
Prognostic value of plasma D-dimer levels in advanced non-small cell lung cancer patients treated with immune checkpoint inhibitors: a retrospective study
Introduction
Lung cancer is one of the most frequently diagnosed cancers and the leading cause of cancer-related deaths worldwide (1). Non-small cell lung cancer (NSCLC) accounts for about 85% of all lung cancers. The majority of NSCLC patients are diagnosed with advanced disease and are treated with chemotherapy (2). However, the overall response rate to chemotherapy in NSCLC patients is only 30–40%, and the median survival time is below 12 months (3). The promising anti-tumor activity of immune checkpoint inhibitors (ICIs), such as programmed cell death-1/programmed cell death-ligand 1 (PD-1/PD-L1) antibodies, has led to regulatory approvals of these agents for the treatment of a variety of malignancies (4). Numerous clinical trials (5-9) have proved that ICIs treatment has brought about a new dawn for NSCLC patients’ treatment, with very durable responses and long-term benefits. However, the benefit brings by ICIs is only limited to a subset of NSCLC patients, of which the overall response rate was about 20% (10), while some even experiencing serious adverse reactions. Therefore, biomarkers that can predict response to NSCLC patients treated with ICIs are being extensively investigated for further advance precision immunotherapy. PD-L1 expression and tumor mutational burden (TMB) have so far been the most widely studied predictors of clinical benefit in advanced NSCLC patients treated with ICIs (11,12), although these biomarkers require pathological tissue specimens and biomarkers cannot accurately predict the response to ICI treatment (13-16). Thus, identification of convenient and noninvasive biomarkers is urgently needed for advanced NSCLC patients receiving ICI therapy.
Inappropriate activation of both coagulation and fibrinolysis is usually discovered in carcinoma patients, especially in those with metastatic disease (17-20). Plasma D-dimer, the smallest cross-linked protein produced in the proteolytic process, is a marker for detecting malignancy and is of great significance for the clinical exclusion of tumor-related thrombosis (21). Previous studies have shown the predictive role of plasma D-dimer in many malignancies treated with chemotherapy, including lung cancer (22-26), colorectal cancer (27), gallbladder carcinoma (20), and breast cancer (28). However, whether pretreatment D-dimer can predict therapeutic efficacy and prognosis in advanced NSCLC patients receiving ICI treatment remains unclear. Hence, we aimed to determine whether pretreatment D-dimer levels could predict clinical benefits from ICIs in advanced NSCLC patients. We present the following article in accordance with the REMARK reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1363/rc).
Methods
Patients and data collection
We retrospectively collected advanced NSCLC patients from the Chinese People’s Liberation Army General Hospital (Beijing, China) between January 2015 and March 2019. Patients were selected by the following inclusion criteria: (I) NSCLC diagnosed by histology evidence; (II) clinical stage IIIB–IV classified according to the 8th edition of the TNM classification for NSCLC; (III) patients received ICIs treatment for at least 6 weeks, and treatment response were evaluated at least once time; (IV) pretreatment D-dimer levels were measured within 5 days before the first ICI treatment.
Radiographic evidences were used to evaluate the treatment responses. Responses were classified into complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 (29). Research indicators including: Progression-free survival (PFS), which was defined as the time from the first ICI treatment to PD or death (whichever occurred first); Overall survival (OS), which was the time between the first ICI treatment and death; Objective response rate (ORR), which was defined as the ratio of patients who reached CR and PR; As well as disease control rate (DCR), which was defined as the ratio of patients who reached CR, PR, and SD. Follow-up of all patients was performed by searching electronic medical records and counseling telephone. The follow-up cut-off date was July 6, 2020.
Patient’s clinical characteristics and blood test results were collected, including age, sex, smoking history, stage, histological type, Eastern Cooperative Oncology Group Performance Status (ECOG PS), lines of treatment, ICI drugs, treatment type (monotherapy, combination therapy), brain metastasis, pretreatment D-dimer levels, and venous thromboembolism (VTE).
D-dimer was a routine clinical examination in our center, for the patients who were newly diagnosed as cancer patients, and the cancer patients who routinely accept anti-tumor treatment, at least 1 day before their anti-cancer therapy. D-dimer was measured by nephelometry immunoassay with the STA-Liatest D-Di kit as instruction. The reference for normal D-dimer level was 0–0.5 µg/mL.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Ethics Committee of the Chinese PLA General Hospital (No. S2018-092-01). The individual consent for this retrospective analysis was waived.
Statistical analysis
Patients were divided into a normal D-dimer group (≤0.5 µg/mL) and high D-dimer group (>0.5 µg/mL) based on the upper limit of the reference for normal pretreatment D-dimer levels. The optimization-based approach was applied to balance baseline covariates between the two groups (30). Each patient was weighted according to the following criteria: (I) absolute value of standardized mean difference less than 0.15, and (II) variance ratio of 0.67 (1/1.5) to 1.5. The PASS software (version 11.0) was used to validate the effective sample size in the weighted sample (α=0.05, 1-β=0.8, proportion in control group =0.3, accrual time =5 years). Chi-square test was used to calculate intergroup differences in ORR. Survival data was analyzed by the Kaplan-Meier method and log-rank test. Cox proportional hazards models calculated hazard ratio (HR) with its 95% confidence interval (CI). All statistical tests were bilateral with a significance level of 0.05. All statistical analyses were performed with R software, using the packages of WeightIt version 0.5.1 (https://cran.r-project.org/web/packages/WeightIt/index.html) for optimization-based methods and survey version 3.36 (https://cran.r-project.org/web/packages/survey/index.html) in the weighted samples.
Results
Patient characteristics
A total of 277 advanced NSCLC patients treated with ICIs at the Chinese PLA General Hospital between January 2015 and March 2019 were included. The last follow-up date was July 6, 2020. The median follow-up time was 15.0 months with a 95% CI of 12.2 months to 17.6 months. Detailed characteristics of patients are shown in Table 1. The median age of this cohort was 61 years (range, 33–91 years). Among the patients, 76.9% were male, 79.4% were stage IV according to the 8th edition of TNM staging by the International Association for the Study of Lung Cancer (31), 64.6% were non-squamous NSCLC patients, 35.4% were squamous cell lung cancer patients, 62.8% had a history of smoking, and about 90% had an ECOG PS of 0−1. Treatment lines 1, 2, and ≥3 accounted for 31.4%, 35.0%, and 33.6% of patients, respectively. Patients receiving ICI monotherapy accounted for 45.5% of the sample, and 54.5% of patients received ICIs in combination with chemotherapy or antiangiogenic agents. A total of 265 (95.7%) patients received PD-1 inhibitor treatment and 12 patients (4.3%) received PD-L1 inhibitor treatment. A total of 207 patients (74.7%) had pretreatment high D-dimer levels. At the start of ICI treatment, 35 patients (12.6%) who had VTE or high risk of thromboembolism (myocardial infarction, cerebral infarction, and surgery) were receiving anticoagulant therapy (Aspirin, Clopidogrel, Rivaroxaban, Ticagrelor, or Nadroparin calcium). The patient size-277 was validated appropriate by PASS software (version 11.0) which showed the sample size should not be less than 260.
Table 1
| Characteristics | No. of patients | Percentage (%) |
|---|---|---|
| Age (year), median (range) | 61 (33–91) | – |
| <70 | 224 | 80.9 |
| ≥70 | 53 | 19.1 |
| Sex | ||
| Male | 213 | 76.9 |
| Female | 64 | 23.1 |
| Stage | ||
| IIIB/C | 57 | 20.6 |
| IV | 220 | 79.4 |
| Histological type | ||
| Non-squamous | 179 | 64.6 |
| Squamous | 98 | 35.4 |
| Smoking history | ||
| No | 103 | 37.2 |
| Yes | 174 | 62.8 |
| ICIs | ||
| PD-1 inhibitor | 265 | 95.7 |
| PD-L1 inhibitor | 12 | 4.3 |
| ECOG PS | ||
| 0–1 | 247 | 89.2 |
| ≥2 | 30 | 10.8 |
| Brain metastasis | ||
| Yes | 46 | 16.6 |
| No | 231 | 83.4 |
| Treatment lines | ||
| 1 line | 87 | 31.4 |
| 2 lines | 97 | 35.0 |
| ≥3 lines | 93 | 33.6 |
| Treatment type | ||
| Monotherapy | 126 | 45.5 |
| Combination therapy | 151 | 54.5 |
| Anticoagulant therapy | ||
| Yes | 35 | 12.6 |
| No | 242 | 87.4 |
| D-dimer level (μg/mL) | ||
| Median (range) | 0.92 (0.09–21.0) | – |
| Normal (≤0.5) | 70 | 25.3 |
| High (>0.5) | 207 | 74.7 |
NSCLC, non-small cell lung cancer; ICIs, immune checkpoint inhibitors; PD-1, programmed cell death-1; PD-L1, programmed cell death-ligand 1; ECOG PS, Eastern Cooperative Oncology Group Performance Status.
Baseline covariates balanced between the 2 groups
An optimization-based approach was used to balance baseline covariates between the normal D-dimer group and the high D-dimer group. We matched a total of 61 patients in the normal D-dimer subset and 204 patients in the high D-dimer subset. The aim was to eliminate some of the differences between the 2 groups during the matching process.
Pretreatment D-dimer associated with clinical outcomes
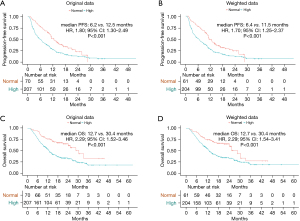
After comparing treatment responses, the results showed that pretreatment normal D-dimer levels were associated with higher ORR (30.0% vs. 15.0%, P=0.005) and DCR (88.6% vs. 64.8%, P<0.001) compared with pretreatment high D-dimer levels (Table 2). Univariate analysis demonstrated that pretreatment high D-dimer levels increased the risk of disease progression (HR, 1.80; 95% CI: 1.30–2.49) and death (HR, 2.29; 95% CI: 1.52–3.46) compared with normal D-dimer levels, and subgroup analysis confirmed that pretreatment high D-dimer levels were associated with worse PFS and OS in most subsets (Figures 1,2). After balancing baseline covariates between the high D-dimer group and normal D-dimer group, the results showed that patients with pretreatment high D-dimer levels had obviously shorter PFS (median: 6.4 vs. 11.5 months; P<0.001) and OS (median: 12.7 vs. 30.4 months; P<0.001) than patients with pretreatment normal D-dimer levels (Figure 3).
Table 2
| Responses | Normal D-dimer group | High D-dimer group | P value |
|---|---|---|---|
| CR, n (%) | 0 (0) | 0 (0) | – |
| PR, n (%) | 21 (30.0) | 31 (15.0) | – |
| SD, n (%) | 41 (58.6) | 103 (49.8) | – |
| PD, n (%) | 8 (11.4) | 73 (35.3) | – |
| ORR, n (%) | 21 (30.0) | 31 (15.0) | 0.005 |
| DCR, n (%) | 62 (88.6) | 134 (64.8) | <0.001 |
CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; ORR, objective response rate; DCR, disease control rate.
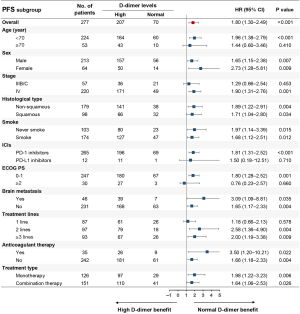
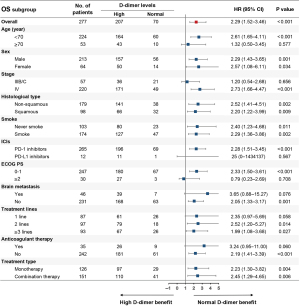
As shown in Table 3, univariate analysis found that age, smoking history, stage, ECOG PS, treatment type, treatment lines, brain metastasis, and pretreatment D-dimer levels were associated with PFS (P<0.05), and multivariate analysis demonstrated that age, ECOG PS, treatment lines, and pretreatment D-dimer levels were independently related to PFS (P<0.05). Baseline variates of age <70, ECOG PS ≥2, ICI monotherapy, later treatment lines, and pretreatment high D-dimer levels were independently associated with shortened PFS (P<0.05). As shown in Table 4, univariate analysis revealed that stage, ECOG PS, treatment type, brain metastasis, treatment lines, and pretreatment D-dimer levels were associated with OS (P<0.05), and multivariate analysis demonstrated that ECOG PS, treatment lines, and pretreatment D-dimer levels were independently related to OS (P<0.05). Baseline variates of ECOG PS ≥2, ICI monotherapy, later treatment lines, and pretreatment high D-dimer levels were independently associated with shortened OS (P<0.05).
Table 3
| Variable | Category | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |||
| Age (year) | ≥70 vs. <70 | 0.70 (0.49–0.99) | 0.044 | 0.67 (0.46–0.98) | 0.039 | |
| Sex | Female vs. Male | 1.26 (0.93–1.71) | 0.144 | – | ||
| Smoking history | Yes vs. No | 0.76 (0.58–0.99) | 0.043 | 0.95 (0.71–1.28) | 0.754 | |
| Histology | Squamous vs. non-squamous | 1.03 (0.79–1.36) | 0.818 | – | ||
| Stage | IV vs. IIIB/C | 1.60 (1.13–2.25) | 0.008 | 1.17 (0.81–1.68) | 0.403 | |
| ECOG PS | ≥2 vs. 0–1 | 1.91 (1.29–2.82) | 0.001 | 1.78 (1.17–2.70) | 0.007 | |
| Treatment type | Combination therapy vs. Monotherapy | 0.75 (0.58–0.97) | 0.031 | 0.80 (0.61–1.05) | 0.104 | |
| Treatment lines | 2 lines vs. 1 line | 2.11 (1.51–2.96) | <0.001 | 1.81 (1.27–2.57) | 0.001 | |
| ≥3 lines vs. 1 line | 2.44 (1.73–3.44) | <0.001 | 2.22 (1.54–3.20) | <0.001 | ||
| Brain metastasis | Yes vs. No | 1.57 (1.11–2.23) | 0.011 | 1.09 (0.76–1.58) | 0.643 | |
| Anticoagulant therapy | Yes vs. No | 1.22 (0.82–1.82) | 0.323 | – | ||
| D-dimer (μg/mL) | High vs. Normal | 1.80 (1.30–2.49) | <0.001 | 1.84 (1.32–2.56) | <0.001 | |
PFS, progression-free survival; HR, hazard ratio; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group Performance Status.
Table 4
| Variable | Category | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |||
| Age (year) | ≥70 vs. <70 | 0.86 (0.58–1.26) | 0.433 | – | ||
| Sex | Female vs. male | 1.18 (0.83–1.67) | 0.352 | – | ||
| Smoking history | Yes vs. No | 0.77 (0.57–1.04) | 0.092 | – | ||
| Histology | Squamous vs. Non-squamous | 1.13 (0.83–1.54) | 0.432 | – | ||
| Stage | IV vs. IIIB/C | 1.62 (1.08–2.45) | 0.021 | 1.20 (0.78–1.84) | 0.405 | |
| ECOG PS | ≥2 vs. 0–1 | 2.38 (1.58–3.57) | <0.001 | 1.94 (1.29–2.93) | 0.002 | |
| Treatment type | Combination therapy vs. Monotherapy | 0.54 (0.40–0.73) | <0.001 | 0.56 (0.41–0.76) | <0.001 | |
| Treatment lines | 2 lines vs. 1 line | 2.39 (1.60–3.59) | <0.001 | 1.85 (1.22–2.82) | 0.004 | |
| ≥3 lines vs. 1 line | 2.24 (1.49–3.39) | <0.001 | 2.09 (1.36–3.21) | 0.001 | ||
| Brain metastasis | Yes vs. No | 1.71 (1.18–2.47) | 0.004 | 1.20 (0.81–1.76) | 0.365 | |
| Anticoagulant therapy | Yes vs. No | 1.42 (0.91–2.23) | 0.124 | – | ||
| D-dimer (μg/mL) | High vs. Normal | 2.29 (1.52–3.46) | <0.001 | 2.13 (1.40–3.25) | <0.001 | |
OS, overall survival; HR, hazard ratio; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group Performance Status.
Discussion
Although progress has been made in cancer immunotherapy, and the use of ICIs has had considerable positive effects on some NSCLC patients, most do not benefit from ICI immunotherapy (7). The predictive ability of some molecules, such as PD-L1 and TMB in advanced NSCLC patients treated with ICIs remains unsatisfactory due to a lack of sensitivity and specificity (32), and thus additional predictive biomarkers are urgently needed in clinical practice to avoid the use of ineffective treatment (10).
Coagulation disorders, which are frequently observed in cancer patients (33), promote tumor angiogenesis, invasion, and metastasis, and ultimately lead to a poor prognosis for tumor patients (34,35). Plasma D-dimer is a stable end product degraded by plasmin-induced fibrinolytic activity and increased by enhanced fibrin formation and fibrinolysis (36). Plasma D-dimer is a useful biomarker for diagnosing VTE, cardiovascular disease, disseminated intravascular coagulation, infectious disease, and cancer (37-39). Previous studies reported that increased plasma D-dimer levels were associated with poor survival in cancer patients through VTE (40,41), which could increase the risk of bleeding during antitumor therapy (42-44). The research of Wang et al. showed that a baseline signature of low D-dimer values was associated with a better survival outcome for early lung cancer (stage I–II) patients treated with surgery (45). Gao et al. found that D-dimer was strongly associated with lymph node metastasis in NSCLC (46-48). Louneva et al. reported that the level of plasma D-dimer was closely related to the prognosis of solid tumors (49). Some clinical studies have also shown that plasma D-dimer level was significantly associated with poor prognosis in lung cancer treated with chemotherapy (50,51). Similarly, a meta-analysis found that for postoperative NSCLC patients, high pretreatment D-dimer level was an independent predictor of poor prognosis (52). Another meta-analysis, which included 7 studies involving 964 patients from China, showed that elevated pretreatment D-dimer level was significantly correlated with worse OS and PFS in patients with small cell lung cancer (53). However, the predictive role of D-dimer in advanced NSCLC patients treated with ICIs remains unclear.
The present study demonstrated the relationship between pretreatment high D-dimer levels and poor clinical outcomes in advanced NSCLC patients treated with ICIs. To our knowledge, this is the first study that addressed the prognostic value of pretreatment D-dimer levels in advanced NSCLC patients treated with ICIs. In this study, although 12.6% of all included patients were receiving different types of anticoagulant therapy at the start of ICI treatment, both univariate analysis and multivariate analysis showed that pretreatment D-dimer levels were independently associated with PFS and OS. Subgroup analysis also confirmed that pretreatment high D-dimer levels were associated with poor prognosis in patients with or without anticoagulant therapy. In patients with advanced NSCLC treated with ICIs, high pretreatment D-dimer levels had a statistically significant association with shortened PFS and OS. Our data provided strong evidence that pretreatment high D-dimer levels were independently associated with poor clinical outcomes in advanced NSCLC patients receiving PD-1/PD-L1 inhibitors.
Chen et al. found that increasing the threshold value of D-dimer from 0.5 to 0.981 µg/mL was statistically significant across different age groups (54). They concluded that the cut-off value of 0.5 µg/mL could not reflect the correlation between age and D-dimer. We did not consider the influence of age factors on D-dimer level and used the critical cut-off value of 0.5 µg/mL. There were other limitations in the present study. First, we only analyzed the prognostic value of pretreatment D-dimer level and not the relationship between changes in D-dimer with efficacy and prognosis of ICI treatment. Second, although we used the optimization-based method to eliminate the bias of baseline covariates between the high D-dimer group and normal D-dimer group, other covariates we did not consider may have also been potential confounders. Third, the retrospective nature of this study may have resulted in unknown selection bias. In addition, how D-dimer affects the efficacy and prognosis of advanced NSCLC patients treated with ICIs remains unclear and needs further investigation.
Conclusions
Pretreatment plasma D-dimer could serve as a predictive biomarker for the efficacy and prognosis of advanced NSCLC patients treated with ICIs. Patients with pretreatment high D-dimer levels may have poor PFS and OS. Further studies are warranted for validation.
Acknowledgments
Funding: This work was supported by funding from the Military Health Special Research Project under grant 20BJZ37.
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1363/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1363/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1363/coif). All authors report that this study was supported by the Military Health Special Research Project under grant 20BJZ37. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Ethics Committee of the Chinese PLA General Hospital (No. S2018-092-01). The individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Thai AA, Solomon BJ, Sequist LV, et al. Lung cancer. Lancet 2021;398:535-54. [Crossref] [PubMed]
- Jett JR, Schild SE, Keith RL, et al. Treatment of non-small cell lung cancer, stage IIIB: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:266S-76S.
- Moro-Sibilot D, Smit E, de Castro Carpeño J, et al. Outcomes and resource use of non-small cell lung cancer (NSCLC) patients treated with first-line platinum-based chemotherapy across Europe: FRAME prospective observational study. Lung Cancer 2015;88:215-22. [Crossref] [PubMed]
- Nishino M, Ramaiya NH, Hatabu H, et al. Monitoring immune-checkpoint blockade: response evaluation and biomarker development. Nat Rev Clin Oncol 2017;14:655-68. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Barlesi F, Vansteenkiste J, Spigel D, et al. Avelumab versus docetaxel in patients with platinum-treated advanced non-small-cell lung cancer (JAVELIN Lung 200): an open-label, randomised, phase 3 study. Lancet Oncol 2018;19:1468-79. [Crossref] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Barbee MS, Ogunniyi A, Horvat TZ, et al. Current status and future directions of the immune checkpoint inhibitors ipilimumab, pembrolizumab, and nivolumab in oncology. Ann Pharmacother 2015;49:907-37. [Crossref] [PubMed]
- Aguiar PN Jr, Santoro IL, Tadokoro H, et al. A pooled analysis of nivolumab for the treatment of advanced non-small-cell lung cancer and the role of PD-L1 as a predictive biomarker. Immunotherapy 2016;8:1011-9. [Crossref] [PubMed]
- Ready N, Hellmann MD, Awad MM, et al. First-Line Nivolumab Plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer (CheckMate 568): Outcomes by Programmed Death Ligand 1 and Tumor Mutational Burden as Biomarkers. J Clin Oncol 2019;37:992-1000. [Crossref] [PubMed]
- Bhaijee F, Anders RA. PD-L1 Expression as a Predictive Biomarker: Is Absence of Proof the Same as Proof of Absence? JAMA Oncol 2016;2:54-5. [Crossref] [PubMed]
- Ilie M, Long-Mira E, Bence C, et al. Comparative study of the PD-L1 status between surgically resected specimens and matched biopsies of NSCLC patients reveal major discordances: a potential issue for anti-PD-L1 therapeutic strategies. Ann Oncol 2016;27:147-53. [Crossref] [PubMed]
- Büttner R, Gosney JR, Skov BG, et al. Programmed Death-Ligand 1 Immunohistochemistry Testing: A Review of Analytical Assays and Clinical Implementation in Non-Small-Cell Lung Cancer. J Clin Oncol 2017;35:3867-76. [Crossref] [PubMed]
- Cristescu R, Mogg R, Ayers M, et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science 2018;362:eaar3593. [Crossref] [PubMed]
- Wang Y, Wang Z. Predictive value of plasma D-dimer levels in patients with advanced non-small-cell lung cancer. Onco Targets Ther 2015;8:805-8. [Crossref] [PubMed]
- Fukumoto K, Taniguchi T, Usami N, et al. Preoperative plasma D-dimer level is an independent prognostic factor in patients with completely resected non-small cell lung cancer. Surg Today 2015;45:63-7. [Crossref] [PubMed]
- Luo YL, Chi PD, Zheng X, et al. Preoperative D-dimers as an independent prognostic marker in cervical carcinoma. Tumour Biol 2015;36:8903-11. [Crossref] [PubMed]
- Kong W, Zhang L, An R, et al. Diagnostic Value of Serum D-Dimer for Detection of Gallbladder Carcinoma. Cancer Manag Res 2021;13:2549-56. [Crossref] [PubMed]
- Knowlson L, Bacchu S, Paneesha S, et al. Elevated D-dimers are also a marker of underlying malignancy and increased mortality in the absence of venous thromboembolism. J Clin Pathol 2010;63:818-22. [Crossref] [PubMed]
- Antoniou D, Pavlakou G, Stathopoulos GP, et al. Predictive value of D-dimer plasma levels in response and progressive disease in patients with lung cancer. Lung Cancer 2006;53:205-10. [Crossref] [PubMed]
- Ge LP, Li J, Bao QL, et al. Prognostic and predictive value of plasma D-dimer in advanced non-small cell lung cancer patients undergoing first-line chemotherapy. Clin Transl Oncol 2015;17:57-64. [Crossref] [PubMed]
- Altiay G, Ciftci A, Demir M, et al. High plasma D-dimer level is associated with decreased survival in patients with lung cancer. Clin Oncol (R Coll Radiol) 2007;19:494-8. [Crossref] [PubMed]
- Zhu LR, Li J, Chen P, et al. Clinical significance of plasma fibrinogen and D-dimer in predicting the chemotherapy efficacy and prognosis for small cell lung cancer patients. Clin Transl Oncol 2016;18:178-88. [Crossref] [PubMed]
- Oikawa M, Yaegashi D, Yokokawa T, et al. D-Dimer Is a Predictive Factor of Cancer Therapeutics-Related Cardiac Dysfunction in Patients Treated With Cardiotoxic Chemotherapy. Front Cardiovasc Med 2021;8:807754. [Crossref] [PubMed]
- Blackwell K, Hurwitz H, Liebérman G, et al. Circulating D-dimer levels are better predictors of overall survival and disease progression than carcinoembryonic antigen levels in patients with metastatic colorectal carcinoma. Cancer 2004;101:77-82. [Crossref] [PubMed]
- Dirix LY, Salgado R, Weytjens R, et al. Plasma fibrin D-dimer levels correlate with tumour volume, progression rate and survival in patients with metastatic breast cancer. Br J Cancer 2002;86:389-95. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Zubizarreta JR. Stable Weights that Balance Covariates for Estimation With Incomplete Outcome Data. Journal of the American Statistical Association 2015;110:910-22. [Crossref]
- Nicholson AG, Chansky K, Crowley J, et al. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals for the Revision of the Clinical and Pathologic Staging of Small Cell Lung Cancer in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:300-11.
- Indovina P, Marcelli E, Maranta P, et al. Lung cancer proteomics: recent advances in biomarker discovery. Int J Proteomics 2011;2011:726869. [Crossref] [PubMed]
- Lyman GH, Khorana AA. Cancer, clots and consensus: new understanding of an old problem. J Clin Oncol 2009;27:4821-6. [Crossref] [PubMed]
- Ay C, Dunkler D, Pirker R, et al. High D-dimer levels are associated with poor prognosis in cancer patients. Haematologica 2012;97:1158-64. [Crossref] [PubMed]
- Pawar NR, Buzza MS, Antalis TM. Membrane-Anchored Serine Proteases and Protease-Activated Receptor-2-Mediated Signaling: Co-Conspirators in Cancer Progression. Cancer Res 2019;79:301-10. [Crossref] [PubMed]
- Hisada Y, Geddings JE, Ay C, et al. Venous thrombosis and cancer: from mouse models to clinical trials. J Thromb Haemost 2015;13:1372-82. [Crossref] [PubMed]
- Hanna DL, White RH, Wun T. Biomolecular markers of cancer-associated thromboembolism. Crit Rev Oncol Hematol 2013;88:19-29. [Crossref] [PubMed]
- Patel P, Patel P, Bhatt M, et al. Systematic review and meta-analysis of outcomes in patients with suspected deep vein thrombosis. Blood Adv 2020;4:2779-88. [Crossref] [PubMed]
- Cho JH, Kim JB, Lee DG. Correlation Between D-Dimer Level and Deep Venous Thrombosis in Patients With Acute Spinal Cord Injuries. Am J Phys Med Rehabil 2020;99:613-6. [Crossref] [PubMed]
- Alcalay A, Wun T, Khatri V, et al. Venous thromboembolism in patients with colorectal cancer: incidence and effect on survival. J Clin Oncol 2006;24:1112-8. [Crossref] [PubMed]
- Chew HK, Wun T, Harvey DJ, et al. Incidence of venous thromboembolism and the impact on survival in breast cancer patients. J Clin Oncol 2007;25:70-6. [Crossref] [PubMed]
- Prandoni P, Lensing AW, Piccioli A, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood 2002;100:3484-8. [Crossref] [PubMed]
- Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med 2003;349:146-53. [Crossref] [PubMed]
- Schulman S, Zondag M, Linkins L, et al. Recurrent venous thromboembolism in anticoagulated patients with cancer: management and short-term prognosis. J Thromb Haemost 2015;13:1010-8. [Crossref] [PubMed]
- Wang J, Li H, Xu R, et al. The MLR, NLR, PLR and D-dimer are associated with clinical outcome in lung cancer patients treated with surgery. BMC Pulm Med 2022;22:104. [Crossref] [PubMed]
- Gao XL, Wang SS, Cao DB, et al. The role of plasma D-dimer levels for predicting lymph node and mediastinal lymph node involvement in non-small cell lung cancer. Clin Respir J 2018;12:2151-6. [Crossref] [PubMed]
- Fan S, Zhao G, An G. High pretreatment plasma D-dimer levels are associated with shorter overall survival in patients with small cell lung cancer. J Int Med Res 2019;47:215-24. [Crossref] [PubMed]
- Mahé I, Elalamy I, Gerotziafas GT, et al. Treatment of Cancer-Associated Thrombosis: Beyond HOKUSAI. TH Open 2019;3:e309-15. [Crossref] [PubMed]
- Louneva N, Maity A, Kennedy AR. Plasma D-Dimer Levels are Elevated in Radiation Oncology Patients. Radiat Res 2020;193:46-53. [Crossref] [PubMed]
- Jones JM, McGonigle NC, McAnespie M, et al. Plasma fibrinogen and serum C-reactive protein are associated with non-small cell lung cancer. Lung Cancer 2006;53:97-101. [Crossref] [PubMed]
- Zhao J, Zhao M, Jin B, et al. Tumor response and survival in patients with advanced non-small-cell lung cancer: the predictive value of chemotherapy-induced changes in fibrinogen. BMC Cancer 2012;12:330. [Crossref] [PubMed]
- Deng HY, Zheng X, Jiang R, et al. Preoperative D-dimer level is an independent prognostic factor for non-small cell lung cancer after surgical resection: a systematic review and meta-analysis. Ann Transl Med 2019;7:366. [Crossref] [PubMed]
- Li J, Wang Y, Li J, et al. Prognostic Value of Pretreatment D-Dimer Level in Small-Cell Lung Cancer: A Meta-Analysis. Technol Cancer Res Treat 2021;20:1533033821989822. [Crossref] [PubMed]
- Chen C, Li J, Li J, et al. Application of an elevated plasma D-dimer cut-off value improves prognosis prediction of advanced non-small cell lung cancer. Ann Transl Med 2020;8:1153. [Crossref] [PubMed]
(English Language Editor: A. Muylwyk)


