The application of postoperative chemotherapy in thymic tumors and its prognostic effect
Introduction
Thymic malignancies are relatively rare tumors most commonly seen in the anterior mediastinum. While most thymic tumors have favorable outcomes after surgical resection (1), around 1/3 of the patients may already have advanced disease upon presentation, making radical surgery inapplicable. As reported by Masaoka et al. (2), the 5-year survival rate was about 67% for locally advanced cases, and only 50% for patients with distant metastasis. There are still controversies concerning chemotherapy for thymic tumors. The purposes of chemotherapy are to reduce the tumor burden to enable the possibility of later surgery or radiotherapy on the one hand, and to extend the time for controlling the diseases on the other. Chemotherapy can be applied at different stages of the treatment, with the normal treatment modes including preoperative chemotherapy and surgery, surgery and postoperative chemotherapy, or chemoradiotherapy. Besides, for thymic tumors patients with distant metastasis, palliative chemotherapy is usually the major therapeutic approach. At present, there are no standard chemotherapy regimens for thymic tumors, and even these limited data comes mostly from the single-institution retrospective reports, with largely varied results. The Chinese Alliance for Research in Thymomas (ChART) retrospectively collected patient data from 18 centers nationwide, and used this collective data to study the management outcomes in thymic tumors patients. The current study aimed at elucidating the value of postoperative chemotherapy in Masaoka-Koga stage III and IV tumors (3), using the ChART database.
Materials and methods
Clinical data and research method
From March 1994 to December 2012, ChART database registered 2,306 thymic tumor cases. After excluding patients with unclear information on staging (224 cases), World Health Organization (WHO) histology types (121 cases), whether radiotherapy was applied (96 cases), and those non-surgical patients (97 cases), or those treated with induction therapy (68 cases), 1,700 patients were finally included in the current study. Among them, 294 (17.3%) patients received chemotherapy after surgery, while the rest 1,406 (82.7%) patients did not. For studying the effect of postoperative chemotherapy on prognosis, only tumors in Masaoka-Koga III and IV were included. Because only de-identified data were used for the study, informed consent was waived by Institutional Review Board (IRB).
Statistical processing
Clinical pathological data and follow-up information entered into the database were retrospectively reviewed. The SPSS 19.0 software was used for statistical analysis. Ratios were compared with χ2 test. Regarding survival analysis, Kaplan-Meier method was used to chart the survival curves, and log-rank test was used for inter-group comparison. Cox regression was used in multivariate analysis to reveal the factors affecting the prognosis, with a 95% confidence interval (CI). To reduce the impact of unbalanced distribution of factors due to the retrospective nature of the study, 1:1 caliper propensity-matched study was used to further compare the survival in patients having or not having chemotherapy after surgery. Differences were considered statistically significant if P<0.05.
Results
Overall incidence of postoperative chemotherapy
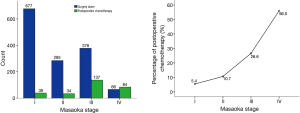
Percentages of patients having postoperative chemotherapy in different tumor stages were listed in Table 1. As can be seen from Figure 1, the use of adjuvant chemotherapy increased significantly with Masaoka-Koga tumor stage (P=0.000). In stage I and II tumors, chemotherapy was used in less than 10% cases.
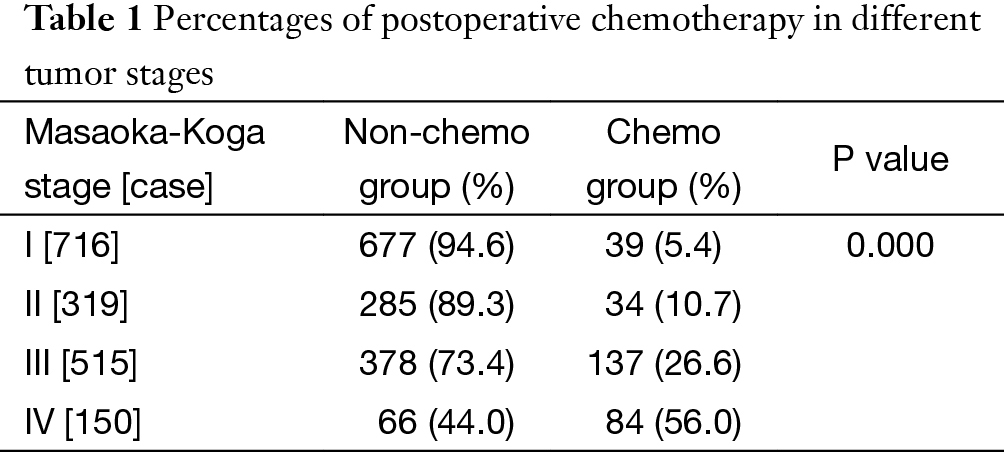
Full table
Postoperative chemotherapy in Masaoka-Koga III and IV patients
For the purpose of the study, patients with stage I (716 cases) and stage II (319 cases) tumors were further excluded. In the remaining 665 Masaoka-Koga III and IV patients, 221 received postoperative chemotherapy (chemo group) and the other 444 did not receive adjuvant chemotherapy (non-chemo group).
Clinical analysis on general data
Clinical features and myasthenia of the patients
The results showed that there was no significant difference in patients’ gender or age between the chemo and non-chemo groups. Significantly fewer patients in the chemo group had concomitant myasthenia gravis than the non-chemo group (P=0.000) (Table 2).
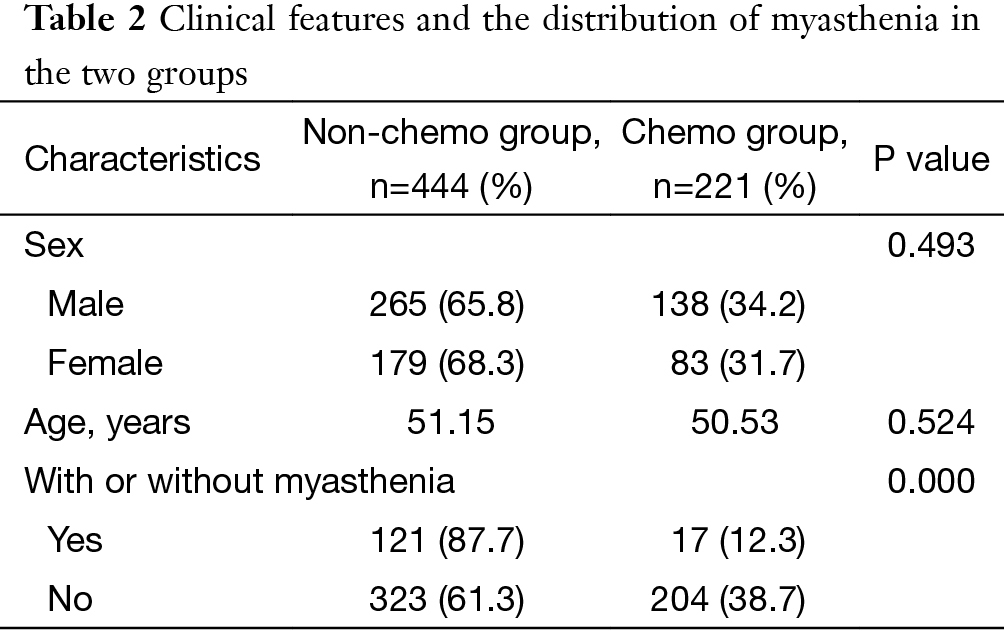
Full table
Type of thymic tumors
Significant difference was detected in the percentages of postoperative chemotherapy among different WHO histology types (P=0.000). Postoperative chemotherapy was used significantly more often in type C or neuroendocrine tumors than in type B1 + B2 + B3 or A + AB tumors (P=0.000) (Table 3).
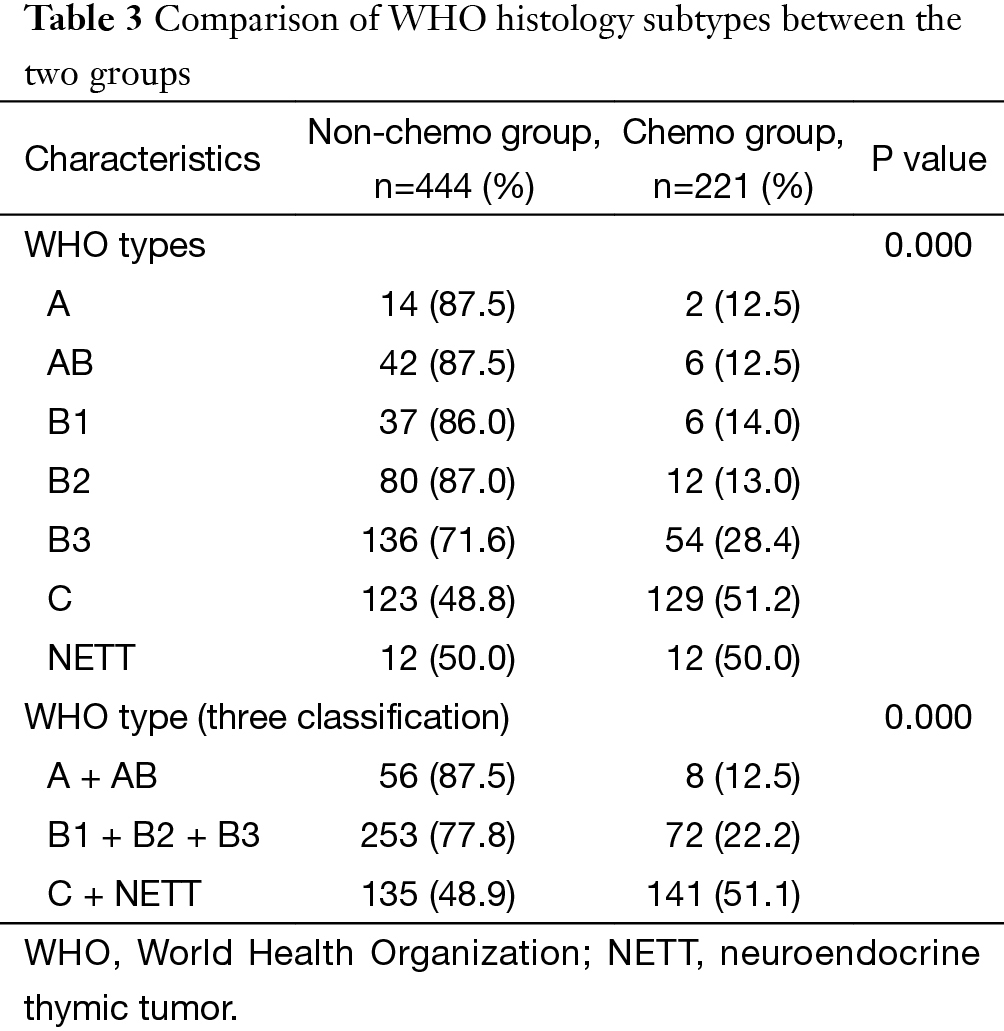
Full table
Comparison between tumor size, pathological staging and resection status
Tumors size was similar between the two groups (P=0.218). The chemo group had significantly more patients with stage IV diseases (P=0.000). Overall radical resection rate in this cohort was 73.1%. It was significantly higher in the non-chemo group than in the chemo group (P=0.000) (Table 4).
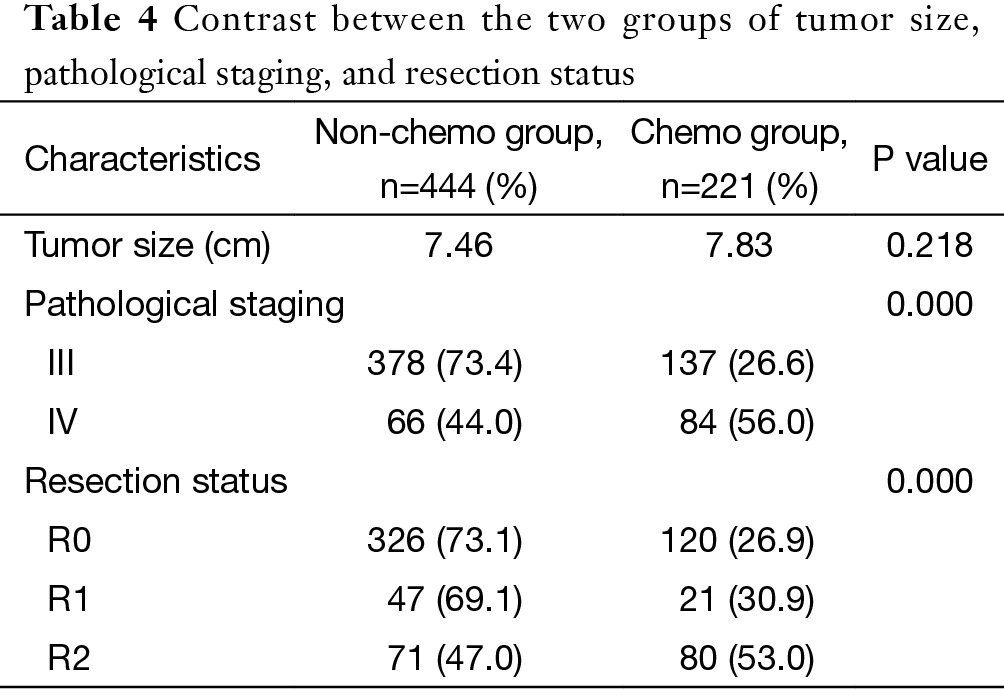
Full table
Modes of adjuvant therapy
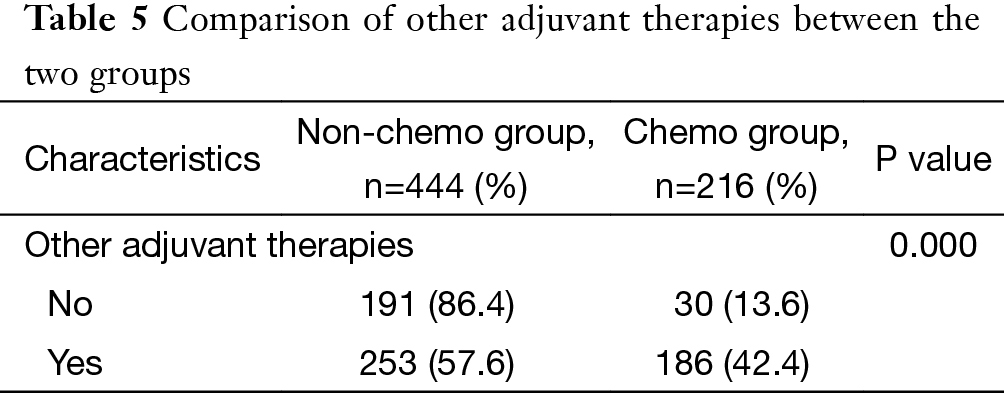
Detailed information on postoperative adjuvant therapy was lacking for further analysis in five patients. Among the remaining 660 patients, 191 patients were treated by surgery alone (191/660, 28.9%), and the rest 469 patients received postoperative adjuvant therapies (71.1%). These included 30 patients having chemotherapy alone (30/660, 4.5%), 253 patients having radiotherapy alone (253/660, 38.3%), and 186 patients having postoperative chemoradiotherapy (186/660, 28.2%). In the chemo group, significantly more patients received chemoradiotherapy than chemotherapy alone (P=0.000) (Table 5).
Full table
Analysis of factors relating to the survival of Masaoka-Koga III and IV patients
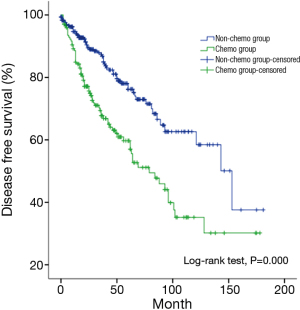
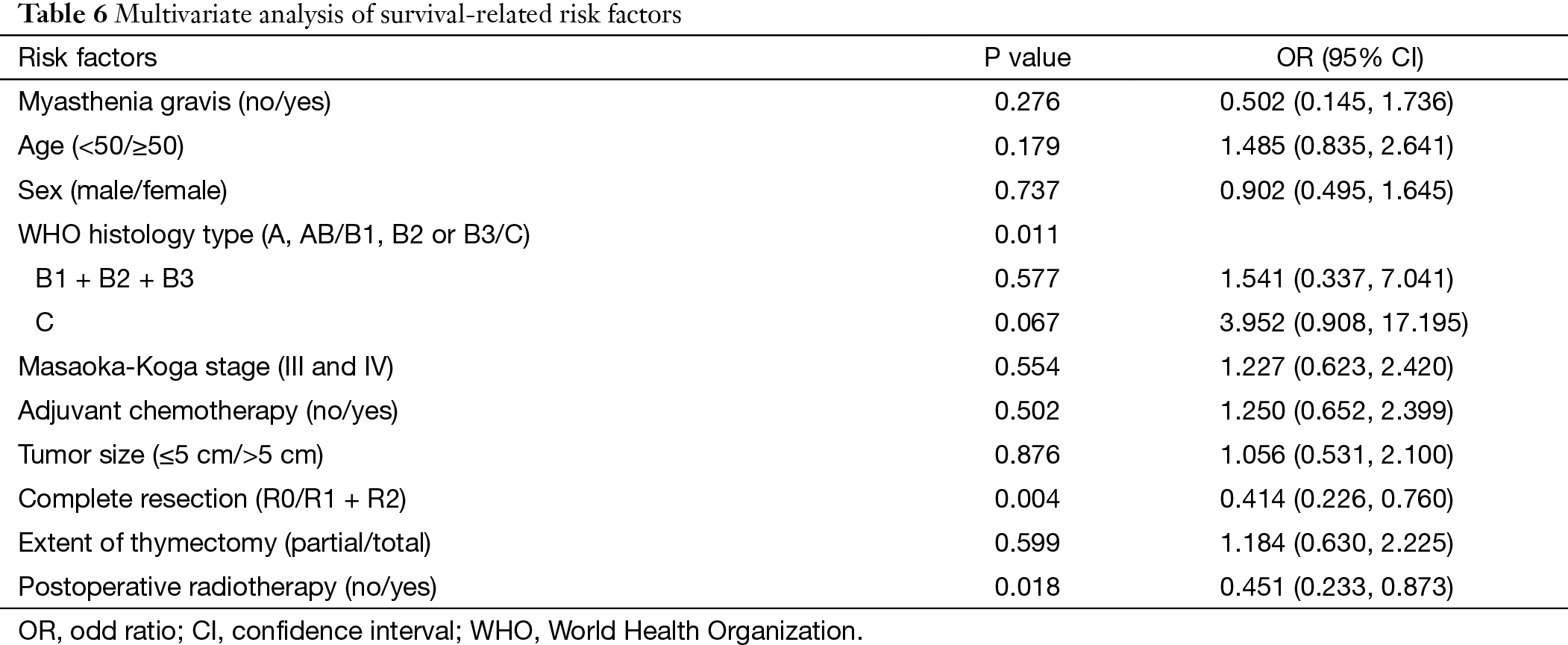
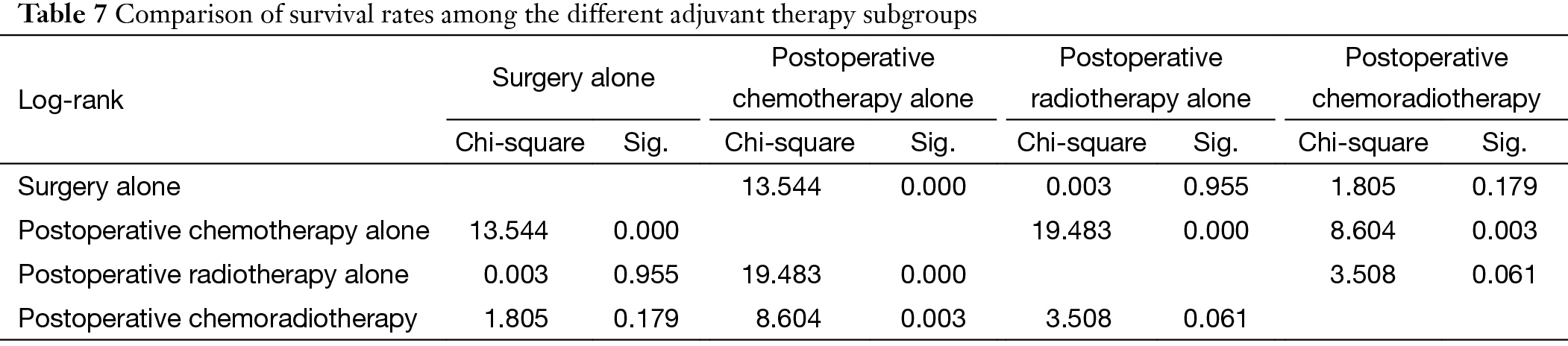
Multivariate analysis showed that histological subtypes, resection status and postoperative radiotherapy were the independent predictive factors for overall survival in patients with stage III and IV tumors. WHO type C tumors, incomplete resection, and use of adjuvant radiation were associated with significantly worse outcome (P=0.011, P=0.004, P=0.018). Five-year and 10-year disease free survivals (DFS) were 73% and 58% for the non-chemo group, and 51% and 30% for the chemo group, with significant differences between the two groups (P=0.000) (Table 6, Table 7) (Figure 2).
Full table
Full table
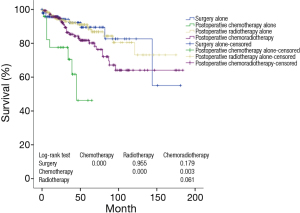
Further stratification analysis showed that the survival rate in Masaoka-Koga III and IV patients having adjuvant chemotherapy alone after surgery was noticeably lower than those having surgery alone, postoperative radiotherapy alone, or postoperative chemoradiotherapy (P=0.000, P=0.000, P=0.003, respectively) (Figure 3).
Multivariate analysis of factors related to recurrence in Masaoka-Koga stage III and IV patients
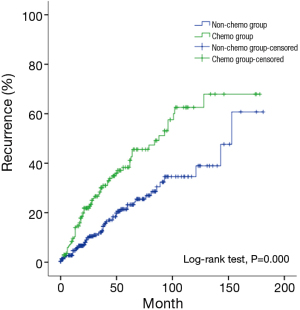
Multivariate analysis showed that histology subtypes, resection status, and postoperative radiotherapy were the independent risk factors predicting recurrence in Masaoka-Koga stage III and IV tumors. Recurrence was significantly more frequent in those patients with WHO type C tumors, incomplete resection, or postoperative radiation (P=0.024, P=0.021, P=0.014, respectively). Five-year and 10-year recurrence rates were 26% and 40% in the non-chemo group, and were 46% and 68% in the chemo group, with statistical significance between the groups (P=0.000) (Table 8) (Figure 4).
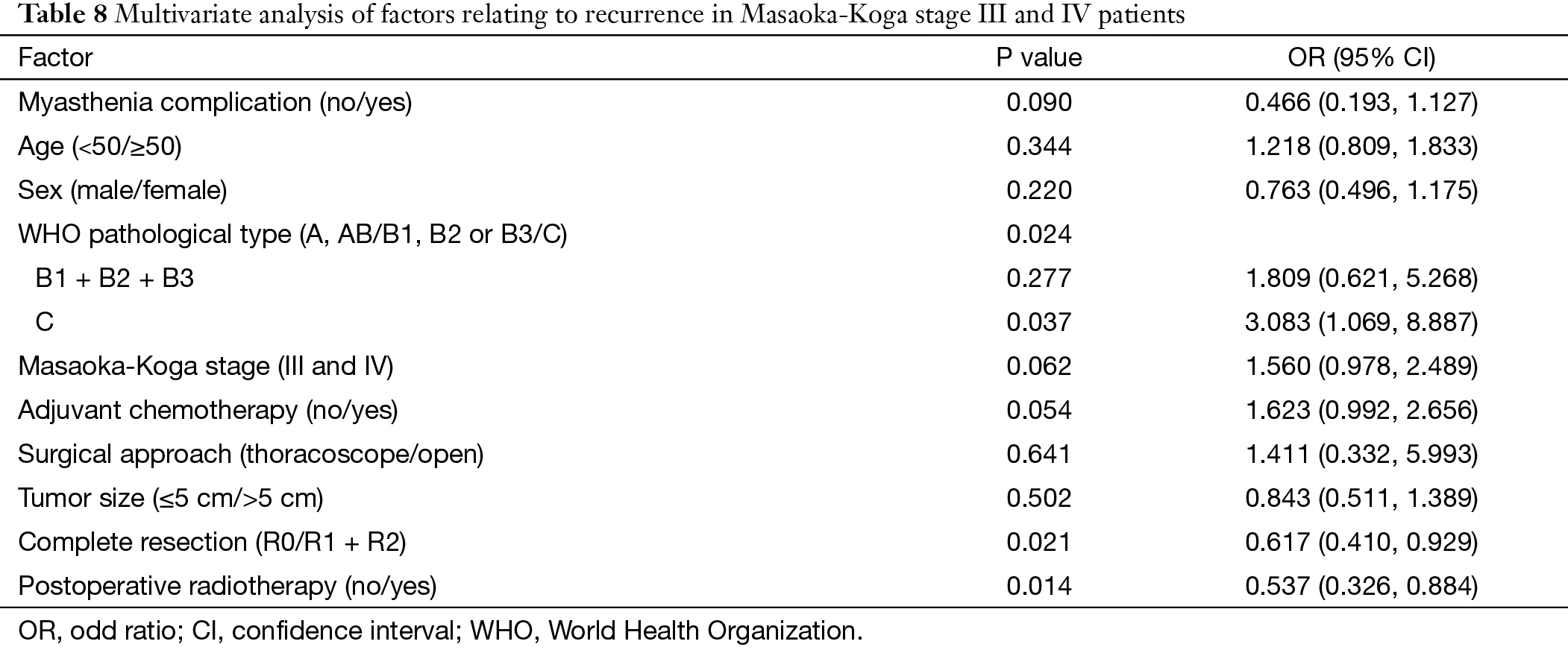
Full table
Further stratification analysis showed that recurrence rate in Masaoka-Koga stage III and IV patients having adjuvant chemotherapy alone after surgery was noticeably higher than those of having surgery alone, postoperative radiotherapy alone, or postoperative chemoradiotherapy (P=0.002, P=0.000, P=0.024, respectively) (Figure 5) (Table 9).
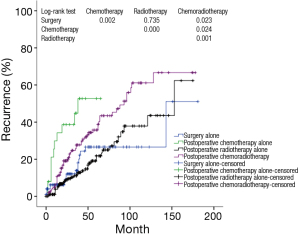
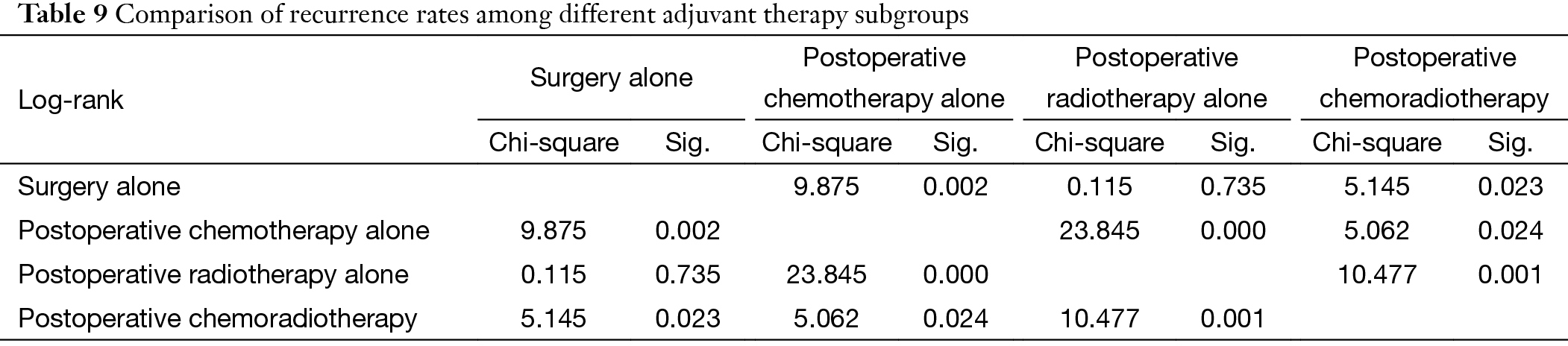
Full table
Propensity-matched study comparing survival in patients having or not having adjuvant chemotherapy
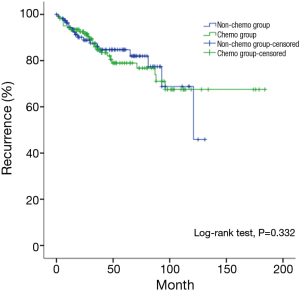
The study was carried out by matching factors including the presence of myasthenia gravis, WHO histology type, pathological staging, resection status, and postoperative adjuvant radiotherapy. Three-hundred and sixteen patients were obtained after matching, with 158 cases each in the chemo and non-chemo groups. Patient characteristics were listed in Table 10. Again no survival benefit from postoperative chemotherapy was detected, although this time the survivals in patients having or not having chemotherapy after surgery became similar (P=0.332, Figure 6).
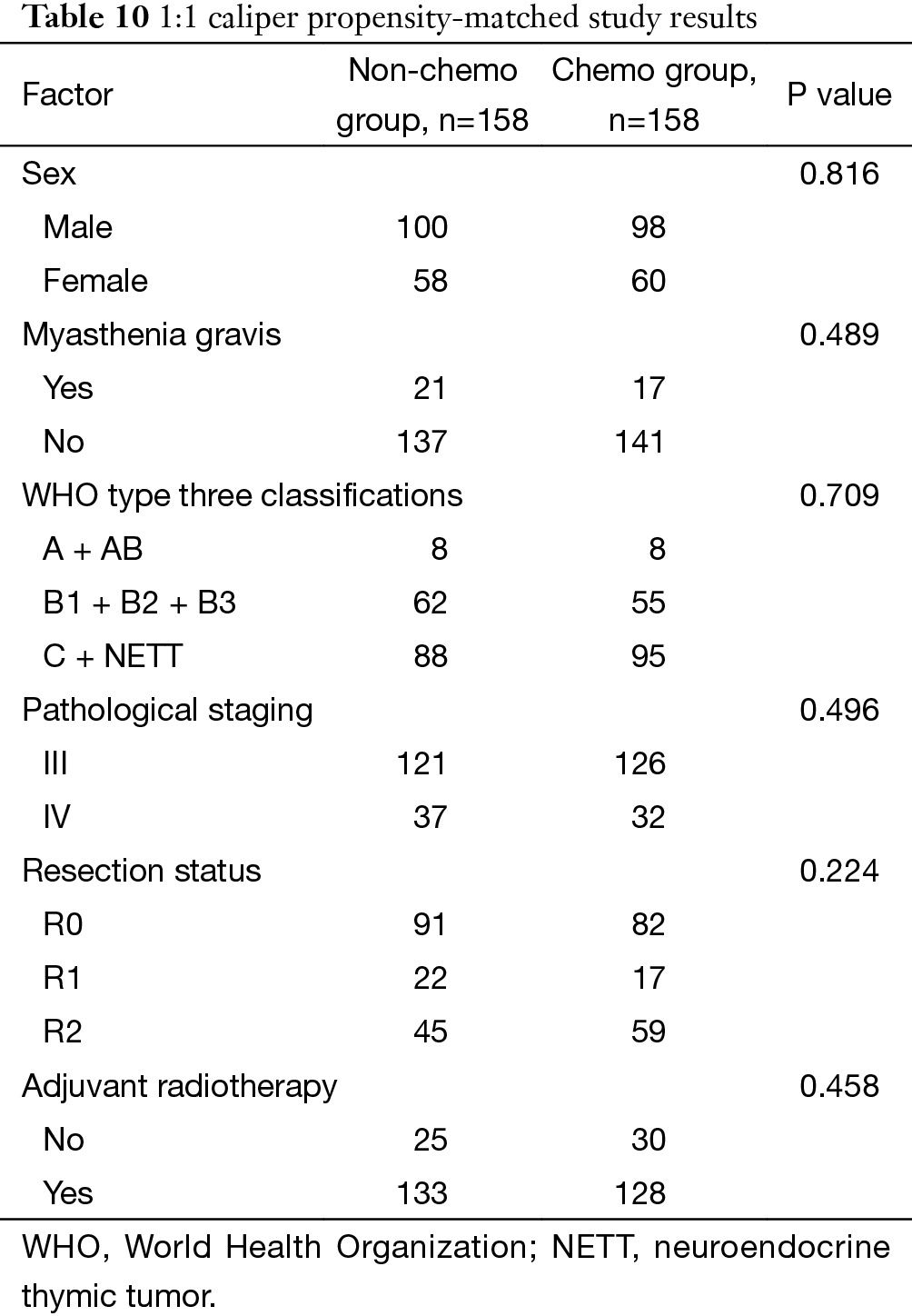
Full table
Discussion
Long-term survival of thymic tumors patients varies according to their histological subtypes, tumor staging, and resection status, which have repeatedly been identified as independent predictive factors for prognosis. As reported by Kondo et al. (4), 5-year survival rates for patients at stages I, II, III and IV were 100%, 98%, 89% and 71% respectively. Most published results showed that for tumors at stages I and II, no postoperative adjuvant therapy is necessary after complete resection (5,6). But in stage III and IV diseases, radical resection rate is much lower due to extensive local invasion or tumor spread. Postoperative adjuvant therapies, including chemotherapy, are often accepted as a common practice. Our results also found that WHO type C tumors, incomplete resection, and postoperative radiotherapy were adversely related to survival and recurrence in thymic tumor patients. The worse outcome in patients receiving adjuvant radiotherapy may be contributed to its use in higher grade tumors in more advanced stages. Unfortunately, we failed to find any survival benefit of adjuvant chemotherapy for stage III and IV tumors in our study.
Currently, postoperative chemotherapy is mainly used with the purpose to reduce tumor burden after incomplete surgery, or to enhance disease control after complete resection. Its prognostic value is highly controversial, as there have been only limited data from retrospective studies of small sample researches (7-11). Ströbel et al. (12) reported a retrospective analysis on their experiences in 228 cases thymomas and thymic squamous cell carcinomas. The results showed that postoperative chemotherapy did not improve long-term survival in type A, AB and B1 thymomas, or in stage II type B2 and B3 tumors, while postoperative radiotherapy seemed to extend survival in stage III patients. Kim et al. (13) analyzed the clinical data from 100 cases of thymic tumors and found that comparing to postoperative radiotherapy alone, adding chemotherapy to radiation failed to show any significant difference in 5-year survival rates of stage II and IV tumor patients. Kondo et al. (4) reported the largest retrospective cohort yet published, including 1,320 cases of thymic tumors treated at 115 medical centers in Japan. Again, they found that neither postoperative radiotherapy nor postoperative chemotherapy could improve the outcomes in stage III and IV thymic tumors after radical surgery. Attaran et al. (14) concluded that although primary chemotherapy and palliative chemotherapy might have favorable therapeutic effect in certain patients, no evidence till now showed that postoperative chemotherapy could help prolong survival in patients with thymic tumors in general. Using the ChART retrospective database, the results from the current study were in consistency with the previous reports. Our results indicate that for Masaoka-Koga stage III and IV thymomas and thymic carcinomas, postoperative chemotherapy has no clear advantage with regard to recurrence and survival time.
The retrospective nature of our study and the lack of consistence in the sources of data from different centers may help explain the causes of relatively unfavorable survival and higher recurrence in patients receiving adjuvant chemotherapy after surgery. The chemo group was found to have higher grade of tumor, more stage IV diseases, and less radical resection comparing to the non-chemo group, all of which have been shown to be associated with worse prognosis in thymic malignancy. The Japanese Association for Research in Thymus (JART) results (4) showed that 10-year survival rates in completely resected stage III and IV thymomas were 70.9%, 70.4%, 77.9% and 95% respectively for the subgroups of postoperative chemotherapy, postoperative chemoradiotherapy, postoperative radiotherapy and surgery alone, with a significant difference between the subgroups of surgery alone and of postoperative chemoradiotherapy (P=0.0353). The 5-year survival rates in completely resected stage III and IV thymic carcinomas were 81.5%, 46.6%, 73.6%, and 72.2% respectively, for the subgroups of postoperative chemotherapy alone, postoperative chemoradiotherapy, postoperative radiotherapy, and surgery alone, with significant differences among the subgroups of postoperative chemoradiotherapy, postoperative chemotherapy and surgery alone (P=0.0213, P=0.0397). In the current study from the ChART database, survival rates in the subgroups of postoperative chemoradiotherapy and of postoperative chemotherapy were even worse compared to the subgroups of surgery alone or surgery followed by radiotherapy. In view of the unbalanced distribution of potential risk factors in each group, we are in no position to conclude that chemotherapy lead to worse outcome. But neither did we found any survival benefit brought by the application of postoperative chemotherapy to our patients with Masaoka-Koga stage III and IV thymic tumors.
To rule out the potential selection bias and other intrinsic bias associated with all retrospective studies, we further carried out a propensity matched study so as to balance the distribution of all risk factors. The 158 pairs of patients having or not having postoperative chemotherapy after surgery were comparable in tumor stage and histology, myasthenia gravis, resection status, and postoperative radiotherapy. Still there was no significant difference in overall survival rates between the subgroups having or not having postoperative chemotherapy (P=0.332). Thus at current stage it is reasonable to conclude that, postoperative chemotherapy may not have any survival benefits for advanced stage thymic tumors.
Because of the multiple centers involved and long time span, the chemotherapy regimens, cycles and dosage used in the current study were highly heterogeneous. It was thus impossible for us to evaluate the difference of any specific regimen. Only prospectively designed study could help answer such questions. However, our study again shows that histological subtypes and completeness of resection play major roles in determining the prognosis of thymic tumors, even for advanced stage diseases. Currently available chemotherapy regimens do not seem likely to provide significant survival benefit for this group of patients. The hope of improving management outcomes would lie in the use of chemotherapy in neoadjuvant setting, or in the search of more effective new agents for thymic tumors.
Acknowledgements
None.
Footnote
Members of Chinese Alliance for Research in Thymomas (ChART): Yi Shen, Yucheng Wei, Affiliated Hospital of Qingdao University, Qingdao, China; Yin Li, Guanghui Liang, Affiliated Cancer Hospital of Zhengzhou University, Zhengzhou, China; Keneng Chen, Hao Fu, Beijing Cancer Hospital, Beijing, China; Hezhong Chen, Shihua Yao, Changhai Hospital, Shanghai, China; Youbin Cui, Yanzhong Xin, First Affiliated Hospital of Jilin University, Changchun, China; Renquan Zhang, Ningning Kang, First Hospital of Anhui Medical University, Hefei, China; Lijie Tan, Jianyong Ding, Hao Wang, Gang Chen, Jie Wu, Zhongshan Hospital, Fudan University, Shanghai, China; Chun Chen, Wei Zheng, Fujian Medical University Union Hospital, Fuzhou, China; Liewen Pang, Fangrui Wang, Huashan Hospital, Fudan University, Shanghai, China; Yangchun Liu, Qing Lin, Jiangxi People’s Hospital, Nanchang, China; Yongyu Liu, Yongkai Wu, Liaoning Cancer Hospital, Shenyang, China; Wentao Fang, Jie Zhang, Yan Shen, Changlu Wang, Lei Zhu, Zhitao Gu, Shanghai Chest Hospital, Shanghai Jiao Tong University, Shanghai, China; Yongtao Han, Lin Peng, Sichuan Cancer Hospital, Chengdu, China; Jianhua Fu, Qianwen Liu, Department of Thoracic Surgery, Guangdong Esophageal Cancer Institute, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center of Cancer Medicine, Guangzhou, China; Zhentao Yu, Jie Yue, Tianjin Cancer Hospital, Tianjin, China; Peng Zhang, Yuan Chen, Tianjin Medical University General Hospital, Tianjin, China; Yun Wang, Yingcai Geng, West China Hospital, Sichuan University, Chengdu, China; Xinming Zhou, Hongguang Zhao, Zhejiang Cancer Hospital, Hangzhou, China.
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Girard N, Mornex F, Van Houtte P, et al. Thymoma: a focus on current therapeutic management. J Thorac Oncol 2009;4:119-26. [Crossref] [PubMed]
- Masaoka A, Monden Y, Nakahara K, et al. Follow-up study of thymomas with special reference to their clinical stages. Cancer 1981;48:2485-92. [Crossref] [PubMed]
- Detterbeck FC, Nicholson AG, Kondo K, et al. The Masaoka-Koga stage classification for thymic malignancies: clarification and definition of terms. J Thorac Oncol 2011;6:S1710-6. [Crossref] [PubMed]
- Kondo K, Monden Y. Therapy for thymic epithelial tumors: a clinical study of 1,320 patients from Japan. Ann Thorac Surg 2003;76:878-84; discussion 884-5. [Crossref] [PubMed]
- Singhal S, Shrager JB, Rosenthal DI, et al. Comparison of stages I-II thymoma treated by complete resection with or without adjuvant radiation. Ann Thorac Surg 2003;76:1635-41; discussion 1641-2. [Crossref] [PubMed]
- Mangi AA, Wright CD, Allan JS, et al. Adjuvant radiation therapy for stage II thymoma. Ann Thorac Surg 2002;74:1033-7. [Crossref] [PubMed]
- Fornasiero A, Daniele O, Ghiotto C, et al. Chemotherapy of invasive thymoma. J Clin Oncol 1990;8:1419-23. [PubMed]
- Bretti S, Berruti A, Loddo C, et al. Multimodal management of stages III-IVa malignant thymoma. Lung Cancer 2004;44:69-77. [Crossref] [PubMed]
- Loehrer PJ Sr, Jiroutek M, Aisner S, et al. Combined etoposide, ifosfamide, and cisplatin in the treatment of patients with advanced thymoma and thymic carcinoma: an intergroup trial. Cancer 2001;91:2010-5. [Crossref] [PubMed]
- Berruti A, Borasio P, Roncari A, et al. Neoadjuvant chemotherapy with adriamycin, cisplatin, vincristine and cyclophosphamide (ADOC) in invasive thymomas: results in six patients. Ann Oncol 1993;4:429-31. [PubMed]
- Berruti A, Borasio P, Gerbino A, et al. Primary chemotherapy with adriamycin, cisplatin, vincristine and cyclophosphamide in locally advanced thymomas: a single institution experience. Br J Cancer 1999;81:841-5. [Crossref] [PubMed]
- Ströbel P, Bauer A, Puppe B, et al. Tumor recurrence and survival in patients treated for thymomas and thymic squamous cell carcinomas: a retrospective analysis. J Clin Oncol 2004;22:1501-9. [Crossref] [PubMed]
- Kim BK, Cho BC, Choi HJ, et al. A single institutional experience of surgically resected thymic epithelial tumors over 10 years: clinical outcomes and clinicopathologic features. Oncol Rep 2008;19:1525-31. [PubMed]
- Attaran S, McCormack D, Pilling J, et al. Which stages of thymoma benefit from adjuvant chemotherapy post-thymectomy? Interact Cardiovasc Thorac Surg 2012;15:273-5. [Crossref] [PubMed]