A study about different findings of PET-CT between neoadjuvant and non-neoadjuvant therapy: SUVmax is not a reliable predictor of lymphatic involvement after neoadjuvant therapy for esophageal cancer
Introduction
Oncologists should evaluate a patient’s exact cancer status when managing esophageal cancer, especially when planning surgery (1). Positron emission tomography computed tomography (PET-CT) has been essential to the evaluation of exact cancer status, especially distant or lymph node (LN) metastases, in esophageal cancer (1-3). Because 18Fludeoxyglucose (18FDG, a radiopharmaceutical for PET-CT) uptake in tissues is a marker for the tissue uptake of glucose, which is in turn closely associated with tissue metabolism, correlations between the maximum standard uptake value (SUVmax) in the lesions and cancer progression are well known (1,3,4). Neoadjuvant therapy (NT) is usually recommended in esophageal cancer when the preoperative clinical stage is T3N1 or greater (5-7). A lesion is usually considered to be positive for malignancy when the SUVmax via PET-CT is more than 2.5 and the lesion is larger than 1 cm in diameter (8,9). However, discrepancies between PET-CT and pathologic findings are common, especially in regional LN status. Thus, preoperative evaluation using PET-CT might provide unreliable information and influence the management plan for esophageal cancer (4,5,10). No definitive findings or established guidelines have been published for the evaluation of esophageal tumors (tumor) and regional LN using PET-CT in patients with esophageal cancer (2,7,8,10). In addition, it remains unclear whether PET-CT findings vary between patients treated with and without NT (2,11). The purpose of the present study is to clarify any variation in PET-CT findings between esophageal cancer patients treated with and without NT and to predict LN metastases for better prognosis.
Methods
Study subjects and methods
We retrospectively compiled and analyzed data from 192 consecutive patients who had undergone curative and complete surgery for intra-thoracic esophageal cancers at a single tertiary Korean hospital from January 2009 to December 2014. Inclusion criteria were complete and curative surgery cases, intra-thoracic esophageal cancer, PET-CT acquisition for initial evaluation and re-evaluation after NT, and preoperatively proven histology of squamous cell carcinoma. Exclusion criteria were palliation or salvage cases, other uncured previous or current primary cancers, and concurrent active inflammation cases. The preoperative assessments included esophagogastroduodenoscopy, esophagography, chest CT, abdominal CT, PET-CT, endoscopic ultrasound, and bone scan. NT and adjuvant therapies were performed following the National Comprehensive Cancer Network (NCCN) guidelines; the recommendations of a multidisciplinary team who assessed cancer status, resectability, or operability; and each patient’s condition (5). NT usually consisted of two cycles of cisplatin and 5-fluorouracil, plus 25 fractions of radiation therapy (over 5 weeks) to a total of 41–45 Gray. Re-evaluation by PET-CT was performed 4 weeks after completion of NT, and further management was determined. The surgery was performed 5 or 6 weeks after completion of NT. In the present study, preoperative stage in neoadjuvant cases was defined as clinical stage by PET-CT reevaluation after NT and before surgery. Surgeries were performed by two surgeons using the Ivor Lewis or the McKeown procedures, depending on cancer status and patient condition. Two-field LN dissections were performed. We retrospectively compiled PET-CT SUVmax data on tumors and LNs. To clarify the different findings between NT and non-neoadjuvant therapy (non-NT) group and to predict LN metastases, we assessed the relationship between SUVmax values and pathologic stage, compared disease-free survival (DFS) and overall survival (OS), investigated the relationship between tumor and LN SUVmax values, evaluated and predicted LN metastases using SUVmax values, compared pathologically negative and positive LNs using SUVmax values, and examined the effects of NT on SUVmax. Those findings were analyzed in the context of the pathologic findings. We analyzed the histopathological findings of specimens using the multiple serial sectional and immunohistochemistry methods and determined cancer stage according to the seventh American Joint Committee on Cancer (AJCC) staging system.
Protocol for PET-CT measurements and evaluations
All subjects gave written informed consent before PET-CT measurement for the possible future use of their clinical evaluations in research. Subjects fasted for at least 6 h before their PET-CT scans, and their blood glucose levels were measured before injection of 18FDG, a radiopharmaceutical. If blood glucose level was greater than 160 mg/dL, the scan was postponed. An hour after the injection of 18FDG (dosage 0.2 mCi/kg), positron emission images were acquired from the orbitomeatal plane to the proximal thigh. A CT scan was acquired concurrently with positron emission scans for exact anatomic localization of any 18FDG-avid lesion. SUVmax calculated by identifying the region of interest on an axial slice with the highest uptake of 18FDG within a lesion, was used to present the uptake of 18FDG within a lesion. Two nuclear medicine physicians independently evaluated the SUVmax values for each scan. Medical records were also reviewed to discriminate malignancy or metastasis from a nonspecific 18FDG-avid lesion. We compiled the SUVmax values as the highest uptake of 18FDG within a tumor and individual LN later pathologically dissected and confirmed. We defined LN SUVmax as the highest SUVmax value among all pathologically dissected and confirmed LNs. Tumors and LNs were regarded as positive for malignancy or metastasis when the SUVmax value was >2.5 on a PET scan and the size was >1 cm on a CT scan, following previous studies and our hospital policy (8).
Statistical considerations and study approval
Due to non-normal distribution of data, we used non-parametric statistical hypothesis tests. The comparisons among subgroups were evaluated using the Mann-Whitney U or the Jonckheere-Terpstra test after propensity score matching methods, if needed. We used the Wilcoxon signed rank test when comparing two matched data and evaluated comparisons for categorical variables with the χ2 test or Fisher’s exact test, as appropriate. Association studies were evaluated using the Spearman’ rho test, and survival analyses were performed using Kaplan-Meier survival estimation, with the log-rank test used to search for differences in survival across these strata. We performed receiver operating characteristic (ROC) analysis for diagnostic evaluation. We used the Statistical Package of Social Sciences version 22.0 (SPSS, Chicago, IL, USA) for all analyses. A P value <0.05 was considered statistically significant. The present study was approved by the Institutional Review Board of Seoul St Mary’s Hospital (Approval number: KC15RISI0763).
Results
Study subjects
We included 192 patients (male 178, female 14; mean age 63.9 years) who had undergone curative and complete surgery for esophageal cancers from January 2009 to December 2014 in this study. Seventy patients received NT (initial clinical stage: IIa 4, IIb 6, IIIa 20, IIIb 20, and IIIc 20 cases). All cancer histologies were squamous cell carcinomas. Tumors were located in the upper thoracic esophagus (32 cases), middle thoracic esophagus (76 cases), and lower thoracic esophagus (84 cases). Mean tumor length and size were 3.2±2.0 cm and 9.3±11.2 cm2, respectively. Mean tumor and regional LN SUVmax were 6.71±5.12 and 2.98±2.33, respectively. The mean number of regional LNs dissected was 25.2±13.8. The mean observation period was 24.7±18.6 months. The overall clinic-pathologic characteristics for the study subjects are summarized in Table 1.
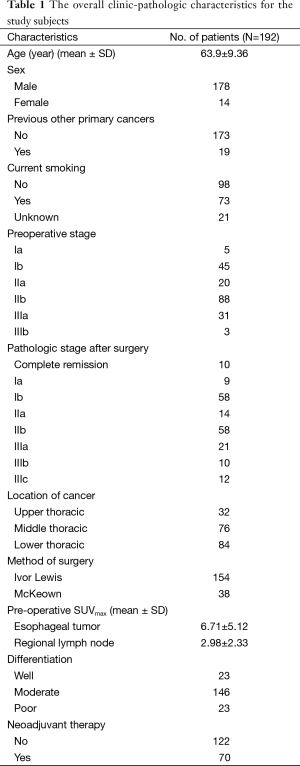
Full table
The relationship between SUVmax and pathologic stage, and other various factors
In NT, we found a positive correlation between pathological T stage and SUVmax (tumor SUVmax P<0.001, LN SUVmax P=0.010); however, we found no relationship between pathological N stage and SUVmax. In non-NT, we found a positive correlation between pathological stage and SUVmax (T stage, tumor SUVmax P<0.001, LN SUVmax P=0.001; N stage, tumor SUVmax P=0.003, LN SUVmax P=0.021) (Figure 1). Tumor SUVmax values in the subgroup with tumor lymphatic invasion were higher than in the subgroup without lymphatic invasion, regardless of NT (NT P<0.001, non-NT P=0.001). We found no significant difference in tumor or LN SUV max with various other factors, chronic lung disease (COPD and bronchiectasis), age, history of previous other primary cancer, and sex, regardless of NT. Preoperative N staging using PET-CT had a tendency of overestimation regardless of NT. However, despite the inaccurate evaluation of individual LN status via PET-CT, we found no significant difference between preoperative and pathologic N stage.
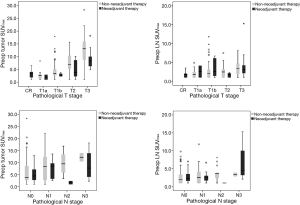
Disease-free survival (DFS) and overall survival (OS) analyses according to SUVmax values
We divided patients into two groups by the mean preoperative SUVmax (NT, tumor SUVmax 6.0, LN SUVmax 3.1; non-NT, tumor SUVmax 7.1, LN SUVmax 2.9). In NT, the low SUVmax group had higher DFS and OS than the high SUVmax group (DFS, tumor SUVmax P<0.001, LN SUVmax P=0.142; OS, tumor SUVmax P<0.001, LN SUVmax P=0.002). In non-NT, the low SUVmax group also had higher DFS and OS than the high SUVmax group (DFS, tumor SUVmax P<0.001, LN SUVmax P=0.008; OS, tumor SUVmax P=0.029, LN SUVmax P=0.016) (Figure 2).
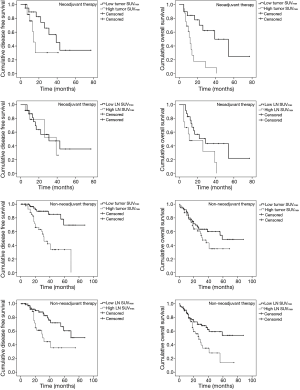
The relationship between tumor and lymph nodes (LN) SUVmax
Tumor SUVmax had a positive correlation with LN SUVmax in both NT and non-NT (P=0.006, P<0.001, respectively). In addition, tumor SUVmax also had a positive correlation with LN SUVmax in both pathologically positive and negative LN (P=0.002, P<0.001, respectively) (Figure 3).
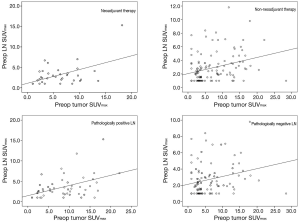
Receiver operating characteristic (ROC) analysis for evaluation of lymph nodes (LN) metastasis using SUVmax
Twenty-eight of the 98 patients with LN SUVmax ≤2.5 had a pathologic N stage of one or greater (pN1–3), and 53 of 94 patients with LN SUVmax >2.5 had a pathologic N stage of zero (pN0). In non-NT, when the ratio of LN SUVmax to tumor SUVmax was >1.0 (when LN SUVmax was larger than tumor SUVmax), the LNs were statistically benign regardless of LN SUVmax (P=0.009). ROC analysis also revealed significant cutoff values for diagnosis of LN metastases using both tumor and LN SUVmax (tumor SUVmax, cutoff value 4.9, sensitivity 71.8%, specificity 56.6%, area =0.650, P=0.008; LN SUVmax, cutoff value 2.5, sensitivity 64.1%, specificity 57.8%, area =0.613, P=0.045). However, in NT, the ratio of LN SUVmax to tumor SUVmax was not related with diagnosis of LN metastases, and ROC analysis showed no significant findings of LN metastases using SUVmax values. ROC analysis for using SUVmax to diagnose LNs metastasis is shown in Figure 4.
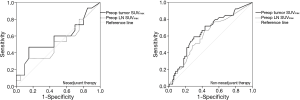
Therefore, we investigated the possibility of predicting LN metastases based on tumor SUVmax in NT. When tumor SUVmax value was >2.5 and larger than LN SUVmax value, ROC analysis revealed the presence of a significant cutoff value for diagnosis of LN metastases using tumor SUVmax: cutoff value 8.2, sensitivity 70.0%, specificity 80.0%, area =0.697, P=0.019. However, if tumor SUVmax was ≤2.5, the value of tumor SUVmax did not provide an appropriate diagnostic value for LN metastasis.
Comparison between neoadjuvant (NT) and non-neoadjuvant (non-NT) therapy cases
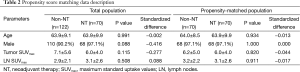
Due to heterogeneity of the data between NT and non-NT, we used the propensity score matching method to compare NT and non-NT cases to overcome this bias (Table 2). SUVmax being equal, we found no significant differences in pathological T and N stage between NT and non-NT. In addition, non-NT had significantly higher DFS and OS than NT (P=0.011, P=0.009, respectively), despite the absence of significant differences in pathological stage between NT and non-NT (Figure 5).
Full table

Neoadjuvant therapy (NT) effects on SUVmax
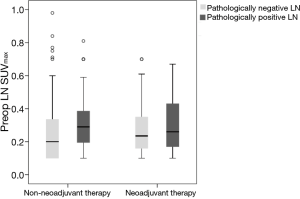
Seventy patients received NT (initial clinical stage: IIa 4, IIb 6, IIIa 20 IIIb 20, and IIIc 20 cases). After NT, clinical stage as determined via PET-CT changed [down staging (56/70, 80%) and no change (14/70, 20%)], with significant decreases in both tumor and LN SUVmax (both, P<0.001). Interestingly, we also found significant decreases of SUVmax in both pathologically positive and negative LN after NT (both, P<0.001). In NT, we found no significant difference of LN SUVmax between pathologically negative and positive LN. However, in non-NT, LN SUVmax of pathologically positive LN was significantly higher than that of pathologically negative LN (P=0.042) (Figure 6). In addition, after NT, we found significant decreases in tumor and LN SUVmax in both complete and non-complete remission cases (both, P<0.001).
Discussion
PET-CT has become an essential tool to assess exact cancer status, especially distant or LNs metastases in esophageal cancer (1). Most thoracic surgeons will consider NT instead of prompt surgery as initial management for esophageal cancer if a preoperative evaluation using PET-CT reveals possible LN metastasis (5). PET-CT thus promotes appropriate decisions in managing esophageal cancer by providing qualitative and quantitative information about a lesion by measuring its metabolic activity (4,5). However, discordance commonly occurs between PET-CT and pathologic findings, especially in LN status (1,3,8). In addition, the assessment of LN status using PET-CT in patients with esophageal cancer varies by institute because no established findings or qualitative features of PET-CT have been published (2,7,8). To clarify PET-CT findings in esophageal cancer, we investigated the different findings of PET-CT between NT and non-NT, especially in assessment of LNs for surgery, from the viewpoint of a thoracic surgeon. For the purposes of this study, we considered a lesion to be malignant or metastatic when its SUVmax was >2.5 and its size on CT scan was >1 cm (8).
Like many previous studies, the present study showed that tumor SUVmax correlated with the progression of esophageal cancer (i.e., pathologic stage and lymphatic invasion) and survival (DFS and OS) regardless of NT (1-4,8,9,12). However, N stage had no correlation with SUVmax following NT (3,13). Preoperative N staging using PET-CT tended toward overestimation regardless of NT (5,13). However, despite inaccurate evaluation of individual LN status using PET-CT, we found no significant difference between preoperative and pathologic N stage. Interestingly, we found a significant decrease in SUVmax in both pathologically positive and negative LNs after NT along with tumor and LN SUVmax decreases in both complete remission and non-complete remission cases. We also found no difference in SUVmax between pathologically positive and negative LNs in NT. However, in non-NT, LN SUVmax of pathologically positive LN was significantly higher than that of pathologically negative LN. Those results indicate the impossibility of distinguishing pathologically positive and negative LNs using only SUVmax levels in NT. SUVmax value is thus not an appropriate diagnosis value for LN metastasis in NT, as shown in the ROC analysis, which indicates the importance of considering other conditions to assess LNs exactly (1,3,8,9,14). We also propose that the impossibility of discriminating complete remission from non-complete remission cases by SUVmax level is caused by the fact that a significant portion of pathologically positive LNs convert to negative ones after NT, and fewer effects of NT develop in pathologically positive LNs than in pathologically negative ones (4,14).
Many studies have investigated the effects of NT in terms of predicting LN metastases based on the SUVmax of the primary lesions and have shown success in conditions such as hepatocellular carcinoma, breast cancer, and lung cancer (10). We showed that when LN SUVmax was higher than tumor SUVmax, the LN was considered to be benign regardless of SUVmax in non-NT and that predicting LN metastases based on the SUVmax of primary lesions was possible only in non-NT. Using SUVmax values of primary lesions to predict LN metastases was difficult in NT (3,4), probably because of the various effects of NT on tumor and LN (9). Therefore, to predict LN metastases for better diagnostic accuracy and prognosis, we attempted to find new findings for NT cases using esophageal tumor SUVmax. We found that if the tumor SUVmax was higher than the LN SUVmax and was higher than the value of 2.5, it was possible to diagnose LNs using the value of the tumor SUVmax. However, if the tumor SUVmax was ≤2.5, it could not provide an appropriate diagnosis value for LN metastases. We attribute this result to the assumption that FDG uptake in more advanced esophageal cancers provides better diagnostic accuracy because FDG uptake of the primary lesion is positively correlated with that of LN (10,13).
Recent studies have shown a survival benefit from initial NT followed by surgery over the prompt surgery in advanced esophageal cancer and have demonstrated that the improved survival rates result from the NT, not more radical surgery (1,4,14). However, we showed that SUVmax values being equal, non-NT had significantly higher DFS and OS than NT, despite the absence of differences in pathological stage between NT and non-NT. These findings could reflect that NT was in more advanced status. Also, the survival superiority of NT followed by surgery over prompt surgery could result from occult metastases undetected by PET-CT (11,15). We also attribute this finding to the survival superiority of NT followed by surgery over prompt surgery.
In summary, the present study showed similar and different PET-CT findings between NT and non-NT in esophageal cancer. The similar findings pertain to the relationship between T stage and SUVmax, DFS and OS, the relationship between tumor and LN SUVmax, and the relationship with various factors (chronic lung disease, age, history of previous other primary cancer, and sex). The different findings pertain to the relationship between N stage and SUVmax, assessment of LN metastases, and any difference in SUVmax between pathologically positive and negative LN. Some studies reported that preoperative staging using PET-CT shows no survival benefit and no improvement in early recurrence following surgery (15-18). We suggest with caution that surgery should not be withheld only due to preoperative N stage using PET-CT after NT because there is no clear method for evaluation LN status after NT, and PET-CT findings differ between NT and non-NT (4). Further large-scale studies about PET-CT findings in NT are needed to draw definitive conclusions or establish guidelines for the use of PET-CT to diagnose patients with esophageal cancer.
The present study has several limitations, including its retrospective and single center design, small sample size and selection bias, and the shine-through phenomenon. Because the present study included only surgical cases, it mostly consisted of early-stage esophageal cancer, thereby reducing the incidence of LN metastases, which might affect the preoperative assessment (13). The small number of NT cases and the heterogeneity of the data could have affected the study findings. To overcome that bias, we used the propensity score matching method to compare NT and non-NT. Because the measurement of the metabolic activity in a lesion by PET-CT involves considerable variability in conditions, various factors can influence the SUVmax level, especially in early-stage esophageal cancer (13,18). In addition, interpreting SUVmax data using only empirical and quantitative standardization can influence the preoperative assessment, especially in the early stages (8,13,18). The findings from the present study should be confirmed with prospective, randomized studies to provide definitive findings or establish guidelines for the use of PET-CT for the preoperative evaluation of the patients with esophageal cancer. Presently, because we regard evaluation of LNs by SUVmax to not be fully reliable, we chose prompt surgery instead of initial NT in a significant number of cases with preoperative high N stage according to PET-CT findings. To the best of our knowledge, the present study is the first and systematic comparison of PET-CT findings between NT and non-NT.
Conclusions
This study showed different PET-CT findings between NT and non-NT cases, which should be clarified for preoperative evaluation and disease management, especially for surgery. Surgery should not be withheld based on ignorance of the different PET-CT findings, which must be carefully considered in conjunction with other conditions. In addition, further studies on the effects of NT on PET-CT findings are required to improve the evaluation of LNs using PET-CT in esophageal cancer.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Bruzzi JF, Munden RF, Truong MT, et al. PET/CT of esophageal cancer: its role in clinical management. Radiographics 2007;27:1635-52. [Crossref] [PubMed]
- You JJ, Wong RK, Darling G, et al. Clinical utility of 18F-fluorodeoxyglucose positron emission tomography/computed tomography in the staging of patients with potentially resectable esophageal cancer. J Thorac Oncol 2013;8:1563-9. [Crossref] [PubMed]
- Devadas M, Mittal A, Lin M, et al. FDG-PET nodal staging does not correlate with histopathological nodal stage for oesophageal cancers. Int J Surg 2015;20:113-7. [Crossref] [PubMed]
- Park JS, Choi JY, Moon SH, et al. Response evaluation after neoadjuvant chemoradiation by positron emission tomography-computed tomography for esophageal squamous cell carcinoma. Cancer Res Treat 2013;45:22-30. [Crossref] [PubMed]
- Berry MF. Esophageal cancer: staging system and guidelines for staging and treatment. J Thorac Dis 2014;6 Suppl 3:S289-97. [PubMed]
- Fuster D, Mayoral M, Rubello D, et al. Is there a role for PET/CT with esophagogastric junction adenocarcinoma? Clin Nucl Med 2015;40:e201-7. [Crossref] [PubMed]
- Putora PM, Bedenne L, Budach W, et al. Oesophageal cancer: exploring controversies overview of experts' opinions of Austria, Germany, France, Netherlands and Switzerland. Radiat Oncol 2015;10:116. [Crossref] [PubMed]
- Yamada H, Hosokawa M, Itoh K, et al. Diagnostic value of 18F-FDG PET/CT for lymph node metastasis of esophageal squamous cell carcinoma. Surg Today 2014;44:1258-65. [Crossref] [PubMed]
- Elimova E, Wang X, Etchebehere E, et al. 18-fluorodeoxy-glucose positron emission computed tomography as predictive of response after chemoradiation in oesophageal cancer patients. Eur J Cancer 2015;51:2545-52. [Crossref] [PubMed]
- Manabe O, Hattori N, Hirata K, et al. Diagnostic accuracy of lymph node metastasis depends on metabolic activity of the primary lesion in thoracic squamous esophageal cancer. J Nucl Med 2013;54:670-6. [Crossref] [PubMed]
- Kukar M, Alnaji RM, Jabi F, et al. Role of repeat 18F-fluorodeoxyglucose positron emission tomography examination in predicting pathologic response following neoadjuvant chemoradiotherapy for esophageal adenocarcinoma. JAMA Surg 2015;150:555-62. [Crossref] [PubMed]
- Li Y, Lin Q, Luo Z, et al. Value of sequential 18F-fluorodeoxyglucose positron emission tomography/computed tomography (FDG PET/CT) in prediction of the overall survival of esophageal cancer patients treated with chemoradiotherapy. Int J Clin Exp Med 2015;8:10947-55. [PubMed]
- Cuellar SL, Carter BW, Macapinlac HA, et al. Clinical staging of patients with early esophageal adenocarcinoma: does FDG-PET/CT have a role? J Thorac Oncol 2014;9:1202-6. [Crossref] [PubMed]
- Blom RL, Steenbakkers IR, Lammering G, et al. PET/CT-based metabolic tumour volume for response prediction of neoadjuvant chemoradiotherapy in oesophageal carcinoma. Eur J Nucl Med Mol Imaging 2013;40:1500-6. [Crossref] [PubMed]
- Torrance AD, Almond LM, Fry J, et al. Has integrated 18F FDG PET/CT improved staging, reduced early recurrence or increased survival in oesophageal cancer? Surgeon 2015;13:19-33. [Crossref] [PubMed]
- Muijs CT, Beukema JC, Woutersen D, et al. Clinical validation of FDG-PET/CT in the radiation treatment planning for patients with oesophageal cancer. Radiother Oncol 2014;113:188-92. [Crossref] [PubMed]
- Bunting DM, Lai WW, Berrisford RG, et al. Positron emission tomography-computed tomography in oesophageal cancer staging: a tailored approach. World J Surg 2015;39:1000-7. [Crossref] [PubMed]
- Shin S, Kim HK, Choi YS, et al. Clinical stage T1-T2N0M0 oesophageal cancer: accuracy of clinical staging and predictive factors for lymph node metastasis. Eur J Cardiothorac Surg 2014;46:274-9. [Crossref] [PubMed]


