fate of distal aorta after acute type A aortic dissection repair: Change and persistency of postoperative false lumen status. Int J Cardiol 2018;266:50-5. [Crossref] [PubMed]
- Li J, Yang L, Wang G, et al. Severe systemic inflammatory response syndrome in patients following Total aortic arch replacement with deep hypothermic circulatory arrest. J Cardiothorac Surg 2019;14:217. [Crossref] [PubMed]
- Sun Y, Ding X, Cui Y, et al. Positive Effects of Neutrophil Elastase Inhibitor (Sivelestat) on Gut Microbiome and Metabolite Profiles of Septic Rats. Front Cell Infect Microbiol 2022;12:818391. [Crossref] [PubMed]
- Goto Y, Hiramatsu Y, Ageyama N, et al. Rolipram plus Sivelestat inhibits bone marrow-derived leukocytic lung recruitment after cardiopulmonary bypass in a primate model. J Artif Organs 2019;22:44-52. [Crossref] [PubMed]
- Iwata K, Doi A, Ohji G, et al. Effect of neutrophil elastase inhibitor (sivelestat sodium) in the treatment of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS): a systematic review and meta-analysis. Intern Med 2010;49:2423-32. [Crossref] [PubMed]
- Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012;307:2526-33. [PubMed]
- Xiao XG, Zu HG, Li QG, et al. Sivelestat sodium hydrate attenuates acute lung injury by decreasing systemic inflammation in a rat model of severe burns. Eur Rev Med Pharmacol Sci 2016;20:528-36. [PubMed]
- Kurz SD, Falk V, Kempfert J, et al. Insight into the incidence of acute aortic dissection in the German region of Berlin and Brandenburg. Int J Cardiol 2017;241:326-9. [Crossref] [PubMed]
- Lee JM, Yeo CD, Lee HY, et al. Inhibition of neutrophil elastase contributes to attenuation of lipopolysaccharide-induced acute lung injury during neutropenia recovery in mice. J Anesth 2017;31:397-404. [Crossref] [PubMed]
- Iba T, Kidokoro A, Fukunaga M, et al. Pretreatment of sivelestat sodium hydrate improves the lung microcirculation and alveolar damage in lipopolysaccharide-induced acute lung inflammation in hamsters. Shock 2006;26:95-8. [Crossref] [PubMed]
- Maki C, Inoue Y, Ishihara T, et al. Evaluation of appropriate indications for the use of sivelestat sodium in acute respiratory distress syndrome: a retrospective cohort study. Acute Med Surg 2020;7:e471. [Crossref] [PubMed]
- Aikawa N, Ishizaka A, Hirasawa H, et al. Reevaluation of the efficacy and safety of the neutrophil elastase inhibitor, Sivelestat, for the treatment of acute lung injury associated with systemic inflammatory response syndrome; a phase IV study. Pulm Pharmacol Ther 2011;24:549-54. [Crossref] [PubMed]
- Kido T, Muramatsu K, Yatera K, et al. Efficacy of early sivelestat administration on acute lung injury and acute respiratory distress syndrome. Respirology 2017;22:708-13. [Crossref] [PubMed]
- Nakamura S, Yanagihara K, Izumikawa K, et al. Efficacy of sivelestat for acute lung injury due to severe bacterial pneumonia with systemic inflammatory response syndrome. Nihon Kokyuki Gakkai Zasshi 2008;46:793-7. [PubMed]
- Morimoto N, Morimoto K, Morimoto Y, et al. Sivelestat attenuates postoperative pulmonary dysfunction after total arch replacement under deep hypothermia. Eur J Cardiothorac Surg 2008;34:798-804. [Crossref] [PubMed]
- Yamaguchi K, Sugasawa Y, Takeuchi K, et al. Effects of sivelestat on bronchial inflammatory responses after esophagectomy. Int J Mol Med 2011;28:187-92. [Crossref] [PubMed]
- Inoue N, Oka N, Kitamura T, et al. Neutrophil elastase inhibitor sivelestat attenuates perioperative inflammatory response in pediatric heart surgery with cardiopulmonary bypass. Int Heart J 2013;54:149-53. [Crossref] [PubMed]
- Tsuboko Y, Takeda S, Mii S, et al. Clinical evaluation of sivelestat for acute lung injury/acute respiratory distress syndrome following surgery for abdominal sepsis. Drug Des Devel Ther 2012;6:273-8. [Crossref] [PubMed]
- Hayakawa M, Katabami K, Wada T, et al. Sivelestat (selective neutrophil elastase inhibitor) improves the mortality rate of sepsis associated with both acute respiratory distress syndrome and disseminated intravascular coagulation patients. Shock 2010;33:14-8. [Crossref] [PubMed]
- Gao X, Zhang R, Lei Z, et al. Efficacy, safety, and pharmacoeconomics of sivelestat sodium in the treatment of septic acute respiratory distress syndrome: a retrospective cohort study. Ann Palliat Med 2021;10:11910-7. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)