
Comparison of the Masaoka-Koga staging and the International Association for the Study of Lung Cancer/the International Thymic Malignancies Interest Group proposal for the TNM staging systems based on the Chinese Alliance for Research in Thymomas retrospective database
Introduction
Up till now, not a single staging system for thymic malignancy has ever been universally adopted. Neither has an official stage classification ever been defined by the Union for International Cancer Control (UICC). The Masaoka staging system, further modified by Koga et al., is most widely used (1,2). Although this staging system appeared to be closely related to prognosis for thymic malignancies in many studies (3), it was based on merely 91 patients treated over 30 years ago at a single institution. And comparing to the staging of most other malignancies, the Masaoka-Koga system is sketchy and does not separate the prognostic impact of lymphatic or hematologic dissemination from direct tumor invasion using TNM components as a common practice. Thus a universally acceptable staging system based on big updated data, preferably using the TNM classifications, is desirable to direct future practice and research (4). In collaboration with the International Thymic Malignancies Interest Group (ITMIG) and the International Association for the Study of Lung Cancer (IASLC), a Thymic Domain of the Staging and Prognostic Factors Committee has recently proposed a new TNM stage classification system (5). We hereby use the Chinese Alliance for Research in Thymomas (ChART) retrospective database to compare these two staging systems.
Materials and methods
Two-thousand three hundred and seventy patients treated at 18 tertiary centers in China during 1992 to 2012 were retrospectively recorded in the ChART database and were reviewed for the purpose of the study. Of these, 1,172 patients were excluded (due to missing information for the new TNM staging proposal in 627, missing Masaoka-Koga stage data in 2, and missing survival data in 543), leaving 1,198 patients for final analysis. Only de-identified data were used for this staging study and informed consent was waived by IRB. Cumulative incidence of recurrence (CIR) was assessed only in R0 patients. Overall survival (OS) was evaluated both in an R0 resected cohort, as well as in all patients (any R status). Results of recurrence and OS were first assessed according to the Masaoka-Koga staging system. And then, they were reevaluated using the new TNM staging proposal for comparison.
Statistical analysis was undertaken using the SPSS 18.0 software. Survival curves were estimated using the Kaplan-Meier method, and the significance of differences was assessed with Log-rank test. The CIR, which accounts for the presence of the competing, was used to estimate recurrence. Cox regression models were used to obtain hazard ratios for OS and recurrence adjusted for diagnosis. A two-sided P value less than 0.05 was considered to be statistically significant.
Results
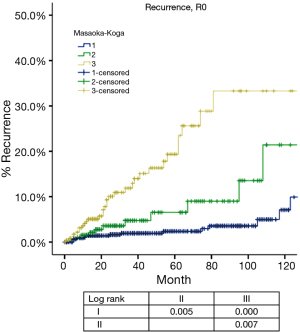
Based on Masaoka-Koga staging system, pathological staging was stage I in 618, stage II in 200, stage III in 319, stage IVa in 23 and stage IVb in 38 patients. Recurrence rate (Table 1) in patients with R0 resection increased with higher tumor stage, while OS (Table 2) in patients with R any resection decreased. CIR in patients with R0 resection was shown in Figure 1 and Table 3. Differences in CIR between stage I and stage II or III were statistically significant (P=0.005, P=0.000; respectively), as well as that between stage II and III (P=0.007). OS of patients with any R resection was shown in Figure 2 and Table 3. Statistical significance was detected in differences of OS between stage I and stage III (P=0.000), and between stage IVb and all other stage categories (P<0.05); whereas differences between stage II and stage I or stage III were not significant (P=0.111, P=0.103; respectively).

Full table

Full table
Full table

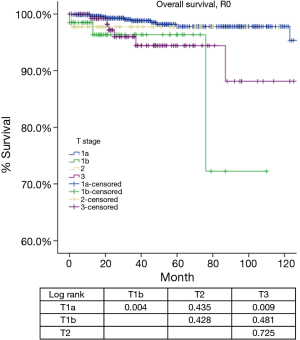
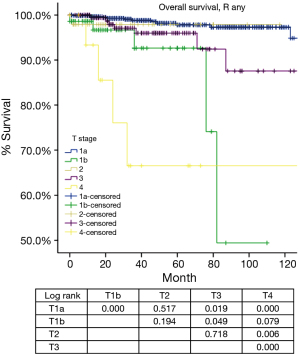
According to the new TNM staging proposal, pathological staging was stage I in 886, stage II in 48, stage III in 205, stage IVa in 38 and stage IVb in 21 patients. Again recurrence rate in patients with R0 resection increased with higher tumor stage (Table 4), while OS in patients with R any resection decreased (Table 5). For T categories, CIR in TxN0M0 R0 patients with T1a was significantly lower compared to patients with other T stages (P<0.05). Especially noticeable was the significant difference in CIR between T1a and T1b tumors (P=0.021). However, differences in CIR between T1b and T2 or T3 were not significant (P=0.315, P=0.215; respectively), neither was the difference between T2 and T3 (P=0.963, Figure 3). For OS in TxN0M0 R0 patients, T1a was significantly better than that of T1b (P=0.004), whereas no statistical difference was detected between T1b and T2 or T3 (P=0.428, P=0.481; respectively, Figure 4). For OS in TxN0M0 R any patients, T4 was significantly worse compared with all other T categories (P<0.05, Figure 5). Upon COX analysis, difference in OS was statistically significant between patients with T1a and T1b tumors (P=0.000), as well as that between T3 and T4 (P=0.001); whereas no statistical difference was detected between T2 and T3 (P=0.72, Table 6).
Full table
Full table


Full table
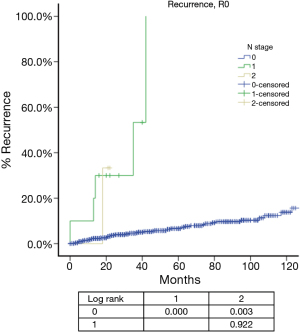
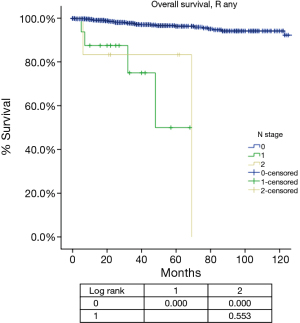
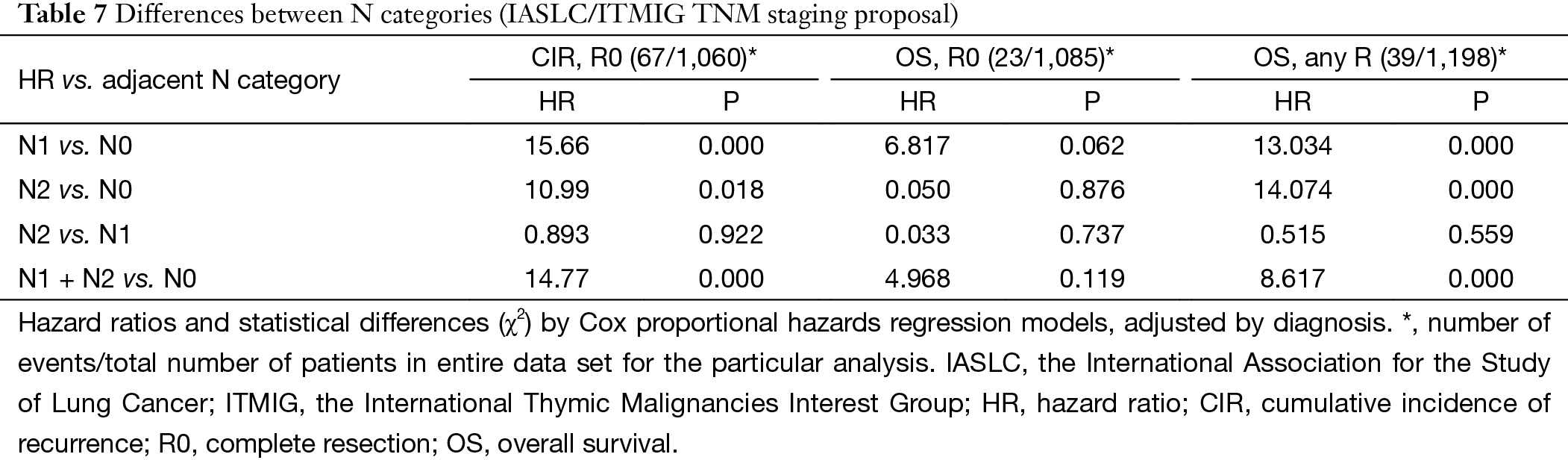
For N categories, CIR in R0 patients was shown in Figure 6 and OS in R any patients was shown in Figure 7. CIR and OS in N negative patients were both better than those of N positive patients (P<0.05), whereas no statistical difference was detected between N1 and N2 (P>0.05). Upon COX analysis, N positive was a significant risk factor for increased CIR in patients with R0 resection and also a significant risk factor for worse OS in patients with any R (Table 7).
Full table
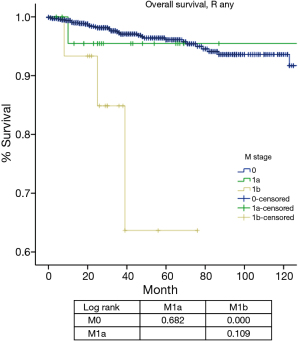
For M categories, CIR or disease progression in R any M negative patients was significantly lower than that in patients with M positive diseases (P<0.05), whereas no statistical difference was detected between M1a and M1b (P=0.263, Figure 8

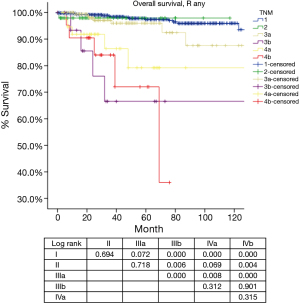
Based on the proposed new TNM staging, CIR in R0 patients with stage I disease was significantly lower than stage II or stage IIIa (P=0.000, P=0.000; respectively), with no statistical difference detected between stage II and stage IIIa (P=0.963). OS in R any patients with stage I and stage II diseases was similar (P=0.694), as well between patients with stage II and stage IIIa (P=0.718). OS in R any patients with stage IIIa was significantly better than in those with stage IIIb tumors (P=0.000). For OS in R any patients, stage IVb was worst among all categories. Moreover, there was no statistical difference detected in OS between stage IIIb and stage IVa (P=0.312), or between stage IVa with stage IVb (P=0.315) (Table 8, Figure 10).
Full table
Discussion
Almost a dozen of different staging systems have been proposed for thymic malignancies (6-17). But few have adopted the TNM approach as in most other solid tumors. The IASLC/ITMIG proposal for the new UICC staging of thymic malignancy is mostly based on the widely used Masaoka-Koga system, but using the TNM components instead. As can be seen from Table 9, stages I–IIIb in this new staging system are classified primarily by the T component, which are corresponding to stages I–III in the Masaoka-Koga system. Stages IVa and IVb are determined by the presence of N1 or M1a disease for IVa and N2 or M1b disease for IVb (5), while in the Masaoka-Koga staging system all lymphatic metastasis were classified as stage IVb.
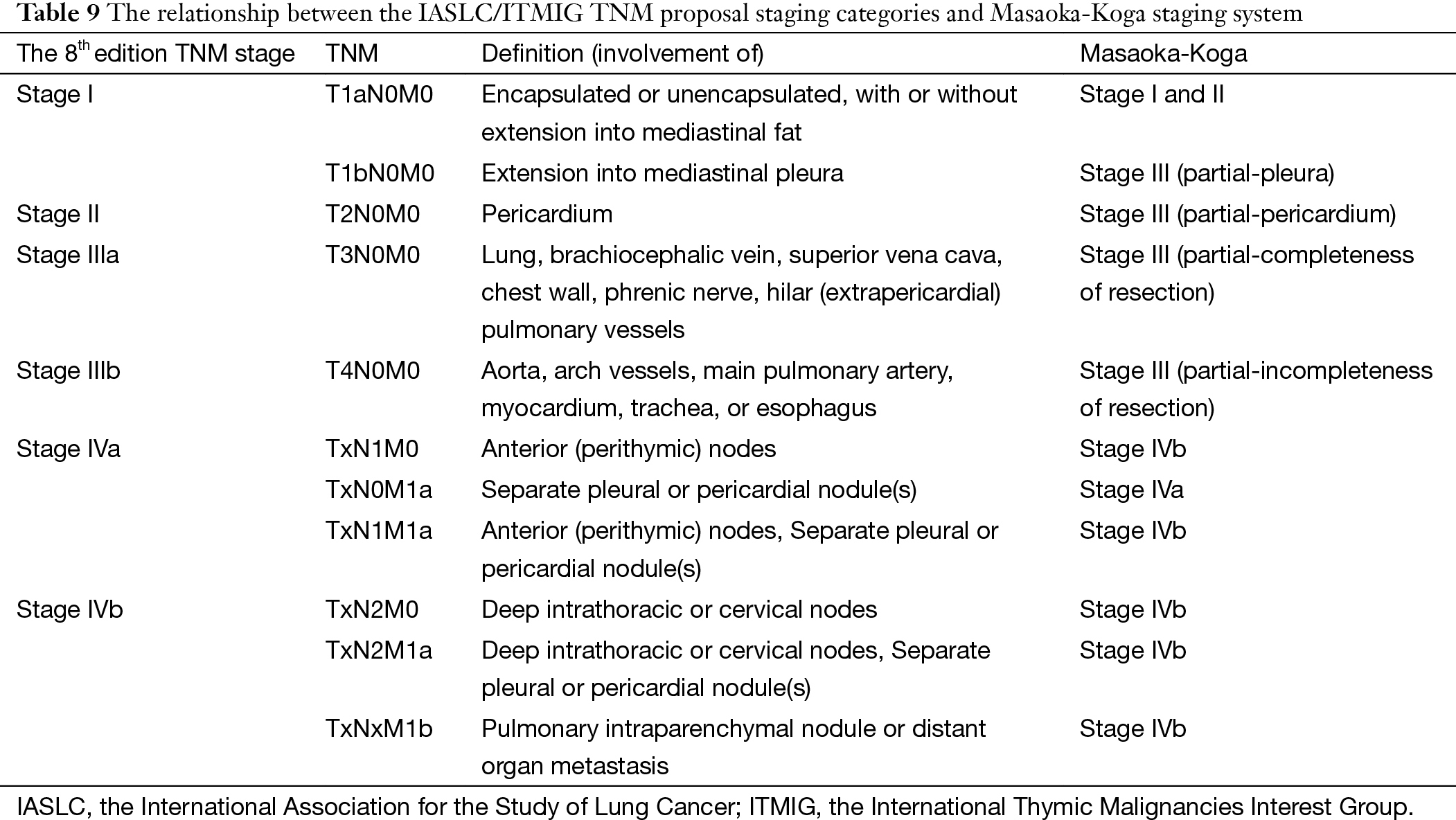
Full table
Our results showed that although there were significant differences in CIR among Masaoka-Koga stage I to III tumors, OS remained similar between stage I and II (Tables 1-3, Figures 1,2). These suggest that combining Masaoka-Koga stage I and II together to become T1a (stage I) as in the ITMIG proposed system may be warranted (18). Still, the difference between recurrence rates in tumors with or without invasion into the capsule or mediastinal fat (Masaoka-Koga stage I and II) leaves the question whether they should be further subdivided in the future, as recurrence is also an important measure in less aggressive tumors (19).
Tumors invading the mediastinal pleura were classified as stage II in the Masaoka and stage III in the Masaoka-Koga systems. They are now included into stage I because no consistent difference in outcomes (recurrence or survival) were detected during the IASLC/ITMIG staging project. Division into T1a and T1b was preserved because there was a slight difference in CIR in patients from Japan submitted by the Japanese Association for Research in the Thymus. Hopefully this could leave a window open for further testing. However, in the present study, there was a significant difference in both CIR and OS between T1aN0M0 and T1bN0M0 patients (Tables 4,5,6, Figures 3,4,5) from the ChART database. Pleural invasion theoretically increase the chance of pleural cavity dissemination, which is the most common type of recurrence in thymic tumors. Given the difficulty in identifying pleural invasion in pathology, it is thus critically important to mark out mediastinal pleura in surgical specimens and prospectively record invasion status for future investigation.
Stage III in the Masaoka-Koga system is highly heterogeneous. Tumors invading mediastinal pleura (T1b), pericardium (T2), or any other structures (T3–4) are all included in a single category. In the current study, we failed to find any survival difference between Masaoka-Koga stage II and III, although CIRs were significantly different (Tables 1,2,3, Figures 1,2). Intuitively, limited invasion into readily resectable structures and those vital organs not readily resectable would carry different prognostic impact. In ChART patients we did not detect any significant difference in OS or CIR among T1b to T3 (stage I to IIIa in the IASLC/ITMIG proposal) diseases, although all were distinct from T1a tumors (Tables 4,5,6, Figures 3,4,5). The separation of recurrence or survival curves between T1 and T2 or T3 could be contributed to the better outcome in T1a diseases. Only in T4 tumors (stage IIIb) did survival and recurrence results became significantly worse. And we failed to find any significant difference between stage I and stage II or IIIa according to the IASLC/ITMIG proposal (Table 8, Figure 10). This echoes with numerous previous studies revealing radical resection as an independent prognostic factor for thymic malignancies (20), as complete tumor removal can readily be achieved in T1 to T3 tumors. Since systemic dissemination is not commonly encountered in this low grade tumor, prognosis may be similar as long as the lesions could be completely resected. Considering that the TNM system is an anatomical classification, differentiating extent of tumor invasion according to the T categories of the IASLC/ITMIG proposal is warranted. However, prognostic grouping should still be based on long-term outcome of the patients. Thus except for stage IIIb (T4), further analysis is necessary to validate the current stage grouping in the IASLC/ITMIG proposal for the new staging system.
Among all the staging proposals for thymic malignancy, only four have used the TNM approach (11,12,15,21). In all others lymphatic involvement was simply considered as a sign of late stage disease. In the IASLC/ITMIG proposal lymph node metastasis was still classified as stage IV. But ITMIG has also proposed a new mediastinum lymph node map (21). This helped to separate the N status into N0 to N1–2 in the proposed new staging (22). However, no significant difference was detected between N1 and N2 diseases in either OS or CIR. Nor was the current study able to reveal any statistical significance between these two nodal statuses, as there were few patients with N (+) diseases and even fewer events in survival or recurrence analysis (Table 7), although there was indeed a significantly increased CIR (Figure 6) and worse OS (Figure 7) in node positive patients as compared to node negative patients. Lymph node dissection has seldom been considered as a necessary part of surgery for thymic tumors. A correct estimation of true incidence or extent of lymphatic involvement would be impossible if systemic nodal dissection or sampling is missing. Only with future studies based on such information could the prognostic impact of lymphatic involvement be correctly addressed.
M categories in the IASLC/ITMIG proposal was divided into M1a (pleural dissemination) and M1b (distant organ metastasis) (22). And they were grouped as stage IVa and IVb, respectively, similar to the stage IVa and IVb classification in the Masaoka-Koga system. However, there was only a visual separation of the survival curves between M1a and M1b during the staging process. In the current study, we did not find a statistical significance in CIR or OS between these two categories, either. Both M1 categories had worse prognosis than M0 patients (Figures 8,9). However, it is interesting to notice that while the M1a group had a significantly higher CIR than the M0 group (Figure 8), its OS was not significantly different from the latter (Figure 9). This may again be attributed to the few events noticed in survival analysis. For tumors with an indolent nature as thymic malignancy, long-term survival could still be expected even if local regional spread like pleural dissemination is present. On the other hand, distant organ metastasis represents a true adverse prognostic factor. Both CIR and OS in the M1b group were significantly worse than the M0 group.
As for prognostic grouping, we found that OS was almost always statistically different when comparison was made between stages I–IIIa and stages IIIb–IVb (Table 8, Figure 10). The differences were of borderline significance in comparison between stage I and IIIa (P=0.072), and between stage II and IVa (P=0.069). However, no statistical difference could be detected among stages IIIb to IVb. Although CIR were significantly lower in stage I as compared to stages II or IIIa, no statistical difference was revealed in OS among the three stages.
Overall, the ISLAC/ITMIG proposal of a new staging for thymic tumors was a major step forward in this relatively rare disease. It was the first time that careful analysis was carried out based on a large multicenter data with worldwide collaboration. The TNM components were adopted to describe tumor invasion as well as dissemination. The inability to discriminate survival difference in advanced stage disease is mostly owing to the nature of a surgically dominated database, and the unique behavior of the disease itself in slow progress and long-term survival. Using the ChART database which is also surgically dominated, we failed to demonstrate prognostic differences between N1 and 2 or M1a and 1b categories, except for a clear difference between N0 and N (+) or M0 and M1b diseases. In T components, T1a and T4 clearly stand for the two extremes of prognosis, while T1b through T3 show no statistical difference in recurrence or OS. This in itself reflects precisely the critical importance of complete resection in the management of thymic tumors. The new staging proposal provides a useful tool for future studies for better prognostic groupings. Careful recording the TNM components separately in each case and in a prospective manner would help revealing their prognostic significance which may not be able to attain with retrospective studies.
Acknowledgements
None.
Footnote
Members of Chinese Alliance for Research in Thymomas (ChART): Yi Shen, Yucheng Wei, Affiliated Hospital of Qingdao University, Qingdao, China; Yin Li, and Guanghui Liang, Affiliated Cancer Hospital of Zhengzhou University, Zhengzhou, China; Keneng Chen and Hao Fu, Beijing Cancer Hospital, Beijing, China; Hezhong Chen and Shihua Yao, Changhai Hospital, Shanghai, China; Youbin Cui and Yanzhong Xin, First Affiliated Hospital of Jilin University, Changchun, China; Renquan Zhang and Ningning Kang, First Hospital of Anhui Medical University, Hefei, China; Lijie Tan, Jianyong Ding, Hao Wang, Gang Chen, and Jie Wu, Zhongshan Hospital, Fudan University, Shanghai, China; Chun Chen and Wei Zheng, Fujian Medical University Union Hospital, Fuzhou, China; Liewen Pang and Fangrui Wang, Huashan Hospital, Fudan University, Shanghai, China; Yangchun Liu and Qing Lin, Jiangxi People’s Hospital, Nanchang, China; Yongyu Liu and Yongkai Wu, Liaoning Cancer Hospital, Shenyang, China; Wentao Fang, Jie Zhang, Yan Shen, Changlu Wang, Lei Zhu and Zhitao Gu, Shanghai Chest Hospital, Shanghai Jiao Tong University, Shanghai, China; Yongtao Han, Lin Peng, Sichuan Cancer Hospital, Chengdu, China; Jianhua Fu and Qianwen Liu, Department of Thoracic Surgery, Guangdong Esophageal Cancer Institute, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center of Cancer Medicine, Guangzhou, China; Zhentao Yu and Jie Yue, Tianjin Cancer Hospital, Tianjin, China; Peng Zhang and Yuan Chen, Tianjin Medical University General Hospital, Tianjin, China; Yun Wang and Yingcai Geng, West China Hospital, Sichuan University, Chengdu, China; Xinming Zhou and Hongguang Zhao, Zhejiang Cancer Hospital, Hangzhou, China.
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Masaoka A, Monden Y, Nakahara K, et al. Follow-up study of thymomas with special reference to their clinical stages. Cancer 1981;48:2485-92. [Crossref] [PubMed]
- Koga K, Matsuno Y, Noguchi M, et al. A review of 79 thymomas: modification of staging system and reappraisal of conventional division into invasive and non-invasive thymoma. Pathol Int 1994;44:359-67. [Crossref] [PubMed]
- Detterbeck F, Youssef S, Ruffini E, et al. A review of prognostic factors in thymic malignancies. J Thorac Oncol 2011;6:S1698-704. [Crossref] [PubMed]
- Detterbeck FC, Asamura H, Crowley J, et al. The IASLC/ITMIG thymic malignancies staging project: development of a stage classification for thymic malignancies. J Thorac Oncol 2013;8:1467-73. [Crossref] [PubMed]
- Detterbeck FC, Stratton K, Giroux D, et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: proposal for an evidence-based stage classification system for the forthcoming (8th) edition of the TNM classification of malignant tumors. J Thorac Oncol 2014;9:S65-72. [Crossref] [PubMed]
- Bergh NP, Gatzinsky P, Larsson S, et al. Tumors of the thymus and thymic region: I. Clinicopathological studies on thymomas. Ann Thorac Surg 1978;25:91-8. [Crossref] [PubMed]
- Wilkins EW Jr, Castleman B. Thymoma: a continuing survey at the Massachusetts General Hospital. Ann Thorac Surg 1979;28:252-6. [Crossref] [PubMed]
- Verley JM, Hollmann KH. Thymoma. A comparative study of clinical stages, histologic features, and survival in 200 cases. Cancer 1985;55:1074-86. [Crossref] [PubMed]
- Regnard JF, Magdeleinat P, Dromer C, et al. Prognostic factors and long-term results after thymoma resection: a series of 307 patients. J Thorac Cardiovasc Surg 1996;112:376-84. [Crossref] [PubMed]
- Weydert JA, De Young BR, Leslie KO, et al. Recommendations for the reporting of surgically resected thymic epithelial tumors. Am J Clin Pathol 2009;132:10-5. [Crossref] [PubMed]
- Gamondès JP, Balawi A, Greenland T, et al. Seventeen years of surgical treatment of thymoma: factors influencing survival. Eur J Cardiothorac Surg 1991;5:124-31. [Crossref] [PubMed]
- Yamakawa Y, Masaoka A, Hashimoto T, et al. A tentative tumor-node-metastasis classification of thymoma. Cancer 1991;68:1984-7. [Crossref] [PubMed]
- Tsuchiya R, Koga K, Matsuno Y, et al. Thymic carcinoma: proposal for pathological TNM and staging. Pathol Int 1994;44:505-12. [Crossref] [PubMed]
- Travis WD, Brambilla E, Muller-Hermelink H, et al. Pathology and genetics of tumors of the lung, pleura, thymus and heart. In: Kleihues P, Sobin L, editors. WHO Classification of Tumors. Second Edition. Lyon: IARC Press, 2004:145-97.
- Bedini AV, Andreani SM, Tavecchio L, et al. Proposal of a novel system for the staging of thymic epithelial tumors. Ann Thorac Surg 2005;80:1994-2000. [Crossref] [PubMed]
- Kondo K, Monden Y. Lymphogenous and hematogenous metastasis of thymic epithelial tumors. Ann Thorac Surg 2003;76:1859-64; discussion 1864-5.
- Kondo K. Tumor-node metastasis staging system for thymic epithelial tumors. J Thorac Oncol 2010;5:S352-6. [Crossref] [PubMed]
- Nicholson AG, Detterbeck FC, Marino M, et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: proposals for the T Component for the forthcoming (8th) edition of the TNM classification of malignant tumors. J Thorac Oncol 2014;9:S73-80. [Crossref] [PubMed]
- Huang J, Detterbeck FC, Wang Z, et al. Standard outcome measures for thymic malignancies. J Thorac Oncol 2010;5:2017-23. [Crossref] [PubMed]
- Fang W, Chen W, Chen G, et al. Surgical management of thymic epithelial tumors: a retrospective review of 204 cases. Ann Thorac Surg 2005;80:2002-7. [Crossref] [PubMed]
- Bhora FY, Chen DJ, Detterbeck FC, et al. The ITMIG/IASLC Thymic Epithelial Tumors Staging Project: A Proposed Lymph Node Map for Thymic Epithelial Tumors in the Forthcoming 8th Edition of the TNM Classification of Malignant Tumors. J Thorac Oncol 2014;9:S88-96.
- Kondo K, Van Schil P, Detterbeck FC, et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: proposals for the N and M components for the forthcoming (8th) edition of the TNM classification of malignant tumors. J Thorac Oncol 2014;9:S81-7. [Crossref] [PubMed]


