Ninety-seven cases of experiences with the left thoracotomy approach for off-pump conventional revascularization: a retrospective cohort study
Introduction
The superiority of coronary artery bypass grafting (CABG) to percutaneous coronary intervention (PCI) in terms of the need for repeat interventions (1,2) and long-term survival has been established, especially in complex multi-vessel disease patients. Globally, CABG remains the standard approach for revascularization and demonstrates good long-term results, especially among patients with multi-vessel disease, left main disease, and left ventricular dysfunction. Median sternotomy is the standard procedure for all open-heart surgeries. However, compared with PCI, the conventional procedure is associated with potential complications and slow recovery. Among the most serious complications is mediastinitis related to sternal dehiscence, particularly in diabetic and obese patients, and the early and in-hospital mortality rates are high (3,4). Furthermore, the long-term survival of patients is also significantly reduced (5). Therefore, procedures are being developed to minimize the invasiveness of the procedure and avoid complications while maintaining the proven advantages of surgical revascularization. Robot-assisted coronary bypass grafting (6), video-assisted coronary bypass grafting, minimally invasive multi-vessel CABG, and hybrid coronary revascularization are all well attempted; yet, some of these procedures may be more expensive.
However, none of these approaches achieve conventional complete revascularization. From this perspective, we attempted minimally invasive coronary surgery-CABG (MICS CABG) via a small left anterior incision which has both the advantage of less invasiveness and complete revascularization with the same configuration as the median sternotomy. Through this incision, revascularization was completed similarly to the sternotomy approach, taking down the left internal mammary artery (LIMA) and performing one to two proximal anastomoses on the aorta and then distal anastomoses. For patients with coronary artery disease (CAD) affecting the left anterior descending artery (LAD), we chose the gold standard, which involves anastomosis of the LIMA to LAD (LIMA-LAD) with a 10–15-year patency of greater than 94% (7). The adjunct conduits were the great saphenous vein and the right internal mammary artery. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1162/rc).
Methods
This study is a retrospective analysis of patients who underwent MICS CABG performed by a single surgeon from January 2020 to March 2022. A total of 97 patients with coronary artery disease received left thoracotomy for off-pump bypass grafting. We explored the feasibility of conventional revascularization using the left thoracotomy (8–10 cm) approach.
The patients signed the individual consent for the operation preoperatively. All patients received coronary angiography, echocardiography, electrocardiography, chest computed tomography, carotid ultrasonography, internal mammary artery ultrasonography, and laboratory examinations before operation.
All patients received echocardiography, electrocardiography, chest X-ray and laboratory examinations every postoperative day until they were transferred out of intensive care unit and on the day before discharge. All patients received dual-source computerized tomographic angiography at 1-week postoperatively to evaluate the graft patency and detect pulmonary embolism and aortic dissection.
Follow-up was performed by phone or via outpatient visits and was available for 92 patients (95%). According to patients’ willingness dual-source computerized tomographic angiography were conducted to evaluate the graft patency.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Tianjin Chest Hospital (No. 2022LW-016). Individual consent for this retrospective analysis was waived.
Exclusion criteria
The exclusion criteria were as follows: (I) Emergency surgery; (II) patients with severe arrhythmia with unstable hemodynamics; (III) those with a porcelain aorta; (IV) patients with decreased cardiac function [left ventricular ejection fraction (LVEF) <40% or left ventricular diastolic dimension (LVDD) >65 mm]; (V) patients with severe chest deformities and pulmonary comorbidities; (VI) those with calcified femoral artery negating potential extracorporeal support; and (VII) combined open-heart surgery.
Patients with a high body mass index (BMI) were considered a relative contraindication, as exposure through the thoracotomy may be difficult. Diffuse lesions and intramyocardial vessels were also difficult for a surgeon in the learning curve for MICS CABG.
Operative technique
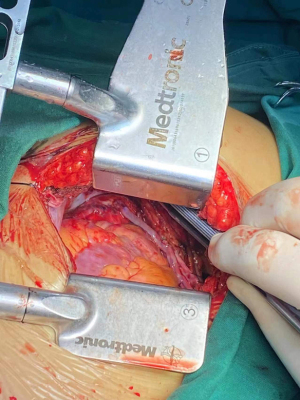
The patient was positioned 20–30 degrees laterally with the left hemithorax elevated and both upper limbs in adduction routinely. External defibrillation pads were attached. A double-lumen endotracheal tube or a single-lumen tube and bronchial blocker were then applied. For pain management, ropivacaine was used for regional or paravertebral block at the beginning of the procedure. After positioning, the midline and intercostal space were marked with a marker pen. It is routine practice to keep the bilateral groin exposed to access femoral vessels for cardiopulmonary bypass cannulation. Cerebral oxygen saturation was monitored intraoperatively. Transesophageal echocardiography (TEE) was used to assess the wall motion and intravascular volume. Right ventricular expansion was avoided. The procedure was performed through an 8–10 cm anterolateral thoracotomy in the fourth or fifth intercostal space. For patients with multi-vessel disease, the fourth intercostal space was chosen to allow for better access to the ascending aorta. Before opening the pericardium, the partial thymus was usually removed to facilitate the exposure. The LIMA was then harvested under direct visualization with a specially designed retractor with single lung ventilation. Appropriate positive end-expiratory pressure (PEEP) and increased tidal volume allowed for the exposure of the heart. We typically harvested the LIMA in a pedicled manner up to the proximal origin of the left subclavian artery and down to the fifth intercostal space. We used titanium clips to cut off the LIMA branch. Heparin (1 mg/kg) was administered to maintain an activated clotting time of over 250 seconds. Subsequently, the LIMA was transected and sprayed with papaverine saline to prevent spasms.
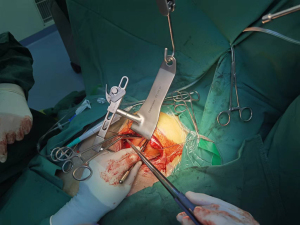
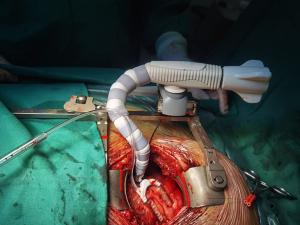
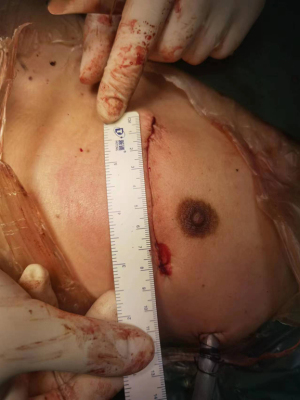
Suspension sutures were placed on the right side of the pericardium close to the ascending aorta, and gauze was placed between the right side of the pericardium and the aorta to improve visualization of the ascending aorta. The proximal anastomoses were usually performed first (Figure 1 and Figure 2). For patients with severe left main stenosis, revascularization of the LAD was urgent. Before the application of the side-biting clamp, systolic pressure was brought down to 85–90 mmHg. Distal anastomoses were performed on a beating heart with a standard stabilizer for off-pump CABG, which was placed on a rib retractor with an external fixator. The anastomosis of the LIMA to the LAD artery was then performed. We applied the gold standard LIMA-LAD rule; no Y graft or sequential graft was related to LAD. Next, the posterior descending artery, diagonal, intermediate, and obtuse marginal were anastomosed. The distal anastomoses were performed with a single running 7-0 or 8-0 polypropylene suture (Figure 3). After completion of anastomosis, TEE provided immediate measurement of wall motion. Following treatment with protamine-neutralized heparin, a chest tube was inserted into the pleural space and the thoracotomy was closed. Figure 4 shows the length of the incision. If there was no evidence of bleeding, aspirin and clopidogrel were started on the first postoperative day. The majority of the patients were extubated in the first 24 hours.
Statistical analysis
For statistical analyses, we used IBM SPSS ver. 22.0 (IBM Corp., Armonk, NY, USA). Quantitative data were presented as the mean± standard deviation (SD) or the median value with interquartile range. For quantitative data, independent samples t-test, paired samples t-test and rank-sum test were conducted. For qualitative data expressed as n (n%), chi square test and Fisher exact probability method were used. The significance level (alpha) was 0.05.
Results
The preoperative characteristics were collected and illustrated in Table 1. A total of 97 patients received MICS CABG. Among the 97 included patients in this study, there were 16 females (16.5%), and the mean age of the cohort was 61.5±8.8 years. Sixty patients (61.9%) had hypertension, 34 (35.1%) had diabetes mellitus, 19 (19.6%) had PCI, 17 (17.5%) had stroke, and 25 (25.8%) had myocardial infarction. Also, 18 (18.6%) patients had the left main disease.
Table 1
| Variables | Value (N=97) |
|---|---|
| Age (years), mean ± SD | 61.5±8.8 |
| Sex, n (%) | |
| Female | 16 (16.5) |
| Male | 81 (83.5) |
| Hypertension, n (%) | 60 (61.9) |
| DM, n (%) | 34 (35.1) |
| Previous PCI, n (%) | 19 (19.6) |
| Previous stroke, n (%) | 17 (17.5) |
| Previous MI, n (%) | 25 (25.8) |
| NYHA class, n (%) | |
| I | 15 (15.5) |
| II | 82 (84.5) |
| III/IV | 0 |
| Left main disease, n (%) | 18 (18.6) |
| LVEF (%) (preoperative), mean ± SD | 59.8±5.6 |
| LVDD (mm) (preoperative), mean ± SD | 51.5±3.6 |
SD, standard deviation; DM, diabetes mellitus; PCI, percutaneous coronary intervention; MI, myocardial infarction; NYHA, New York Heart Association; LVEF, left ventricle ejection fraction; LVDD, left ventricle diastolic dimension.
The patients’ postoperative characteristics are illustrated in Table 2. Among the included patients, 4 (4.1%) had perioperative myocardial infarction, and 32 (33.0%) had new onset atrial fibrillation. The 30-day mortality rate (death due to any cause during the first 30 days after surgery) was 1%; 1 patient (1.0%) who received a single graft (LIMA-LAD) experienced a stroke and aspiration pneumonia and died. Six patients (6.2%) required blood transfusion, and 6 (6.2%) were ventilated over 24 hours.
Table 2
| Variables | N (%) |
|---|---|
| Perioperative MI | 4 (4.1) |
| New onset of AF | 32 (33.0) |
| Neurological complication | 1 (1.0) |
| Blood transfusion | 6 (6.2) |
| Atelectasis | 28 (28.9) |
| Ventilation >24 hours | 6 (6.2) |
| Re-exploration for bleeding | 1 (1.0) |
| IABP support | 1 (1.0) |
| 30-day mortality | 1 (1.0) |
MI, myocardial infarction; AF, atrial fibrillation; IABP, intra-aortic balloon pump.
As shown in Tables 3,4, we also compared the single graft and non-single graft groups. Patients in both groups had BMIs slightly above the normal level (18.5–23.9 kg/m2), which reflected our patient selection standard. Obese patients may hinder visualization. As we become more familiar with the procedure, our selection standards may be broadened. There were more patients with previous MI in the non-single graft group. Furthermore, patients in the non-single graft group had a longer operative duration and more intraoperative blood loss. Other parameters, including the length of intensive care unit (ICU) stay, duration of ventilation, new onset of stroke, new onset of atrial fibrillation, conversion to sternotomy, re-exploration for bleeding, and 30-day mortality, were not significantly different between the two groups. The combined operation included one lobectomy, one local excision of the lung tumor, and one coronary arterio-pulmonary artery fistula.
Table 3
| Variables | Single graft group (n=32) | Non-single graft group (n=65) | P value |
|---|---|---|---|
| Female, n (%) | 8 (25.0) | 8 (12.3) | 0.113 |
| Age (years), mean ± SD | 60.5±9.3 | 61.9±8.6 | 0.472 |
| Height (cm), mean ± SD | 169.4±7.3 | 170.6±6.0 | 0.411 |
| Weight (kg), mean ± SD | 72.7±10.3 | 70.9±7.8 | 0.383 |
| BMI (kg/m2), mean ± SD | 25.3±2.7 | 24.3±1.9 | 0.089 |
| LVEF (%), mean ± SD (preoperative) | 59.7±6.6 | 59.9±5.1 | 0.906 |
| LVDD (mm), mean ± SD (preoperative) | 51.1±4.0 | 51.8±3.5 | 0.382 |
| Previous MI, n (%) | 4 (12.5) | 21 (32.3) | 0.036 |
| Diabetes mellitus, n (%) | 8 (25.0) | 26 (40.0) | 0.145 |
| Hypertension, n (%) | 21 (65.6) | 39 (60.0) | 0.592 |
| COPD, n (%) | 1 (3.1) | 0 | 0.330 (Fisher) |
| Brain infarction history, n (%) | 7 (21.9) | 10 (15.4) | 0.429 |
SD, standard deviation; BMI, body mass index; LVEF, left ventricle ejection fraction; LVDD, left ventricle diastolic dimension; MI, myocardial infarction; COPD, chronic obstructive pulmonary disease.
Table 4
| Variables | Single graft group (n=32) | Non-single graft group (n=65) | P value |
|---|---|---|---|
| Operative duration (hours), mean ± SD | 3.08±0.81 | 5.17±1.13 | 0.000 |
| Combined operation, n (%) | 2 (6.3) | 1 (1.5) | 0.252 |
| Intraoperative blood loss (mL) | |||
| Mean ± SD | 242.8±133.8 | 679.1±402.1 | 0.000 |
| Median [IQR] | 220 [155, 300] | 600 [455, 800] | 0.000 |
| Intraoperative blood transfusion, n (%) | 0 | 6 (9.2) | 0.173 |
| Postoperative ventilation (hours) | |||
| Mean ± SD | 12.4±4.7 | 18.1±25.1 | 0.208 |
| Median [IQR] | 12 [8.5, 16] | 13 [10.0, 15.0] | 0.642 |
| Length of ICU stay (hours) | |||
| Mean ± SD | 54.0±24.8 | 67.2±46.5 | 0.136 |
| Median [IQR] | 45.00 [42.00, 65.75] | 45.0 [43.0, 79.0] | 0.469 |
| LVEF (%), mean ± SD (postoperative) | 59.0±5.8 | 57.2±4.3 | 0.094 |
| LVDD (mm), mean ± SD (postoperative) | 48.2±4.2 | 47.7±3.5 | 0.660 |
| IABP, n (%) | 0 | 1 (1.5) | 1.000 (Fisher) |
| New onset of stroke, n (%) | 1 (3.1) | 0 | 0.330 (Fisher) |
| New onset of atrial fibrillation, n (%) | 11 (34.4) | 21 (32.3) | 1.000 (Fisher) |
| Conversion to sternotomy, n (%) | 0 | 0 | 1.000 |
| Re-exploration for bleeding, n (%) | 0 | 1 (1.5) | 1.000 (Fisher) |
| 30-day mortality, n (%) | 1 (3.1) | 0 | 0.330 (Fisher) |
SD, standard deviation; IQR, interquartile range; ICU, intensive care unit; LVEF, left ventricle ejection fraction; LVDD, left ventricle diastolic dimension; IABP, intra-aortic balloon pump.
A total of 191 grafts were performed. The average number of grafts in the cohort and non-single graft groups were 1.9±0.9 and 2.5±0.6, respectively. Also, 32 patients (33.0%) received one graft, 40 patients (41.2%) received two grafts, 21 patients (21.6%) received three grafts, and 4 patients (4.1%) received four grafts. The LAD branch was grafted in 96 patients. Moreover, 26 patients received a graft to the posterior descending branch (PDA) and 41 grafts to the obtuse marginal branches (OM), 8 to the ramus intermedius artery (RI), 16 to the diagonal branch (DIAG), 3 to the left posterior ventricular branch (PL), and 1 to right coronary artery (RCA) were done. In patients who received more than two grafts involving both the left and right coronary systems, two proximal anastomoses were hand-sewn under direct visualization to enable coronary revascularization with a similar configuration to median sternotomy CABG. In other words, we never perform sequential or Y grafts composed of the left and right coronary branches. Also, there was no conversion to median sternotomy and no conversion to on-pump CABG. One patient in the non-single graft group had unstable hemodynamics during surgery, and an intra-aortic balloon pump (IABP) was utilized. The 30-day mortality rate was 1.0%; one patient in the single graft group (LIMA-LAD) died of aspiration pneumonia after a stroke. Meanwhile, in the non-single graft group, no postoperative cerebral complications, such as transient delirium, were observed. No postoperative aortic dissection was detected. One patient had a pulmonary embolism and received anticoagulation therapy. All grafts were unobstructed except for one graft to the OM (0.5%). One patient in the non-single graft group had wound dehiscence and needed secondary suturing. No deep wound infection was found.
The patients were followed up for 3–27 months. The major adverse cardiovascular and cerebral events (MACCE) were also analyzed. During the follow-up period, one patient (1.1%) suffered from acute myocardial infarction and received PCI. One patient (1.1%) who had suspected angina was examined by dual-source CT and revealed all grafts unobstructed. After careful evaluation, the patient was recommended to psycho-cardiology. All patients are alive and angina-free.
Discussion
Median sternotomy coronary revascularization is a safe and effective procedure for treating multi-vessel disease, with good long-term outcomes and reproducibility. However, the associated trauma and slow recovery of this procedure are concerning. In recent years, with the development of MICS CABG, an increasing number of patients have chosen to accept the procedure when possible. First introduced in 1995, MICS CABG has been adopted by many surgeons in Europe and America (8,9). Previous reports indicate that MICS CABG not only has comparable patency rates with traditional median sternotomy CABG (10-12), it also avoids sternotomy-related complications, such as wound dehiscence and decreased physical ability, and provides favorable outcomes including shorter hospitalization, faster recovery, and decreased need for blood transfusion. Long-term clinical data on MICS CABG is limited, but several observational studies have demonstrated promising clinical results (11-13). Furthermore, it is widely acknowledged that there is a learning curve in MICS CABG. Compared to conventional median sternotomy CABG, there may be a longer operative duration, increased intraoperative blood loss, and even ICU stays (especially in earlier cases). This does not affect the graft patency or the clinical outcomes. The early and mid-term outcomes of MICS CABG are promising and satisfy patient preferences for a less invasive procedure.
Studies have reported a perioperative mortality rate ranging from 0% to 1.3% (11,14). One of our patients in the single graft group suffered from a stroke and died of aspiration pneumonia. In the non-single graft group, no proximal anastomosis-related complications such as stroke or aortic dissection were observed. In our patient cohort, the total 30-day mortality rate was 1%. No patients were transferred to on-pump or median sternotomy. The in-hospital complications with MICS CABG procedures may include new onset of atrial fibrillation (AF), stroke, renal failure, and incision complications. In this study, the rate of postoperative AF was 33%, and no renal failure was observed.
We chose the LIMA and veins as the grafts for most of our patients. RIMA was used in only one patient, since RIMA harvesting through a small incision may be difficult. In terms of survival, a previous report on BIMA use has shown that patients receiving BIMA grafts did not exhibit a significant difference compared to those receiving SIMA grafts (15). Based on the current evidence, LIMA is still the best arterial-coronary conduit in terms of patency and clinical outcome (7,16,17).
Although multi-arterial grafting is being increasingly emphasized in recent years, saphenous vein graft (SVG) is still universally selected as the CABG conduit (18). There remains controversy regarding the patency of sequential and individual grafts. Multiple studies have reported superior mid- and long-term patency of sequential grafts compared with individual grafts, especially in small coronaries (18,19). Also, there is still some concern about sequential grafts, as the possibility of proximal occlusion may affect the perfusion of several coronary branches (20,21).
From our perspective, no more than two distal anastomoses should be performed for each sequential graft, and no Y-graft is related to LIMA-LAD. We pursue the conventional configuration of CABG, which involves one to two anastomoses on the aorta. Given that it provides good long-term results, no aorta-related complications were observed in our experience.
It is well known that patients with multi-vessel lesions, severe LV dysfunction and ample viable myocardium benefit most from CABG. Meanwhile, for patients with a preserved baseline LVEF, research on the changes of LVEF after CABG is scarce. Papestiev et al. reported that in patients with preoperative LV dysfunction, there is an improvement in systolic function, whereas, in patients with preserved preoperative myocardial function, a decline in postoperative LVEF was observed (22). This is consistent with our findings, which showed statistical differences in the non-single graft group and no statistical differences in the single graft group (not illustrated in table).
Limitations
Firstly, this was a retrospective observational study, and our follow-up duration was relatively short. Also, we did not perform transit-time flow measurements to assess the intraoperative blood flow after vascular anastomosis. Moreover, one patient experienced graft occlusion without revision; the patient had no ischemic symptoms, and intensive medication was applied.
Conclusions
Conventional coronary artery bypass grafting using the left thoracotomy (8–10cm) approach can be performed safely with low rates of postoperative morbidity and mortality and acceptable early and mid-term results in properly selected patients. In multi-vessel coronary artery disease, the procedure achieves complete myocardial revascularization while avoiding sternotomy. In proximal anastomosis, blood pressure control helps to avoid aortic manipulation-related complications. We applied the standard LIMA-LAD rule and traditional configuration, which separates the left and right coronary territories. As more cases are performed, our selection criteria may be broadened and more patients may benefit from this procedure.
Acknowledgments
Funding: This study was funded by the Tianjin Key Medical Discipline (Specialty) Construction Project (No. TJYXZDXK-042A).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1162/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1162/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1162/coif). All authors report that this study was funded by the Tianjin Key Medical Discipline (Specialty) Construction Project (No. TJYXZDXK-042A). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Tianjin Chest Hospital (No. 2022LW-016). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Serruys PW, Ong AT, van Herwerden LA, et al. Five-year outcomes after coronary stenting versus bypass surgery for the treatment of multivessel disease: the final analysis of the Arterial Revascularization Therapies Study (ARTS) randomized trial. J Am Coll Cardiol 2005;46:575-81. [Crossref] [PubMed]
- From AM, Al Badarin FJ, Cha SS, et al. Percutaneous coronary intervention with drug-eluting stents versus coronary artery bypass surgery for multivessel coronary artery disease: a meta-analysis of data from the ARTS II, CARDia, ERACI III, and SYNTAX studies and systematic review of observational data. EuroIntervention 2010;6:269-76. [Crossref] [PubMed]
- Loop FD, Lytle BW, Cosgrove DM, et al. J. Maxwell Chamberlain memorial paper. Sternal wound complications after isolated coronary artery bypass grafting: early and late mortality, morbidity, and cost of care. Ann Thorac Surg 1990;49:179-87. [Crossref] [PubMed]
- Braxton JH, Marrin CA, McGrath PD, et al. 10-year follow-up of patients with and without mediastinitis. Semin Thorac Cardiovasc Surg 2004;16:70-6. [Crossref] [PubMed]
- Risnes I, Abdelnoor M, Almdahl SM, et al. Mediastinitis after coronary artery bypass grafting risk factors and long-term survival. Ann Thorac Surg 2010;89:1502-9. [Crossref] [PubMed]
- Lin TH, Wang CW, Shen CH, et al. Clinical outcomes of multivessel coronary artery disease patients revascularized by robot-assisted vs conventional standard coronary artery bypass graft surgeries in real-world practice. Medicine (Baltimore) 2021;100:e23830. [Crossref] [PubMed]
- Loop FD, Lytle BW, Cosgrove DM, et al. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med 1986;314:1-6. [Crossref] [PubMed]
- Iribarne A, Easterwood R, Chan EY, et al. The golden age of minimally invasive cardiothoracic surgery: current and future perspectives. Future Cardiol 2011;7:333-46. [Crossref] [PubMed]
- Hartz RS. Minimally invasive heart surgery. Executive Committee of the Council on Cardio-Thoracic and Vascular Surgery. Circulation 1996;94:2669-70. [Crossref] [PubMed]
- Van Praet KM, Kofler M, Shafti TZN, et al. Minimally Invasive Coronary Revascularisation Surgery: A Focused Review of the Available Literature. Interv Cardiol 2021;16:e08. [Crossref] [PubMed]
- Kofidis T, Emmert MY, Paeschke HG, et al. Long-term follow-up after minimal invasive direct coronary artery bypass grafting procedure: a multi-factorial retrospective analysis at 1000 patient-years. Interact Cardiovasc Thorac Surg 2009;9:990-4. [Crossref] [PubMed]
- Nambala S, Mishra YK, Ruel M. Less invasive multivessel coronary artery bypass grafting: now is the time. Curr Opin Cardiol 2021;36:735-9. [Crossref] [PubMed]
- Liu JJ, Kong QY, You B, et al. Surgical Challenges in Multi-Vessel Minimally Invasive Coronary Artery Bypass Grafting. J Interv Cardiol 2021;2021:1195613. [Crossref] [PubMed]
- Guida GA, Guida GA, Bruno VD, et al. Left thoracotomy approach for off-pump coronary artery bypass grafting surgery: 15 years of experience in 2500 consecutive patients. Eur J Cardiothorac Surg 2020;57:271-6. [PubMed]
- Narayan P. Has Arterial Revascularization Trial ART burst the BITA bubble? Indian J Thorac Cardiovasc Surg 2020;36:78-80. [Crossref] [PubMed]
- Taggart DP, Altman DG, Gray AM, et al. Randomized Trial of Bilateral versus Single Internal-Thoracic-Artery Grafts. N Engl J Med 2016;375:2540-9. [Crossref] [PubMed]
- Nappi F, Sutherland FW, Al-Attar N, et al. Incomplete Revascularization in PCI and CABG: When Two Plus Two Does Not Make Four. J Am Coll Cardiol 2016;68:877-8. [Crossref] [PubMed]
- Park SJ, Kim HJ, Kim JB, et al. Sequential Versus Individual Saphenous Vein Grafting During Coronary Arterial Bypass Surgery. Ann Thorac Surg 2020;109:1165-73. [Crossref] [PubMed]
- Joshi MM, Paul S, Bhosle KN, et al. Individual Versus Sequential Saphenous Vein Grafts for on-Pump Coronary Artery Bypass Grafting - Does Smaller Coronaries in Indians Affect Graft Choice? - A Mid-Term Patency Comparison Study. J Saudi Heart Assoc 2021;33:109-16. [Crossref] [PubMed]
- Ouzounian M, Hassan A, Yip AM, et al. The impact of sequential grafting on clinical outcomes following coronary artery bypass grafting. Eur J Cardiothorac Surg 2010;38:579-84. [Crossref] [PubMed]
- Meeter K, Veldkamp R, Tijssen JG, et al. Clinical outcome of single versus sequential grafts in coronary bypass operations at ten years' follow-up. J Thorac Cardiovasc Surg 1991;101:1076-81. [Crossref] [PubMed]
- Papestiev V, Jovev S, Sokarovski M, et al. Changes of Left Ventricular Systolic Function in Patients Undergoing Coronary Artery Bypass Grafting. Open Access Maced J Med Sci 2019;7:3574-8. [Crossref] [PubMed]
(English Language Editor: A. Kassem)